Graphical abstract
Poly(imide siloxane) exhibits good thermal and mechanical properties with enhanced flexibility and hydrophobicity.

Abstract
A series of poly(imide siloxane) (PIS) copolymers were synthesized using 1,3-bis(aminopropyl)tetradimethylsiloxane, naphthalene-1,4,5,8-tetracarboxylic dianhydride, and 4,4-oxydianiline as building blocks, with a siloxane content of 20–50 wt%, showing high solubility in organic solvents. Attenuated total reflectance-Fourier transform infrared and proton nuclear magnetic resonance spectroscopy were used to structurally characterize the polymers. The thermal properties of the copolymers were measured by thermogravimetric analysis and differential scanning calorimetry. Dynamic mechanical analysis measured the thermomechanical dynamics of polymers. The addition of siloxane boosted the material’s stretchability and water resistance but decreased its stiffness and pulling force resistance due to the plasticizing effect of the dimethylsiloxane moiety. Polymers with naphthalene moieties in the backbone also have different thermal and mechanical properties. PIS films were compared with the flexible bisphenol-A core having 30 wt% siloxane loading (PIS-BPADA-30). The morphological properties were examined using scanning electron microscopy and atomic force microscopy. The polymer backbone became more flexible and hydrophobic when siloxane was included. For hydrophobic and durable applications, siloxane-modified polyimides showed potential.
1 Introduction
The exploration of advanced materials has gained significant momentum in various fields, including electronics, aerospace, and biomedical engineering, where there is a continuous demand for polymers that exhibit superior thermal and mechanical properties (1–3). Among these materials, non-sulfonated poly(imide siloxane) (PIS) copolymers have emerged as a compelling choice due to their unique combination of flexibility, thermal stability, and chemical resistance (4,5). Non-sulfonated PIS copolymers have made notable progress in the last few decades, characterized by major achievements in material synthesis and use. Over the past few decades, researchers have synthesized different types of PIS copolymers (6,7). In a typical preparation procedure, the polysiloxane diamine, mostly bis(γ-aminopropyl)polydimethylsiloxane (APPS), was initially combined with the dianhydride solution. Subsequently, the non-siloxane diamine was added to the dianhydride and anhydride-capped polysiloxane (6–9). The polymer chain contained a random distribution of the polysiloxane diamine. The length of the polysiloxane soft block was dictated by the molecular weight of the polysiloxane diamine, while the length of the polyimide hard segment was dependent on its composition. The copolymers that were developed were referred to as randomly segmented PIS copolymers. This resulted in the creation of a new group of materials that showed great potential for many uses, such as membrane technology for separating gases (10). The development of membrane technology reveals that non-sulfonated polyimide membranes possess notable benefits compared to sulfonated membranes, especially in terms of stability and performance under specified circumstances (10).
Polysiloxanes, commonly known as silicones, are a class of synthetic polymers characterized by their unique chemical structure consisting of alternating silicon and oxygen atoms with organic substituents attached to the silicon atoms (11–16). Their inorganic–organic hybrid nature endows them with a range of desirable properties, including thermal and oxidative stability, easy to process, relatively low water absorptivity, enhanced adhesion to a wide range of surfaces, flexibility, and gas permeability, making them valuable in various industrial applications (9,17–22). Some of the recent research papers aim to provide a detailed investigation into the thermal stability and mechanical properties of aminopropyl-terminated polysiloxane copolymers with varying siloxane content, which is of particular interest due to the potential applications of these materials in diverse fields such as aerospace, automotive, and biomedical industries (23,24). The chemical structure and composition of aminopropyl-terminated dimethylsiloxane moieties in the copolymer backbone significantly influence their thermal and mechanical characteristics (25). Incorporating organic functional groups, such as the aminopropyl moiety, can further enhance the versatility of these materials by imparting additional functionality and allowing for the tailoring of their properties to meet specific requirements. The influence of the siloxane content on the thermal stability and mechanical behavior of these copolymers is crucial to understand, as it can provide valuable insights for optimizing their performance in different applications (26).
As a result of their adaptable features, non-sulfonated PIS copolymers have widespread applications across a variety of industrial sectors. One of the most important uses is in the field of membrane technology, where these copolymers are used as substrates for gas separation membranes. As a result of their capacity to improve gas permeability while preserving structural integrity, they are ideally suited for applications that aim to reduce emissions and improve the energy efficiency (10,27). In addition, these copolymers are utilized extensively in the production of membranes for a variety of processes, such as the treatment of water and the separation of chemicals, because their resistance to solvents and thermal stability play an important role (27,28). Because they can be developed to satisfy specific needs for medical devices and drug delivery systems, non-sulfonated PIS copolymers are gaining popularity in the biomedical industry for applications that require biocompatibility and environmental friendliness (10). This is because these copolymers can be engineered to suit specific requirements. Furthermore, their one-of-a-kind mechanical and thermal qualities make them important in the aerospace and automotive industries, which are two sectors in which lightweight and long-lasting materials are vital for ensuring both performance and safety (10). In the ongoing research and development that is being conducted for the synthesis and functionalization of these copolymers, the application potential of these copolymers is continuing to expand across a variety of industries. A variety of commercially available siloxane polymers and copolymers have been used in membrane technology, but these polymers have a number of disadvantages like polydimethylsiloxane (PDMS), which shows very low glass transition temperature (T g) (−120°C), is susceptible to swelling in organic solvents, and has poor mechanical stability. Polymethylphenylsiloxane (PMPS) has reduced gas permeability compared to PDMS and has limited mechanical robustness. Polytrifluoropropylmethylsiloxane (PTFPMS) is expensive due to fluorination and has reduced mechanical flexibility, while polydiphenylsiloxane (PDPS) is brittle and has poor mechanical properties (29–33).
PIS copolymers could be positioned as a cornerstone in the future of sustainable energy solutions by addressing the limitations of existing materials and exploring the design of hybrid or composite systems as the field continues to evolve. This could further enhance the efficacy and applicability of those materials. Siloxane polymers, despite providing excellent permeability and flexibility, frequently exhibit limited selectivity, solvent-induced swelling, and inadequate mechanical strength, hence constraining their utilization in membrane technologies without modifications or composite structures (31,34). This study details the results of synthesizing and characterizing PISs with different siloxane loadings in the main chain, along with a rigid naphthalene moiety, and its effects on the thermal, mechanical, optical, contact angle, and morphological properties.
2 Materials and methods
2.1 Materials
The reagent 1,3-bis(aminopropyl)tetradimethylsiloxane (BPTS) was purchased from Alfa Aesar. Naphthalene-1,4,5,8-tetracarboxylic dianhydride (NTDA) and (4,4′-(4,4′-isopropylidenediphenoxy)bis(phthalic anhydride) (BPADA) were purchased from TCI, India, and they were preheated at 120°C for 12 h prior to use. 4,4-Oxydianiline (ODA) was purchased from Sigma Aldrich and was used as received. Chloroform (CHCl3), dichloromethane (CH2Cl2), 1,2-dichlorobenzene (ODCB), dimethylformamide (DMF), N,N-dimethyl acetamide (DMAc), and N-methyl-2-pyrrolidinone (NMP), dimethyl sulfoxide, and acetone were purchased from E. Merck and were used as received.
