Abstract
This study facilitates the synthesis process of a novel graft copolymer (flocculant) using carboxymethyl cellulose, acrylonitrile and N-vinyl formamide as raw materials. The carboxymethyl cellulose graft polyamidine (CMC-g-PAMD) can be used as new flocculant to replace the traditional polyacrylamide flocculant, which manifested its excellent flocculation and degradation efficiency. A five-membered cyclic copolymer was prepared by the graft copolymerization, and the synthesized flocculants were characterized by EA, TG-DTG, FT-IR, SEM and NMR, confirming the successful synthesis of the desired copolymers. The operation conditions for copolymerization were experimentally investigated, and the results indicated that the optimal initiator dosage, copolymerization temperature, amidinization temperature, acidification time and flocculant dosage were 4 g/L, 50°C, 90°C, 3 h and 60 mg/L, respectively. Compared with the traditional polyacrylamide flocculant, the CMC-g-PAMD presented an outstanding flocculation ability of 96.1% under its optimal operation conditions, which showed an enormous potential in the application of coalmine waste-water treatment.
Graphical Abstract

1 Introduction
Nowadays the world’s economic and social development have a great dependence on fossil energy, which is still increasing with rapid scientific and technological progress (1,2). Coal is one of the fundamental fossil energy sources in the world. In 2016, China’s coal production and consumption were 2.41 billion tons and 2.70 billion tons, respectively (3). Although coal as a traditional energy has favored and improved human life greatly, it will inevitably produce large amounts of sewage water during the mining & utilization process (4,5). If the mentioned wastewater were emitted directly without any treatment, the natural environment would suffer serious or even irreparable damage (6,7). The most convenient and effective method is to use flocculant for wastewater treatment (8), and the conventional flocculants are divided into two major categories: inorganic (9) and organic flocculants (10). Inorganic flocculants (11) include iron salt (12), aluminum salt (13), polyaluminum chloride (PAC) (14) and Polysilicate (15). Although inorganic flocculants are usually cheap and economic, they have several disadvantages such as large dosage, low flocculation efficiency and strong corrosion. Organic flocculants are divided into natural polymers and artificially synthesized polymers. Meanwhile the artificially synthesized polymers mainly include polyacrylate (16), polydiallyldimethylammonium chloride (PDADMAC) (17) and polyacrylamide (PAM) (18), which behave a high turbidity removal effect, small dosage and weak corrosion. However, most synthesized polymers using as flocculants are poisonous and harmful, which are not environmental-friendly and difficult to be degraded naturally. Faced with these situations, many copolymers can be produced by grafting artificial polymers (19,20) onto the hydroxyl groups of natural polymers (21,22). Zhou et al. (23) synthesized a bio-copolymer by starch grafting with acrylamide (AM) and dimethyl diallyl ammonium chloride (DMDAAC) through an initiator system of KMnO4/HIO4. The results showed the graft starch can remove reactive dyes and disperse dyes from wastewater effectively, and the dye removal efficiency of the grafted starch was nearly 10% higher than PAM. Huang et al. (24) prepared the quaternary ammonium salt grafted starch flocculants, starch-graft-poly(2-methacryloyloxyethyl) trimethyl ammonium chloride (St-g-PDMC), and the efficiencies St-g-PDMCs for flocculation of kaolin and Escherichia coli suspensions as well as their mixtures were systematically examined in laboratory scale. Experimental results indicated that St- g-PDMCs exhibited dual functionality of high flocculation effects and antibacterial properties.
In our previous work, Zou et al. (19) proposed a cationic pea starch grafted acrylamide as a flocculant, and investigated its application in coalmine wastewater treatment. The results showed that the graft polymer has several superior application properties compared with PAM flocculant; Li et al. (20) developed a few of graft copolymers of different legume starches and acrylamide, and the experimental results indicated the graft copolymer of acrylamide and mung bean starch behaved an outstanding flocculation effect than other flocculants. It was revealed that legume starch with higher amylose content can achieve better flocculation performance. Compared with traditional PAM flocculant, these St-g-AM copolymers improved both flocculation and biodegradation ability significantly. However, starch is one of the important foods for mankind and animals, and when starch is used as a raw material to produce St-g-AM flocculant, it would inevitably reduce the global food supply and restrict its further application. Additionally, during the copolymerization of St-g-AM, small amount of AM could reside inside the flocculant which is toxic and harmful to people’s health. Since cellulose is characterized by its wide material sources which has similar structure and property with starch, it can be considered to replace starch and prepare novel cellulose grafted copolymer using as flocculant.