2.2 Synthesis of non-sulfonated poly(imide siloxane) (PIS) copolymers
A series of non-sulfonated PIS copolymers were synthesized by polycondensation reactions using NTDA as the dianhydride and two different diamines ODA and BPTS, containing siloxane contents of 20, 30, 40, and 50%, as shown in Scheme 1. Also, a BPADA moiety-based PIS with 30 wt% siloxane loading (PIS-BPADA-30) was synthesized.

Reaction scheme of non-sulfonated PISs.
The synthesis procedure for PIS-30 is explained below. A Dean–Stark apparatus equipped with a condenser, a magnetic stirrer, and a 50 mL three-necked round-bottomed flask with a nitrogen inlet was charged with 1.200 g of NTDA, 0.627 g of ODA, 0.333 g of BPTS, and 20 mL of ODCB. The mixture was continuously stirred under a nitrogen atmosphere. The temperature of the reaction mixture was gradually increased from ambient to 180°C. The reaction was maintained at 180°C for 16 h. When the temperature was increased, the solution became more viscous, which is a sign that polymerization had taken place. Subsequently, the mixture was cooled to room temperature, and the viscous polymer solution was precipitated in 300 mL of methanol. The fibrous polymer product was then filtered and dried overnight at around 100°C in a vacuum.
2.2.1 PIS-20
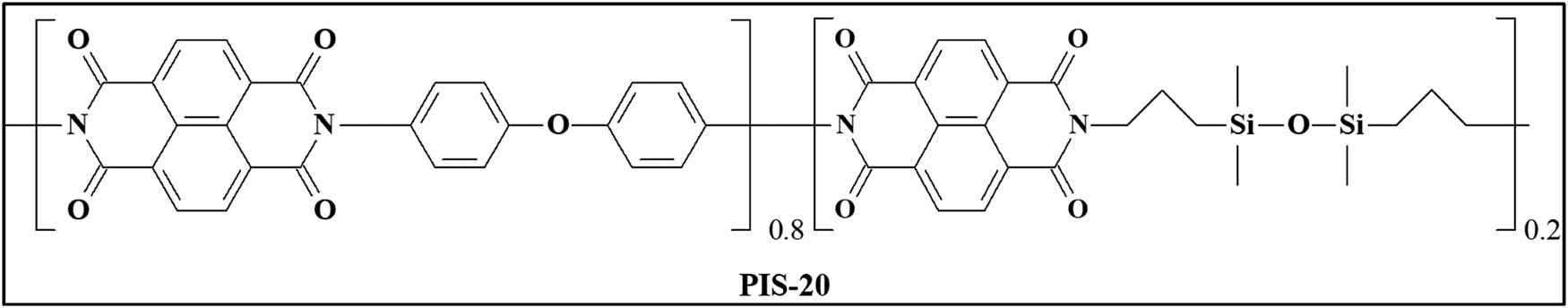
ATR-FTIR (KBr) (cm −1 ): 2,960 (–CH3 group present in BPTS); 1,750–1,800 (–CO– stretch); 1,376 (C–N stretch); 1,000–1,500 (Si–O–Si stretching); 750–850 (801 (Si–C).
1 H NMR (CDCl 3 ): δ 8.75 (8H, Ar–H); 7.6–7.3 (6.4H, Ar–H); 3.62 (s, 0.8H, –CH2–N); 1.75 (s, 0.8H, –CH2–); 0.91–0.54 (m, 0.8H, –CH2–Si); 0.19–0.02 ppm (m, 2.4H, Si–CH3).
2.2.2 PIS-30

ATR-FTIR (KBr) (cm −1 ): 2,960 (–CH3 group present in BPTS); 1,750–1,800 (–CO– stretch); 1,376 (C–N stretch); 1,000–1,500 (Si–O–Si stretching); 750–850 (801 (Si–C).
1 H NMR(CDCl 3 ): δ 8.75 (8H, Ar–H); 7.6–7.3 (5.6H, Ar–H); 3.62 (s, 1.2H, –CH2–N); 1.75 (s, 1.2H, –CH2–); 0.91–0.54 (m, 1.2H, –CH2–Si); 0.19–0.02 ppm (m, 3.6H, Si–CH3).
2.2.3 PIS-40
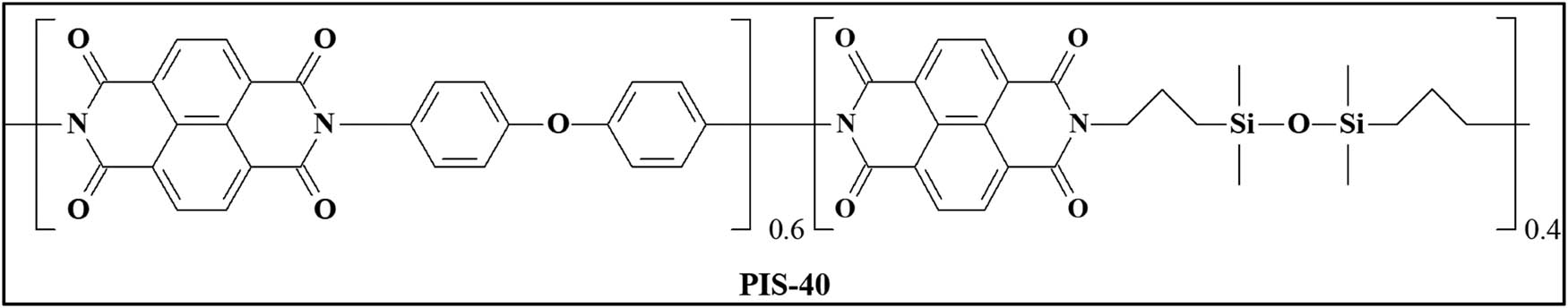
ATR-FTIR (KBr) (cm −1 ): 2,960 (–CH3 group present in BPTS); 1,750–1,800 (–CO– stretch); 1,376 (C–N stretch); 1,000–1,500 (Si–O–Si stretching); 750–850 (801 (Si–C).
1 H NMR(CDCl 3 ): δ 8.75 (8H, Ar-H); 7.6–7.3 (4.8, Ar-H); 3.62 (s, 1.6H, –CH2–N, 1.75 (s, 1.6H, –CH2–); 0.91–0.54 (m, 1.6H, –CH2–Si); 0.19–0.02 ppm (m, 4.8H, Si–CH3).
2.2.4 PIS-50

ATR-FTIR (KBr) (cm −1 ): 2,960 (–CH3 group present in BPTS); 1,750–1,800 (–CO– stretch); 1,376 (C–N stretch); 1,000–1,500 (Si–O–Si stretching); 750–850 (801 (Si–C).
1 H NMR(CDCl 3 ): δ 8.75 (8H, Ar-H); 7.6–7.3 (4.5H, Ar-H); 3.62 (s, 2.0H, –CH2–N); 1.75 (s, 2.0H, –CH2–); 0.91–0.54 (m, 2.0H, –CH2–Si); 0.19–0.02 ppm (m, 6.0H, Si–CH3).