Polyamidine (PAMD) is an organic compound containing amidine groups in polymer molecular chain, which possesses high charge density and has been used in many fields (25), such as the capture of CO2 (26) and photosensitive material (27). According to the current literature (28), PAMD performed a better treatment effect than PAM in water treatment field, avoiding the toxicity and pollution of acrylamide monomer as an ideal flocculant. Therefore, using carboxymethylcellulose (CMC) & PAMD for copolymerization can solve the aforementioned difficulties and produce a high efficiency and environmental-friendly flocculant.
In this work, the CMC-g-PAMD was prepared and investigated, and the related work has applied for China invention patent (29). The copolymer was characterized by elemental analysis (EA), thermogravimetric analysis (TG-DTG), Fourier transform infrared (FT-IR), scanning electron microscopy (SEM) and nuclear magnetic resonance (NMR). Moreover, the flocculation performance was experimentally evaluated and compared by coalmine wastewater treatment.
2 Experimental
2.1 Materials and instruments
The following reagents were used in this experiment: carboxymethyl cellulose (CMC;Henan Weidao Chemical Products Co., Ltd.), ceric ammonium nitrate (CAN; Sinopharm Chemical Reagent Co., Ltd.), N-vinyl formamide (NVF; Sigma-Aldrich Co., Ltd.), acrylonitrile (AN; Sinopharm Chemical Reagent Co., Ltd.), 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AIBA, Aladdin Reagents Co., Ltd.), hydrochloric acid (Sichuan Xilong Chemical Co., Ltd.), anhydrous ethanol (Tianjin Fuyu Fine Chemical Co., Ltd.), polyacrylamide (PAM; Sinopharm Chemical Reagent Co., Ltd.), sodium hydroxide (Tianjin Hongyan Reagent Factory). The instruments used for the sample analysis were listed as follows: EA (Vario Macro Cube, Germany Element, Inc.), TGA-DTG (Discovery, U.S. TA Instruments), FT-IR spectrometer (Vertex 7.0, Germany Brukern Optics), SEM (EVO MA10, Germany Zeiss, Inc.), NMR (liquid-phase AV 400 MHz and solid-phase AV 600 MHz, Germany Bruker Optics, Inc.). The 1H-NMR spectrum was recorded with dimethyl sulfoxide (DMSO) as the solvent, and 1H MAS and 13C CP/MAS NMR were experimentally performed using a 4 mm MAS probe and a spinning rate of 12 kHz at a resonance frequency of 600.1 MHz and 150.9 MHz, respectively. TGA-DTG was constructed within 50-600°C at a heating rate of 10°C/min under a certain constant N2 gas flow, and both mass of the substance and the thermal decomposition were determined as a function of temperature.
The procedure and mechanism of CMC-g-PAMD synthesis were described in Figure 1. Copolymerization and amidinization can proceed smoothly in water under a CO2 atmosphere. 1 g CMC and 50 mL deionized water were added into a 250-mL three-neck flask equipped with blender, condenser and CO2 inlet pipe, and the mixture was stirred under a thermostat water bath for 1 h at 40–60°C. Then the NVF, AN and initiator (CAN:AIBA = 1:1)

The procedure and mechanism of grafting copolymerization (a); amidinization (b).
were injected into the flask and kept stirring at 40-60°C for 5 h. Afterwards the concentrated hydrochloric acid was dropped in the reactants at desired times during the copolymerization, and kept the amidinization of copolymers at 80-100°C for 3 h. Later the copolymer samples were extracted using anhydrous ethanol, and the precipitated crude products were obtained after vacuum drying.
Finally the new flocculant (CMC-g-PAMD) samples could be obtained after removing the impurities inside the crude products by the following process: firstly the crude products were washed with acetone; secondly the samples was smashed into powder after drying under vacuum; finally the “Varma method” was used to reflux the crude samples in a Soxhlet extractor for 12 h, using a mixed solvent of glacial acetic acid and ethylene glycol (1:1 v/v), which could remove the unreacted PAMD and monomers from the crude products effectively.
2.2 Evaluation of the grafting efficiency (GE)
GE was calculated according to the following equation:
where Mc denotes the mass of CMC (g), Mp represents the mass of the product purified by Soxhlet extractor (g).