2.3 Fabrication of polymer films
The polymer was purified by dissolving the fibrous product in 20 mL of CH2Cl2 and was re-precipitated using methanol. A measured amount of the purified polymer was dissolved in CH2Cl2 and transferred into Petri dishes, where the solvent was allowed to evaporate at 30°C overnight. The Petri dishes were then placed in a vacuum oven, gradually heated to 120°C, and kept under continuous vacuum for 5–6 h to ensure complete removal of the residual solvent. Finally, the transparent, flexible, and free-standing polymer film was obtained by submerging the Petri dishes in boiling water (Figure 1). Also, the photographs of PIS-BPADA-30 are depicted in the Supporting Information (Figure S1).

Images of transparent, flexible, non-sulfonated PIS films: (a) free-standing, (b) and (c) bending, and (d) twisting.
2.4 Polymer and polymer film characterization
The chemical structure of the polymers was characterized by attenuated total reflectance-Fourier transform infrared (ATR-FTIR) and nuclear magnetic resonance (NMR) spectroscopy. The ATR-FTIR spectra were recorded using a NEXUS 870 Thermo Nicolet spectrometer (USA). 1H NMR spectral data were recorded using a Bruker Advance (600 MHz), and CDCl3 was used as a solvent. Powder X-ray diffraction (PXRD) was performed using a Malvern Panalytical 3rd generation Empyrean X-ray diffractometer with a Cu anode (λ = 1.54 Å). The measurements were carried out at 40 kV and 30 mA with a step size of 5°·s−1.
Thermogravimetric analysis (TGA) was carried out on TGA Q50 from TA Instruments (USA) to check the thermal stability of the polymers. It was carried out under synthetic air (N2:O2 = 80:20) at a heating rate of 10°C·min−1. Differential scanning calorimetry (DSC) was carried out on a DSC Q20 from TA Instruments at a scan rate of 10°C·min−1 under nitrogen.
DMA 242 E Artemis instrument (NETZSCH, Germany) was used for dynamic mechanical analysis (DMA) to study the viscoelastic characteristics of the PIS films at 10 Hz in the range of about 30–320°C at a heating rate of 5°C·min−1. The polymer films were around 60–80 µm thick, having 30 mm length and 5 mm width. The mechanical stability of the polymer films was investigated using a Tinius Olsen (UK) universal testing machine at room temperature using a 100 N load cell with a 5 mm·min−1 crosshead speed (35,36). The contact angles were measured using a Surface Electro-Optics (S.E.O.) – Pheonix contact angle analyzer. The measurements were conducted at ambient temperature in distilled water using the sessile drop technique. Each sample was subject to a minimum of three measurements, and the resulting average was calculated. UV–vis spectra were recorded in solution form using an Agilent Cary Series UV–vis spectrophotometer.
The field emission scanning electron microscopy (FE-SEM) technique was employed to study the surface morphology of the polymer films. The analysis was conducted using a ZEISS EVO 60 scanning electron microscope manufactured by Carl ZEISS SMT in Germany. Before imaging, a sputter coater was used to provide a coating of gold–palladium to each of the samples (35,37). Atomic force microscopy (AFM) analysis was done to study the surface morphology in intermittent contact mode (tapping mode) using an AFM 5500 (Agilent Technology).
3 Results and discussion
3.1 Synthesis of PIS copolymers
Copolymerization is a widely employed and effective method for synthesizing novel materials with targeted features, achieved by integrating two distinct structures with varying chemical or physical characteristics into a single polymer chain (38). Polyimides modified with polysiloxane demonstrate numerous advantageous properties, including excellent solubility, elevated hydrophobicity, facile processability, resistance to degradation in harsh oxygen environments, superior adhesion, low dielectric constant, minimal stress, high flexibility, and impact resistance (33,39). The primary characteristic of copolymers is their capacity for phase separation, resulting in the formation of thermoplastic elastomers (30).
The high-temperature PIS copolymers were synthesized using the condensation polymerization process of a diamine and a dianhydride leading to random copolymers. This process was chosen because it was the most appropriate and cost-effective method among various polymerization procedures for synthesizing copolymer materials (40–48).
3.2 Characterization of PIS copolymers
The chemical structure of the PIS copolymers was confirmed by the ATR-FTIR spectrum in Figure 2. The lack of the peak at 3,450 cm−1 in all spectra suggested the complete imidization. The films showed an absorption band in the range of 1,750–1,800 cm−1, which corresponds to the C═O stretching corresponding to the imide bond (49). Additionally, incorporation of siloxane was supported by the presence of significant peaks that can be seen at 1,020 cm−1, which corresponds to Si–O–Si, around 750–850 cm−1 corresponding to the Si–C bond (49), and a characteristic absorption peak at about 2,960 cm−1, which was attributed to the C–H stretching bond (50). A slight increase in the intensities could be detected with the increase in the siloxane content. These results confirm the successful synthesis of the intended final products.

ATR-FTIR spectra of non-sulfonated PIS films.
3.3 Polymer solubility
The solubility of PISs for different organic solvents was tested. At room temperature, these polymers showed very good solubility in CHCl3, CH2Cl2, ODCB, DMF, DMAc, and NMP (51). The solubility of the copolymers in DMF, ODCB, and CHCl3 changed from being easily soluble at ambient temperature to becoming soluble or partially swelling when heated as the lengths of the polyimide and siloxane portions in the polymer backbone increased. This is likely due to the fact that the solubility of the PIS hard segment decreased as its length increased, which, in turn, had an impact on the solubility of the copolymers (52).
3.4 Thermal and mechanical properties of PISs
TGA and DSC measurements were used to evaluate the thermal properties of polymers. For TGA, the samples were first isothermal at 120°C for 2 min to remove moisture, cooled to 40°C, and then heated again at the same rate to 800°C (35). The TGA plot shown in Figure 3a demonstrates the naphthalene moiety-based PIS copolymer’s good thermal stability. The 10 wt% decomposition took place at T d (decomposition temperature) values around 480°C for PIS-20, 452°C for PIS-30, 438°C for PIS-40, and 400°C for PIS-50, which was comparable to that of commercially available PDMS at around 450°C. Naphthalene moiety-based siloxane-imide copolymers exhibited two distinct T ds originating from their siloxane and aromatic polyimide segments. The first T d of approximately 300–500°C is attributed to the presence of weak alkyl linkages (38,53). The second T d was associated with the degradation of the polyimide segment. An increase of the siloxane content in the PISs resulted in a somewhat decreased T d (6). The aromatic nature of the structure, as well as the imide and siloxane units, can be the reason for the high thermal stability of the polymers (54). At higher temperatures, the residual weight of polymers increased due to the inorganic character of silicon and the development of char from its presence (54). Also, the BPADA-based PIS copolymer (BPADA-PIS-30) exhibited similar thermal characteristics (Figure S3). The naphthalene moiety-based PIS copolymers exhibited T gs and no melting or crystallization temperatures which are due to their amorphous nature. There is an observed decrease in the T g values as the loading of siloxane in the PISs is increased, which can be attributed to the increase in the flexibility of the polymer backbone, as shown in Figure 3b. When compared with PIS-BPADA-30 (Figure S2), it was observed that the T g was reduced than that of the corresponding naphthalene moiety-based PIS with the same siloxane loading (49).

(a) TGA thermogram, (b) DSC curves, and (c) stress–strain curves of naphthalene moiety-based PIS films.
The stress–strain curves of the naphthalene moiety-based PIS films are shown in Figure 3c. The mechanical properties of thin PIS films and some commercial siloxane polymers are shown in Table 1. The siloxane-containing polymers have increased elongation at break (EB) sequentially with an increase in siloxane loading but show a lowering of the tensile strength. The films containing 50 wt% siloxane loading (PIS-50) show the highest EB of 45%. The tensile strength of the films ranges from 196 to 163 MPa, and the EB ranges from 20% to 45%. This is obvious since flexibility is generated by the plasticization effect of PDMS units. It was observed that the tensile strength values of the synthesized PIS were comparatively higher than that of the commercially available polymer like PDMS (4.9 MPa) and SILTEM STM1700 (62 MPa), while the Young’s modulus values were lower than those of PDMS (1.13 MPa) and SILTEM STM1700 (2.4 GPa), which make the synthesized PIS suitable for applications that require dimensional stability (57,58). The PIS-BPADA-30 film (Figure S4) showed an EB of around 26% and tensile strength and storage modulus of around 35.9 MPa and 1.35 GPa, respectively, which are higher than those of the corresponding naphthalene moiety-based PIS film. This can be attributed to the increase in siloxane content, which resulted in an increase in the EB but a decrease in both storage modulus and tensile strength. However, the presence of naphthalene moieties in the polymer backbone considerably contributes toward enhancing the modulus values of the polymers depending on the siloxane loading. These naphthalene-based PISs exhibited superior properties when compared to some other PIS series containing different anhydride moieties (Table 1). The well-known strong intermolecular forces and enhanced mechanical strength of the PI homopolymer surely contributed to the increased tensile strength of PIS copolymers. On the other hand, the flexibility of the polysiloxane soft segmented to an increase in the EB (38,59).
Tensile strength, Young’s modulus, EB, maximum storage modulus, T g values, and contact angles of PIS films
| Polymer | Tensile strength (MPa) | Young’s modulus (GPa) | EB (%) | Maximum storage modulus (MPa) | T g from tan δ (°C) | Water contact angle (°) | References |
|---|---|---|---|---|---|---|---|
| PIS-20 | 196 ± 1.2 | 3.39 ± 0.05 | 20 ± 1.5 | 4,416 | 250 | 76.3 ± 0.4 | |
| PIS-30 | 177 ± 1.1 | 3.17 ± 0.02 | 24 ± 0.9 | 1,288 | 234 | 78.2 ± 1.1 | |
| PIS-40 | 172 ± 1.3 | 2.47 ± 0.01 | 40 ± 0.8 | 1,179 | 224 | 85.6 ± 1.4 | |
| PIS-50 | 163 ± 1.1 | 2.38 ± 0.04 | 45 ± 1.2 | 760 | 220 | 94.7 ± 1.2 | |
| PIS-BPADA-30 | 35.9 ± 1.5 | 1.35 ± 0.03 | 26 ± 1.1 | — | — | 72.4 ± 1.5 | |
| 1 h (20 wt%APPS) | 48.40 | 1.30 | 18 | 1,522 | — | — | (55) |
| 1a | 61 | 2.30 | 4 | — | — | — | (56) |
| PDMS | 4.92 | 0.00113 | 461 | — | −125 to −150 | — | (57) |
| SILTEM STM1700 | 62 | 2.4 | 5 | — | 200* | — | (58) |
*T g value from DSC.
DMA was used to assess the viscoelastic properties of the polymer samples to obtain the T g values and changes in the modulus of the polymers with temperature (Figure 4). The figure shows the storage modulus versus temperature curves and the loss tangent (tan δ) versus temperature curves of the naphthalene moiety-based PIS films (Figure 4a and b). The T g taken from the tan δ peaks at 10 Hz is shown in Figure 4b. Because of the low mixing entropy between the PDMS soft segment and the polyimide hard segment, PISs always exhibit a microphase-separated structure. A decreasing trend in the T g can be attributed to the increasing siloxane content in the copolymers (Table 1) (60). It was observed that the T g values obtained from DSC and DMA were somewhat different. For DSC, T g corresponds to the onset of segmental motion, occurring at lower energy states, while DMA requires higher energy input to induce significant motion, resulting in a higher T g. Additionally, the difference in T g values could be due to the difference in the heating rates with DSC conducted at a relatively fast heating rate which limits the relaxation time for molecular segments, while DMA was performed at a slower heating rate or under oscillatory stress with varying frequency, which allows more time for molecular relaxation pushing T g to higher values (61). There is a significant reduction in storage moduli observed in PISs when they reach the T g of the polyimide backbone, which may be due to greater flexibility generated by increasing the soft siloxane part of the copolymers (Figure 4b) (62). This suggests that the material’s structural integrity is compromised when exposed to thermal stress (38). In general, the mechanical properties of the PIS films are very good.

(a) Storage moduli and (b) loss tangents (tan δ) of naphthalene moiety-based PIS films as a function of temperature.
3.5 Contact angle study
It is critically important to measure contact angles in polymer films in order to know about surface properties and interactions. To precisely assess these angles, several different methodologies were utilized, each of which provides a different perspective on the behavior of polymeric surfaces. One method that is frequently used involves calculating contact angles in both air and water (Figure 5). This method offers a full assessment of the wettability of the polymer in a variety of environmental situations (63). Proper surface preparation is essential for precise measurement of contact angles in non-sulfonated PIS copolymers because it affects the hydrophilic–hydrophobic balance and interfacial characteristics.

Contact angle study of (a) PIS-20, (b) PIS-30, (c) PIS-40, and (d) PIS-50.
The water contact angles of the naphthalene moiety-based PIS films were found to increase with the increasing amount of siloxane segments incorporated in the polyimide backbone from 76° to 95° (Table 1). The integration of siloxane segments into the structure of the copolymer tends to increase the hydrophobicity and flexibility of the material, which in turn affects the measurements of the contact angle. Also, the water contact angle value of the bisphenol-A moiety-based PIS (BPADA-PIS-30) film was found to be 72° (Figure S5). The PIS-30 film showed a higher contact angle value than the BPADA-PIS-30 film due to the lower surface energy of the naphthalene moieties. Furthermore, the selection of non-sulfonated aromatic diamines that are utilized in the process of copolymer production has an impact on the overall surface properties, which in turn alters the contact angle response (64). Changes in the contact angle measurements are brought about as a consequence of the surface tension, and the wettability of the polyimide film is dramatically altered when the siloxane content increases (65). The water repellence of PISs was significantly enhanced due to the low free energy of siloxane segments and the increased concentration of siloxane fraction on the film surface, which is attributed to its hydrophobic character (66). As a consequence of this, the insertion of siloxane not only alters the adhesive behavior of the copolymer but also contributes to the overall mechanical properties of the copolymer, such as the percentages of EB (67). These discoveries highlight how important it is to adapt the ratio of polyimide to siloxane to get the necessary wettability qualities in a variety of applications.