2.3 Flocculation performance evaluation
A certain amount of the flocculant and 50 ml of coalmine wastewater were put into a 50 ml beaker and stirred for 1 min, and the transmittance of supernatant was measured with spectrophotometer at the wavelength of 610 nm. The coalmine wastewater sample was taken from Shanxi Ximing Coal Group Co., Ltd., and the detailed information was given below: pH = 7.90, turbid degree = 4800 NTU, COD (chemical oxygen demand) = 89 mg/L, SS (suspended substance) = 1120 mg/L, iron ion = 8.93 mg/L, manganese ion = 7.75 mg/L.
3 Characterizations of the CMC-g-PAMD
3.1 EA
The EA results of AN, NVF, CMC, and the new flocculant (CMC-g-PAMD) were given in Table 1. It was apparent from the table that the C, H, O and N contents of the new flocculant were among those of AN, NVF, CMC and the prepared sample. Compared with CMC, a significant increase of nitrogen percentage inside the prepared sample can be observed, suggesting the successful grafting of AN and NVF onto the backbone of CMC. It can be concluded from Table 1 that PAMD was actually grafted onto CMC during the copolymerization, and produced the new flocculant with specific features and performances.
Elemental analysis.
| Item | C (%) | H (%) | N (%) | O (%) |
|---|---|---|---|---|
| AN | 67.77 | 4.910 | 27.53 | 0 |
| NVF | 47.42 | 6.286 | 18.60 | 27.69 |
| CMC | 36.40 | 5.638 | 0 | 57.95 |
| Prepared sample | 56.47 | 5.466 | 17.51 | 20.55 |
3.2 TG-DTG
The TGA-DTG curves of CMC and the new flocculant (CMC-g-PAMD) under a nitrogen atmosphere were shown in Figure 2. From the curves we can clearly see the loss of humidity, phase transition and degradation processes. For CMC, three weight loss stages were observed: the first stage of weight loss from room temperature to 180°C was supposed to be derived from water loss; the next weight loss stage within 200–400°C was due to broken of hydroxyl group; and the last weight loss stage at 400–530°C was attributed to the rupture of the main chains of CMC and key bonds. In the case of CMC-g-PAMD, the weight loss during the first region (46–190°C) was related to moisture removal; a sudden large weight loss occurred within 200-360°C as the temperature rose, indicating the thermal decomposition of CMC; and the final weight loss stage (360–520°C) could be explained as the degradation of PAMD chains. As shown in Figure 2, the new flocculant had more residue (37%) than that of CMC (29%) after high temperature heating, and performed a better thermal stability, which illustrated the successful grafting of PAMD onto CMC.
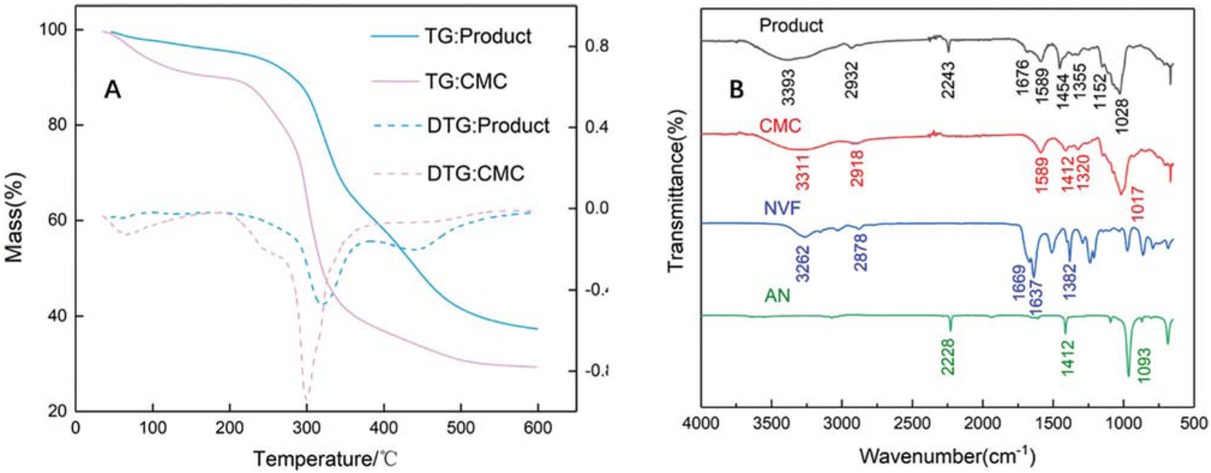
(a) TG-DTG curves of the prepared sample; (b) FT-IR of CMC, NVF, AN and the product.