3.6 Optical properties
The PIS polymers in this study contain a naphthalene core, which is an aromatic ring system. Aromatic systems possess delocalized π-electrons capable of absorbing UV light, hence inducing electronic changes. Naphthalene’s structure enables its excellent absorption of UV light by strong π → π* transitions. The UV–vis spectra of PIS-30 were recorded along with PIS-BPADA-30 in NMP solution by excitation with UV light of different wavelengths to have a comparative idea of their light-emitting behavior. PIS-30 displayed a strong UV absorption maximum (λ max) in the range of 270–300 nm, whereas PIS-BPADA-30 showed a lower intensity of absorption, which can be due to the electronic conjugation between the two rings, which is less effective compared to naphthalene because of the single bond that connects them (Figure 6). This leads to less effective π → π* transitions (68,69).

UV–vis absorption spectra of naphthalene and bisphenol-A moiety-based PIS polymers.
3.7 Morphological analysis
The surface morphology of siloxane-containing PIS films was thoroughly examined using AFM in tapping mode. The surface topography images, shown in Figure 7, demonstrate a highly uniform, featureless structure of the films. After careful examination at different magnification levels, it was found that the films displayed a consistent and flawless structure devoid of any visible pores, fractures, or imperfections. This indicates that the morphology of the films is uniform and free from defects.

AFM images of naphthalene moiety-based PIS-30 and -50.
The morphology of the PIS films was also observed using a scanning electron microscope (Figure 8). The smoothness of the surface suggests a successful and consistent integration of siloxane into the polyimide matrix, resulting in a seamless appearance. The results highlight the effectiveness of the solvent casting method in creating polyimide films that contain the siloxane content evenly distributed throughout. This enhances their structural strength and makes them suitable for use in high-performance materials that require uniform and flawless surfaces. The NTDA/ODA polyimide films possess characteristics that make them highly suitable for applications that demand durable and dependable materials. This further confirms the efficacy of the synthesis and processing techniques utilized in this study.

SEM images of (a) PIS-20, (b) PIS-30, (c) PIS-40, and (d) PIS-50.
4 Conclusions
A set of four PIS copolymers was synthesized by polycondensation reactions. In these polymers, the siloxane content varied from 20 to 30, 40, and 50%. They were characterized by 1H NMR and ATR-FTIR spectroscopy. These copolymers showed two-step thermal degradation. Few observations were made regarding the tensile strength and T d. With an increase in siloxane content, the copolymer exhibited enhanced EB of 8–48% for PIS-20 to PIS-50; however, both the tensile strength and modulus of the copolymer showed a decrease due to the plasticization effect of the dimethylsiloxane units. However, the PIS films with naphthalene moieties showed very high tensile modulus values (up to 5.11 GPa). The flexible siloxane unit reduces the T g and affects the thermal stability of the polymers to some extent. However, the thermal and mechanical properties of the polymers are still in the range that these polymers may find application in high-temperature applications. The study also observed that the addition of siloxane segments to the polyimide backbone greatly enhanced the water contact angles, which ranged from 76° to 95°. This increase in the contact angle indicates improved hydrophobicity, indicating that the material’s surface has become more water-repellent. Incorporating siloxane into the copolymer enhances both the hydrophobic properties and flexibility of the polyimide structure. The observed increase in hydrophobicity can be ascribed to the intrinsic low surface energy of the siloxane constituents, leading to a decrease in the material’s ability to be wetted. The results emphasize the significance of siloxane in modifying the physical characteristics of polyimides, rendering them more appropriate for applications that need hydrophobicity and flexibility and shows great potential for the advancement of specialized polymeric materials with specific properties. The UV absorption properties might make these polymers suitable for optoelectronic applications, where control over light absorption and emission is critical, such as in organic light-emitting diodes or photovoltaic devices. The morphological analysis using AFM demonstrates the uniform integration of siloxane into the polyimide matrix, yielding a smooth structure that was supported by scanning electron microscopy (SEM). Briefly, the PISs containing naphthalene dianhydride were found to have enhanced thermal stability, improved mechanical properties, superior UV stability, and higher T gs. The naphthalene-based PISs were found to have high EB and strong mechanical characteristics, which make them well-suited for use in advanced gas separation membranes, especially in applications that demand stability at elevated temperatures or under chemically harsh conditions. Future research should explore the optimization of copolymer structures, particularly the interplay between sulfonated and non-sulfonated components, to enhance mechanical properties and chemical stability further.
Acknowledgements
The authors acknowledge the financial support from Wacker Metroark Chemicals Pvt. Ltd. (Project No. WACKER-IIT KGP 2020/03) for this work. The authors are also grateful to IIT Kharagpur for providing the necessary research facilities.
-
Author contributions: Riddhi Kamble: conceptualization, methodology, investigation, data curation, formal analysis, writing – original draft. Bholanath Ghanti: investigation, formal analysis. Rahul Badri: investigation, editing. Susanta Banerjee: supervision, conceptualization, formal analysis, writing – original draft, funding acquisition, project administration.
-
Conflict of interest: The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests. Prof. Susanta Banerjee reports that administrative support and equipment, drugs, or supplies were provided by Wacker Metroark Chemicals Pvt. Ltd.
-
Data availability statement: The raw data can be available upon request.