3.3 FT-IR
FT-IR was used to characterize the functional groups of product (CMC-g-PAMD), and the results were shown in Figure 2b. From the figure we can witness that the broad peak of CMC occurred at 3311 cm-1, which was assigned to the stretching vibrations of O–H (30). The peak at 1017 cm-1 represented the functional groups of . For the NVF spectrum in Figure 2b, we can observe that thebands at 3262 and 1382 cm-1 were corresponding to N–H and C-N stretching vibration peaks in amide group respectively, and a stretching vibration peak of C=O appeared at 1669 cm-1 (31). For the AN spectrum in Figure 2b, the peak at 2228 cm-1 (AN) confirmed the existence of C≡N (32). Obviously, compared with CMC, NVF and AN, there are some differences in the infrared spectrum of the product. From the infrared spectrum of the product, the O-H stretching band of CMC backbone and the N-H stretching band of the NVF chain overlapped each other, leading to a combined peak of O-H and N-H groups for the prepared flocculant at 3393 cm-1. Meanwhile, the peak of product at 2243 cm-1 was mainly due to the characteristic absorption of C≡N, confirming the participation of AN in the graft copolymerization. Compared with peak at 1669 cm-1 (NVF), the peak at 1676 cm-1 of the new flocculant was ascribed to the C=O stretching vibrations (29), and the peak area decrease could also verify the hydrolysis of C=O in NVF. Moreover, the stretching vibrations of C–N and C=N were observed at 1454 cm−1 and 1152 cm−1, respectively, demonstrating the occurrence and development of amidinization reaction as expected (33).
3.4 SEM
Figure 3 showed the SEM micrographs of the new flocculant (Figures 3a and 3b) and CMC (Figures 3c and 3d). It was obvious that the morphological characteristics of the product varied significantly after the reaction (Figure 3b vs. Figure 3d), and the original crystal form of CMC (Figure 3b vs. Figure 3d) turned into the amorphous state of the product (Figures 3a and 3b). It can be revealed from the Figure 3b that the new flocculant was uniform fine powder and these small granules showed a net-like structure under greater magnification. Since the smaller particles of flocculant samples tended to present higher diffusion rate in aqueous solution, the net structure of the product may promote the net capture effect remarkably, resulting in an outstanding flocculation performance accordingly.
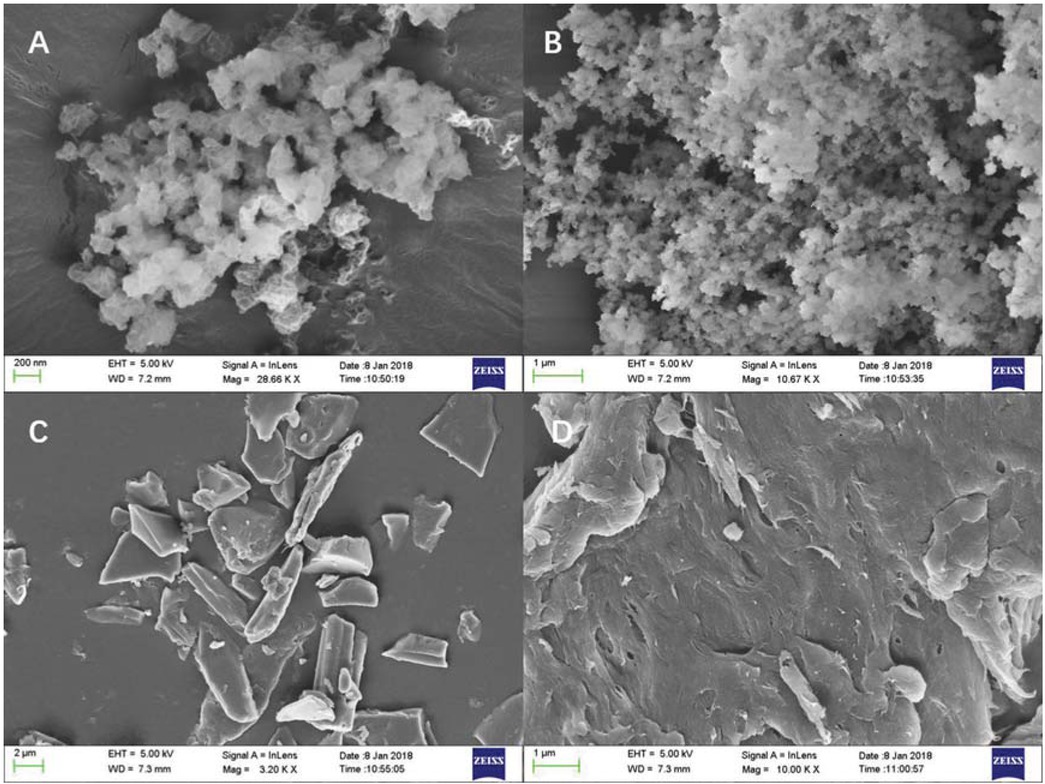
SEM photographs of (a) CMC-g-PAMD (×50000); (b) CMC-g-PAMD (×10000); (c) CMC (×5000); (d) CMC (×10000).
3.5 NMR
The 13C NMR and 1H NMR spectra of the new flocculant were experimentally conducted, and the results were shown in Figure 4. It can be revealed from the figure that the highest chemical shift (δ 3.7) in the 1H NMR spectrum (Figure 4a) could be assigned to —NH2 group. The peaks at chemical shifts (δ 3.2) can be ascribed to H2, H5 protons on the CMC backbone, and the chemical shift (δ 3.4) could be described as the protons of H7 on the carboxymethyl. The peak at chemical shifts of δ 2.1 can be corresponding to H1, H3 and H4; δ 1.3, δ 1.4, δ 2.0 and δ 2.5 ascribed to H10, H11, H6 and H9 respectively. For the 13C NMR spectrum (Figure 4b), the peaks at δ 108.6 and δ 76.0 were assigned to C1 and C10 respectively, and the peak at δ 121.6 ascribed to C3 and C4. Meanwhile the peak at δ 201.4 attributed to C8 and C13, and the peak at δ 44.1 ascribed to C2, C5, C7, C9 and C12.
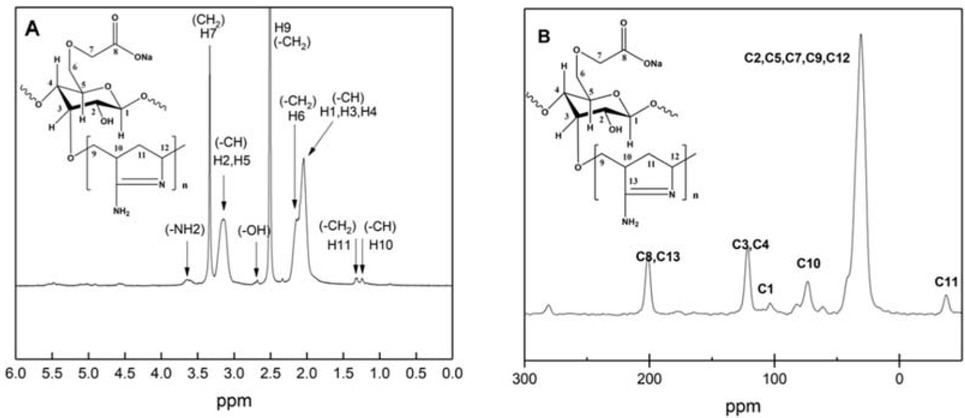
(a) 1H-NMR of the new flocculant; (b) 13C-NMR of the new flocculant.