References
(1) Hasegawa M, Horie K. Photophysics, photochemistry, and optical properties of polyimides. Prog Polym Sci. 2001 Mar;26(2):259–335.10.1016/S0079-6700(00)00042-3Search in Google Scholar
(2) Harvey BG, Yandek GR, Lamb JT, Eck WS, Garrison MD, Davis MC. Synthesis and characterization of a high temperature thermosetting polyimide oligomer derived from a non-toxic, sustainable bisaniline. RSC Adv. 2017;7(37):23149–56.10.1039/C7RA02182HSearch in Google Scholar
(3) Qin S, Chen C, Cui M, Zhang A, Zhao H, Wang L. Facile preparation of polyimide/graphene nanocomposites via an in situ polymerization approach. RSC Adv. 2017;7(5):3003–11.10.1039/C6RA25168DSearch in Google Scholar
(4) Stovall BJ, Long TE. Synthesis and characterization of wholly aromatic, water-soluble polyimides and poly(amic acid)s towards fire suppression foams. Blacksburg, Virginia: Virginia Polytechnic Institute and State University; 2021.Search in Google Scholar
(5) Yang P, Long J, Xuan S, Wang Y, Zhang Y, Li J, et al. Branched sulfonated polyimide membrane with ionic cross-linking for vanadium redox flow battery application. J Power Sources. 2019 Oct;438:226993.10.1016/j.jpowsour.2019.226993Search in Google Scholar
(6) Yamada Y, Furukawa N. Preparation and characterization of siloxane-imide block copolymers based on 3,3′,4,4′-benzophenonetetracarboxylic dianhydride. Polym J. 1997 Nov;29(11):923–30.10.1295/polymj.29.923Search in Google Scholar
(7) Ku CK, Lee YD. Microphase separation in amorphous poly(imide siloxane) segmented copolymers. Polymer (Guildf). 2007 Jun;48(12):3565–73.Search in Google Scholar
(8) Hou Y. Syntheses and surface analysis of novel poly(dimethylsiloxane) containing amphiphilic graft copolymers. Buffalo: University of New York; 2002.Search in Google Scholar
(9) McGrath JE, Dunson DL, Mecham SJ, Hedrick JL. Synthesis and characterization of segmented polyimide-polyorganosiloxane copolymers. In Progress in polyimide chemistry I. Berlin, Heidelberg: Springer; p. 61–105.10.1007/3-540-49815-X_3Search in Google Scholar
(10) Favvas EP, Katsaros FK, Papageorgiou SK, Sapalidis AA, Mitropoulos ACh. A review of the latest development of polyimide based membranes for CO2 separations. React Funct Polym. 2017 Nov;120:104–30.10.1016/j.reactfunctpolym.2017.09.002Search in Google Scholar
(11) Vavrenyuk SV, Efimenko YV, Farafonov AE. The influence of the technological order for modifiers introduction on the physical and mechanical properties of cement-mineral systems. IOP Conf Ser Mater Sci Eng. 2021 Mar;1079(7):072044.10.1088/1757-899X/1079/7/072044Search in Google Scholar
(12) Jafari T, Noshadi I, Khakpash N, Suib SL. Superhydrophobic and stable mesoporous polymeric adsorbent for siloxane removal: D4 super-adsorbent. J Mater Chem A Mater. 2015;3(9):5023–30.10.1039/C4TA06593JSearch in Google Scholar
(13) de Arespacochaga N, Valderrama C, Mesa C, Bouchy L, Cortina JL. Biogas deep clean-up based on adsorption technologies for Solid Oxide Fuel Cell applications. Chem Eng J. 2014 Nov;255:593–603.10.1016/j.cej.2014.06.072Search in Google Scholar
(14) Mark JE. Some interesting things about polysiloxanes. Acc Chem Res. 2004 Dec;37(12):946–53.10.1021/ar030279zSearch in Google Scholar PubMed
(15) Ścibiorek M, Gladkova NK, Chojnowski J. Controlled synthesis of amphiphilic siloxane-siloxane block copolymers with carboxyl functions. Polym Bull. 2000 May;44(4):377–84.10.1007/s002890070087Search in Google Scholar
(16) Macko JA, Ishida H. Structural effects of phenols on the photooxidative degradation of polybenzoxazines. Polymer (Guildf). 2001 Jan;42(1):227–40.10.1016/S0032-3861(00)00323-2Search in Google Scholar
(17) Shea KJ, Loy DA. Bridged polysilsesquioxanes. Molecular-engineered hybrid organic−inorganic materials. Chem Mater. 2001 Oct;13(10):3306–19.10.1021/cm011074sSearch in Google Scholar
(18) Wu Z, He J, Yang H, Yang S. Progress in aromatic polyimide films for electronic applications: Preparation, structure and properties. Polymers (Basel). 2022 Mar;14(6):1269.10.3390/polym14061269Search in Google Scholar PubMed PubMed Central
(19) Arnold CA, Summers JD, McGrath JE. Synthesis and physical behavior of siloxane modified polyimides. Polym Eng Sci. 1989 Oct;29(20):1413–8.10.1002/pen.760292002Search in Google Scholar
(20) Tiwari A, Sugamoto R, Hihara LH. Analysis of molecular morphology and permeation behavior of polyimide-siloxane molecular composites for their possible coatings application. Prog Org Coat. 2006 Nov;57(3):259–72.10.1016/j.porgcoat.2006.09.009Search in Google Scholar
(21) Krea M. New copolyimide membranes with high siloxane content designed to remove polar organics from water by pervaporation. J Membr Sci. 2004 Sep;241(1):55–64.10.1016/j.memsci.2004.03.040Search in Google Scholar
(22) Rimdusit S, Benjapan W, Assabumrungrat S, Takeichi T, Yokota R. Surface segregation of siloxane containing component in polysiloxane‐ block ‐polyimide and s ‐BPDA/ODA polyimide blends. Polym Eng Sci. 2007 Apr;47(4):489–98.10.1002/pen.20723Search in Google Scholar
(23) Wu L, Wang W, Lu J, Sun R, Wong CP. Study of the interfacial adhesion properties of a novel Self-healable siloxane polymer material via molecular dynamics simulation. Appl Surf Sci. 2022 May;583:152471.10.1016/j.apsusc.2022.152471Search in Google Scholar
(24) Shi J, Liu Z, Zhao N, Liu S, Li Z. Controlled ring-opening polymerization of hexamethylcyclotrisiloxane catalyzed by trisphosphazene organobase to well-defined poly(dimethylsiloxane)s. Macromolecules. 2022 Apr;55(7):2844–53.10.1021/acs.macromol.1c02654Search in Google Scholar
(25) Xu CA, Lu M, Tan Z, Qu Z, Wu K, Shi J. Study on the surface properties and thermal stability of polysiloxane-based polyurethane elastomers with aliphatic and aromatic diisocyanate structures. Colloid Polym Sci. 2020 Sep;298(9):1215–26.10.1007/s00396-020-04695-4Search in Google Scholar
(26) Chuang FS, Tsen WC, Shu YC. The effect of different siloxane chain-extenders on the thermal degradation and stability of segmented polyurethanes. Polym Degrad Stab. 2004 Apr;84(1):69–77.10.1016/j.polymdegradstab.2003.10.002Search in Google Scholar
(27) Powell CE, Qiao GG. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J Membr Sci. 2006 Aug;279(1–2):1–49.10.1016/j.memsci.2005.12.062Search in Google Scholar
(28) Tariq AR, Tariq SR, Mahmud T, Sultan M. Selective capture of CO2 from mixture of different gases using cellulose acetate, polyimide, polysulfone and some other polymers based mixed matrix membranes: A review. J Innov Sci. 2020;6(2):157–88.10.17582/journal.jis/2020/6.2.157.188Search in Google Scholar