4 Optimization of reaction conditions
4.1 Effect of initiator dosage
Since initiator is crucial for graft copolymerization, the proper type and dosage of initiator can significantly
promote the reaction and should be investigated intensively. Zhao et al. (34) studied different initiators for the copolymerization of NVF, AN and starch, and the optimal initiator for copolymer synthesis was determined as a mixture of CAN + AIBN (m:m = 1:1). Hence we used the initiator in this work, and further investigated its optimal addition amount for the graft copolymerization of the new flocculant. Different initiator dosage from 1 g/L to 5 g/L was experimentally studied, and results were shown in Figure 5a. It can be seen from the figure that the transmittance of wastewater samples climbed gradually with increasing initiator dosage at initial stage, and the flocculation effect reached its peak at the optimal initiator dosage of 4 g/L; afterwards the transmittance tended to drop. This is because the radical polymerization can proceed efficiently when the initiator concentrationis sufficiently high, and the initiator concentration of 4 g/L was proved to have the most excellent flocculation performance. Moreover, the double bond (C=C) activity of AN was obviously higher than that of NVF in the free radical polymerization due to its relatively small steric hindrance, so the excessive initiator concentration could enhance the homopolymerization of AN and hinder the copolymerization between NVF and AN, resulting in the reduced amidine content and poor flocculation effect. Similarly, as shown in Figure 5b, the product gained the largest GE of 156% under the initiator dosage of 4 g/L, meanwhile the over high initiator addition would impel the premature termination of polymerization chains, causing the GE decrease of the CMC-g-PAMD.
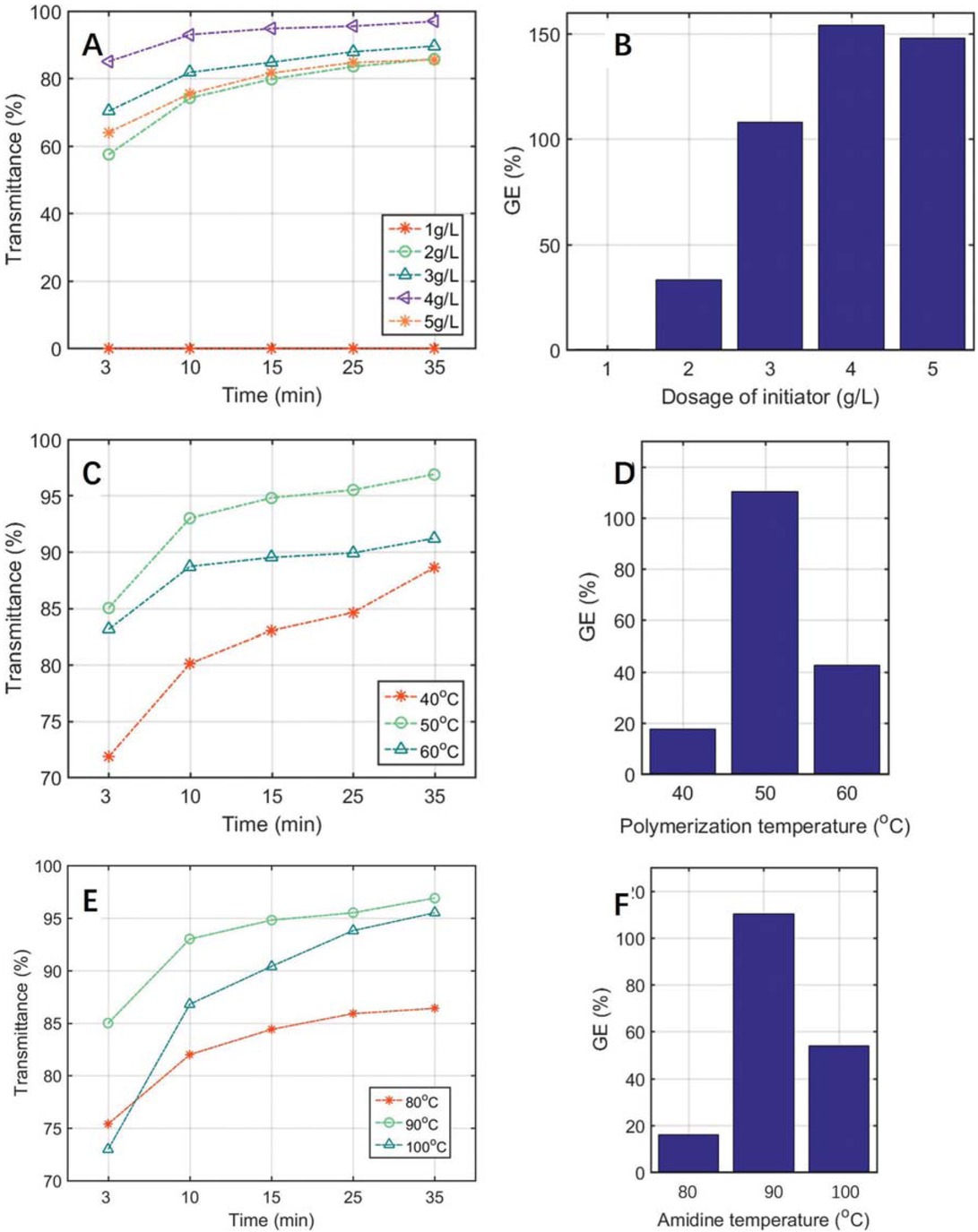
Effect of initiator dosage on transmittance (a) and GE (b); effect of polymerization temperature on transmittance (c) and GE (d); effect of amidinization temperature on transmittance (e) and GE (f).
4.2 Effect of copolymerization temperature
In Figures 5c and 5d, the influence of copolymerization temperature was presented. From the figure we can witness that both GE and transmittance showed the same trend: increase first and then decrease. Before 50°C, the reaction rate ascended steadily as graft copolymerization temperature extended. When the temperature exceeded 50°C, the side reactions including homopolymerization was accelerated remarkably, which can greatly affect the copolymerization and flocculation ability, leading to the evident decline of GE and transmittance accordingly. Thus, 50°C was determined as the optimal copolymerization temperature.
4.3 Effect of amidinization temperature
The amidinization temperature has great impact on the molecular diffusion, GE and flocculation performance, and a few experiments were carried out to explore the optimal temperature. As shown in Figures 5e and 5f, when the amidine temperature was below 90°C, the GE and flocculation performance of the CMC-g-PAMD ascended greatly as temperature extended. This was largely due to the increase of both molecular diffusion and amide hydrolysis (to produce amino group) rates, which could promote the driving force for the copolymerization. Whereas, both GE and flocculation efficiency tended to decline when the amidinization temperature exceeds 90°C. This could be because an excessively high temperature might cause a cross-linking reaction of the amino group generated by hydrolysis, consuming the amino group as the raw material for amidinization reaction, and thus the amidine content of the product could be reduced and the flocculation effect was deteriorated evidently. Additionally, high amidinization temperature may also cause the chain breaking of the copolymerized products, resulting in an obvious decrease of GE. Hence 90°C was selected as the optimal amidinization temperature.
4.4 Effect of acidification time
The acidification time is defined as the actual copolymerization time after the addition of hydrochloric acid. As shown in Figures 6a and 6b, we can clearly observe the optimal acidification time was confirmed to be 3 h, which demonstrated an overwhelming flocculation performance than any other acidification time. More or less acid treat time was unfavorable to the transmittance and GE, and both copolymerization efficiency and flocculation ability dropped significantly when the acid treat time exceeded 3 h. The reason was because NVF has extremely poor water solubility, and the ammonium salt was formed and the NVF solubility can be improved significantly after the treatment of hydrochloric acid, causing the stimulating effect on the copolymerization and flocculation. However, the polysaccharide in the product tended to hydrolyze apparently when the acidification time exceeded 3 h, leading to the decrease of GE and flocculation performance of the prepared samples.