(29) Gevers LEM, Vankelecom IFJ, Jacobs PA. Solvent-resistant nanofiltration with filled polydimethylsiloxane (PDMS) membranes. J Membr Sci. 2006;278(1–2):199–204.10.1016/j.memsci.2005.10.056Search in Google Scholar
(30) Gädda TM, Weber WP. Polydiphenylsiloxane-polydimethylsiloxane-polydiphenylsiloxane triblock copolymers. J Polym Sci A Polym Chem. 2006;44(11):3629–39.10.1002/pola.21468Search in Google Scholar
(31) Yilgör E, Yilgör I. Silicone containing copolymers: Synthesis, properties and applications. Prog Polym Sci. 2014;39:1165–95.10.1016/j.progpolymsci.2013.11.003Search in Google Scholar
(32) Leitner L, Harscoat-Schiavo C, Vallières C. Experimental contribution to the understanding of transport through polydimethylsiloxanenanofiltration membranes: Influence of swelling, compaction and solvent on permeation properties. Polym Test. 2014;33:88–96.10.1016/j.polymertesting.2013.10.016Search in Google Scholar
(33) Damaceanu MD, Musteata VE, Cristea M, Bruma M. Viscoelastic and dielectric behaviour of thin films made from siloxane-containing poly(oxadiazole-imide)s. Eur Polym J. 2010;46(5):1048–62.10.1016/j.eurpolymj.2010.01.020Search in Google Scholar
(34) González Calderón JA, Contreras López D, Pérez E, Vallejo Montesinos J. Polysiloxanes as polymer matrices in biomedical engineering: their interesting properties as the reason for the use in medical sciences. Polym Bull. 2020;77:2749–817.10.1007/s00289-019-02869-xSearch in Google Scholar
(35) Kamble R, Ghorai A, Ghanti B, Pradhan D, Banerjee S. Fabrication of high proton conducting composite membranes from amino group functionalized MOF and semi-fluorinated sulfonated poly(arylene ether sulfone)s. Eur Polym J. 2022 Oct;179:111574.10.1016/j.eurpolymj.2022.111574Search in Google Scholar
(36) Ghanti B, Kamble R, Roy S, Banerjee S. Synthesis and characterization of sulfonated polytriazoles utilizing 1,4‐bis(4‐azido‐2‐(trifluoromethyl)phenoxy)benzene for the proton exchange membrane applications. J Polym Sci. 2023 Aug;61(16):1792–806.10.1002/pol.20220769Search in Google Scholar
(37) Ghanti B, Kamble R, Komber H, Voit B, Banerjee S. Synergistically functionalized pyridinyl- and phosphine-oxide-based semifluoro-sulfonated copolytriazole membrane preparation via “Click” polymerization for proton exchange membrane applications. Macromolecules. 2024 May;57(9):4584–98.10.1021/acs.macromol.4c00050Search in Google Scholar
(38) Pei X, Chen G, Liu J, Fang X. Influence of crystalline polyimide hard block on the properties of poly(imide siloxane) copolymers. Polymer (Guildf). 2015 Jan;56:229–36.10.1016/j.polymer.2014.11.056Search in Google Scholar
(39) Ku CK, Lee YD. Microphase separation in amorphous poly(imide siloxane) segmented copolymers. Polymer (Guildf). 2007;48(12):3565–73.10.1016/j.polymer.2007.04.028Search in Google Scholar
(40) Sysel P, Hynek V, Šípek M. Water transport and sorption in polyimide-polysiloxane copolymers. Collect Czechoslov Chem Commun. 1998;63(1):53–6.10.1135/cccc19980053Search in Google Scholar
(41) Lü C, Wang Z, Liu F, Yan J, Gao L. Microstructure and properties of new polyimide/polysiloxane composite films. J Appl Polym Sci. 2006 Apr;100(1):124–32.10.1002/app.22532Search in Google Scholar
(42) Vora RH, Goh SH. Designed poly(ether-imide)s and fluoro-copoly(ether-imide)s: Synthesis, characterization and their film properties. Mater Sci Eng: B. 2006 Jul;132(1–2):24–33.10.1016/j.mseb.2006.02.049Search in Google Scholar
(43) Constantin CP, Asandulesa M, Varganici C, Melinte V, Bruma M, Jankowski A, et al. Exploring the potential of thin films made from poly(imide-amide-sulfone)s for engineering applications. Mater Sci Eng: B. 2021 Aug;270:115217.10.1016/j.mseb.2021.115217Search in Google Scholar
(44) Pichaimani P, Krishnan S, Song JK, Muthukaruppan A. Bio-silicon reinforced siloxane core polyimide green nanocomposite with multifunctional behavior. High Perform Polym. 2018 Jun;30(5):549–60.10.1177/0954008317709891Search in Google Scholar
(45) Köken N, Karagöz S, Kızılcan N, Ustamehmetoğlu B. Block copolymers of acrylonitrile and poly(dimethylsiloxane)s. J Appl Polym Sci. 2013 Mar;127(5):3790–7.10.1002/app.37649Search in Google Scholar
(46) Anastasiadis SH, Chrissopoulou K, Frick B. Structure and dynamics in polymer/layered silicate nanocomposites. Mater Sci Eng: B. 2008 Aug;152(1–3):33–9.10.1016/j.mseb.2008.06.008Search in Google Scholar
(47) Kızılcan N, Ustamehmetoğlu B, Öz N, Sezai Saraç A, Akar A. Soluble and conductive polypyrrole copolymers containing silicone tegomers. J Appl Polym Sci. 2003 Sep;89(11):2896–901.10.1002/app.12140Search in Google Scholar
(48) Kızılcan N, Öz NK, Ustamehmetoğlu B, Akar A. High conductive copolymers of polypyrrole-α,ω-diamine polydimethylsiloxane. Eur Polym J. 2006 Oct;42(10):2361–8.10.1016/j.eurpolymj.2006.05.016Search in Google Scholar
(49) Ghosh A, Sen SK, Dasgupta B, Banerjee S, Voit B. Synthesis, characterization and gas transport properties of new poly(imide siloxane) copolymers from 4,4′-(4,4′-isopropylidenediphenoxy)bis(phthalic anhydride). J Membr Sci. 2010 Nov;364(1–2):211–8.10.1016/j.memsci.2010.08.015Search in Google Scholar
(50) Peter J, Khalyavina A, Kříž J, Bleha M. Synthesis and gas transport properties of ODPA–TAP–ODA hyperbranched polyimides with various comonomer ratios. Eur Polym J. 2009 Jun;45(6):1716–27.10.1016/j.eurpolymj.2009.03.003Search in Google Scholar
(51) Ghosh A, Sen SK, Banerjee S, Voit B. Solubility improvements in aromatic polyimides by macromolecular engineering. RSC Adv. 2012;2(14):5900.10.1039/c2ra20175eSearch in Google Scholar
(52) Pei X, Chen G, Fang X. Synthesis and properties of poly(imide siloxane) block copolymers with different block lengths. J Appl Polym Sci. 2013;129(6):3718–27.10.1002/app.38918Search in Google Scholar
(53) Arnold CA, Summers JD, Chen YP, Bott RH, Chen D, McGrath JE. Structure-property behaviour of soluble polyimide-polydimethylsiloxane segmented copolymers. Polymer (Guildf). 1989 Jun;30(6):986–95.10.1016/0032-3861(89)90068-2Search in Google Scholar
(54) Jalalian E, Mehdipour-Ataei S, Babanzadeh S, Khodabakhshi F. Silicon-containing poly(amide-imide)s: preparation, characterization, and properties. Des Monomers Polym. 2015 Nov;18(8):714–22.10.1080/15685551.2015.1070502Search in Google Scholar
(55) Ghosh A, Banerjee S. Thermal, mechanical, and dielectric properties of novel fluorinated copoly(imide siloxane)s. J Appl Polym Sci. 2008 Aug;109(4):2329–40.10.1002/app.28298Search in Google Scholar
(56) Ghosh A, Banerjee S, Häußler L, Voit B. New fluorinated poly(imide siloxane) random and block copolymers with variation of siloxane loading. J Macromol Sci Part A. 2010 May;47(7):671–80.10.1080/10601325.2010.483364Search in Google Scholar
(57) Gupta NS, Lee KS, Labouriau A. Tuning thermal and mechanical properties of polydimethylsiloxane with carbon fibers. Polymer (Basel). 2021;13(7):1141.10.3390/polym13071141Search in Google Scholar PubMed PubMed Central
(58) Babkin AV, Erdni-Goryaev EM, Solopchenko AV, Kepman AV, Avdeev VV. Mechanical and thermal properties of modified bismaleimide matrices toughened by polyetherimides and polyimide. Polym Adv Technol. 2016;27(6):774–80.10.1002/pat.3711Search in Google Scholar
(59) Liaw WC, Chang-Chien J, Kang H, Cheng YL, FU LW. A straightforward synthesis and characterization of a new poly(imide siloxane)-based thermoplastic elastomer. Polym J. 2008 Feb;40(2):116–25.10.1295/polymj.PJ2007128Search in Google Scholar
(60) Ghosh A, Banerjee S. Structure–property co‐relationship of fluorinated poly(imide‐siloxane)s. Polym Adv Technol. 2008 Nov;19(11):1486–94.10.1002/pat.1152Search in Google Scholar
(61) Zhao R, Li H, Chen C, Luo X, Li X, Tan D, et al. Effect of temperature and frequency on dynamic mechanical properties of poly(methyl methacrylate). Advanced materials research. 2011;311:1081–6.10.4028/www.scientific.net/AMR.311-313.1081Search in Google Scholar
(62) Pei X, Chen G, Fang X. Preparation and characterization of poly(imide siloxane) block copolymers based on diphenylthioether dianhydride isomer mixtures. High Perform Polym. 2011 Dec;23(8):625–32.10.1177/0954008311424236Search in Google Scholar
(63) Ko YC, Ratner BD, Hoffman AS. Characterization of hydrophilic—hydrophobic polymeric surfaces by contact angle measurements. J Colloid Interface Sci. 1981 Jul;82(1):25–37.10.1016/0021-9797(81)90120-XSearch in Google Scholar
(64) Zou L. Synthesis and characterization of nanostructured sulfonated polyimides for proton exchange membrane fuel cells. Rochester, New York: University of Rochester; 2009.Search in Google Scholar
(65) Jwo SL, Whang WT, Hsieh TE, Pan FM, Liaw WC. Effects of morphology and surface characteristics of poly(imide siloxane)s and deep UV/O3 surface treatment on the interfacial adhesion of poly(imide siloxane)/alloy-42 leadframe joints. J Polym Res. 1999 Jul;6(3):175–89.10.1007/s10965-006-0086-zSearch in Google Scholar
(66) Xiong L, Wang X, Qi H, Liu F. Synthesis of a new siloxane‐containing alicyclic dianhydride and the derived polyimides with improved solubility and hydrophobicity. J Appl Polym Sci. 2013 Feb;127(3):1493–501.10.1002/app.37563Search in Google Scholar
(67) Wohl CJ, Atkins BM, Belcher MA, Connell JW. Synthesis, characterization, topographical modification, and surface properties of copoly(imide siloxane)s. High Perform Polym. 2012 Feb;24(1):40–9.10.1177/0954008311431113Search in Google Scholar
(68) Alexiou MS, Tychopoulos V, Ghorbanian S, Tyman JHP, Brown RG, Brittain PI. The UV–visible absorption and fluorescence of some substituted 1,8-naphthalimides and naphthalic anhydrides. J Chem Soc, Perkin Trans 2. 1990;(5):837–42.10.1039/P29900000837Search in Google Scholar
(69) Wang S, Zhou H, Dang G, Chen C. Synthesis and characterization of thermally stable, high‐modulus polyimides containing benzimidazole moieties. J Polym Sci A Polym Chem. 2009 Apr;47(8):2024–31.10.1002/pola.23306Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”
Articles in the same Issue
- Research Articles
- Flow-induced fiber orientation in gas-powered projectile-assisted injection molded parts
- Research on thermal aging characteristics of silicone rubber composite materials for dry-type distribution transformers
- Kinetics of acryloyloxyethyl trimethyl ammonium chloride polymerization in aqueous solutions
- Influence of siloxane content on the material performance and functional properties of polydimethylsiloxane copolymers containing naphthalene moieties
- Enhancement effect of electron beam irradiation on acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment by adding 1,3-PBO: A potential way for waste ABS reuse
- Model construction and property study of poly(ether-ether-ketone) by molecular dynamics simulation with meta-modeling methods
- Zinc–gallic acid–polylysine nanocomplexes with enhanced bactericidal activity for the treatment of bacterial keratitis
- Effect of pyrogallol compounds dosage on mechanical properties of epoxy coating
- Preparation of in situ polymerized polypyrrole-modified braided cord and its electrical conductivity investigation under varied mechanical conditions
- Hydrophobicity, UV resistance, and antioxidant properties of carnauba wax-reinforced CG bio-polymer film
- Janus nanofiber membrane films loading with bioactive calcium silicate for the promotion of burn wound healing
- Synthesis of migration-resistant antioxidant and its application in natural rubber composites
- Influence of the flow rate on the die swell for polymer micro coextrusion process
- Fatty acid filled polyaniline nanofibres with dual electrical conductivity and thermo-regulatory characteristics: Futuristic material for thermal energy storage
- Hydrolytic depolymerization of major fibrous wastes
- Performance of epoxy hexagonal boron nitrate underfill materials: Single and mixed systems
- Blend electrospinning of citronella or thyme oil-loaded polyurethane nanofibers and evaluating their release behaviors
- Efficiency of flexible shielding materials against gamma rays: Silicon rubber with different sizes of Bi2O3 and SnO
- A comprehensive approach for the production of carbon fibre-reinforced polylactic acid filaments with enhanced wear and mechanical behaviour
- Electret melt-blown nonwovens with charge stability for high-performance PM0.3 purification under extreme environmental conditions
- Study on the failure mechanism of suture CFRP T-joints under/after the low-velocity impact loading
- Experimental testing and finite element analysis of polyurethane adhesive joints under Mode I loading and degradation conditions
- Optimizing recycled PET 3D printing using Taguchi method for improved mechanical properties and dimensional precision
- Effect of stacking sequence of the hybrid composite armor on ballistic performance and damage mechanism
- Bending crack propagation and delamination damage behavior of orthogonal ply laminates under positive and negative loads
- Rapid Communication
- RAFT-mediated polymerization-induced self-assembly of poly(ionic liquid) block copolymers in a green solvent
- Corrigendum
- Corrigendum to “High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing”