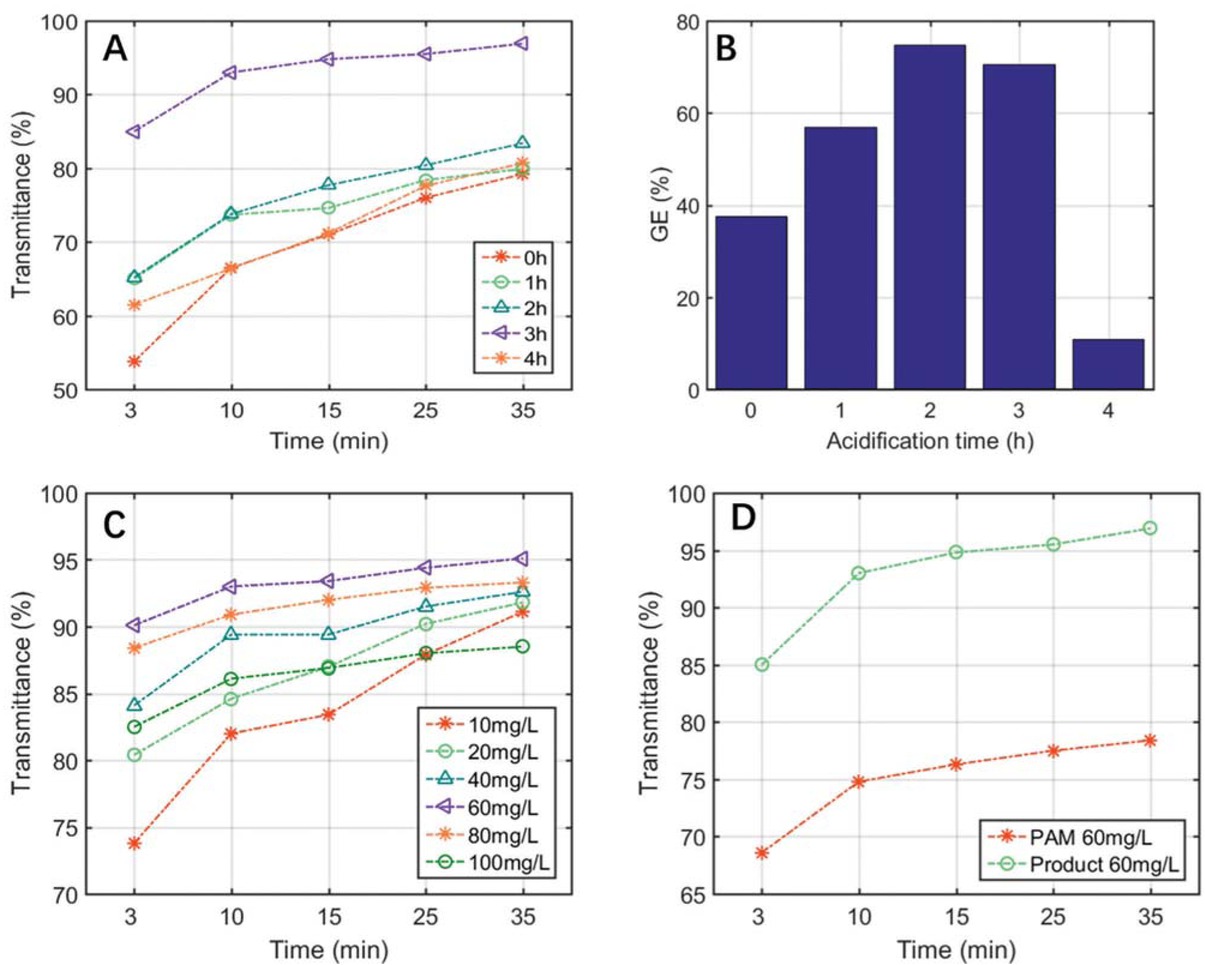
Effect of acidification time on transmittance (a) and GE (b); effect of flocculant dosage on transmittance (c); flocculation ability comparison (d).
4.5 Effect of flocculant dosage
Figure 6c showed the impact of dosage of the synthesized flocculant on its transmittance under the optimal operation conditions. As show in Figure 6c, the flocculation performance increased steadily with the rise of the flocculant dosage at initial stage, afterwards the transmittance tended to decline obviously, and the experimental results indicated that the transmittance was over 95% under the optimal flocculant dosage of 60 mg/L. This could be explained as follows: since the new flocculant has dual effects on neutralization and flocculation, it is more prone to collect the suspended particles inside coalmine wastewater with increasing adding amount (35). Meanwhile the flocculation effect tended to decline when the flocculant dosage exceeded its optimal amount, which was not only because the over-neutralized electric charges of wastewater can make the like charges repelled each other, but also the impurity particle surface would be covered by superfluous copolymer chains, leading to the obstacle for bridging flocculation (36).
Furthermore, the flocculation efficiency between the product and conventional PAM were experimentally compared, and the results were shown in Figure 6d. It can be concluded from the figure that the new flocculant behaved a dominate treatment effect for coalmine waste water than PAM under its optimal operation conditions, which would have a great potential and promising application for waste water treatment in the future.
5 Conclusion
In this work, a new flocculant (CMC-g-PAMD) was produced by the graft copolymerization of AN, NVF andCMC, which was confirmed by the characterizations. The results of EA, TG-DTG and SEM indicated that PAMD was actually grafted onto CMC, and FT-IR and NMR spectra demonstrated the successful formation of the polyamidine structure (five-membered cyclic copolymer). The optimal synthesis conditions of CMC-g-PAMD were experimentally determined: 4 g initiator dosage, 50°C polymerization temperature, 5 h polymerization time, 4 h acidification time, 90°C amidinization temperature and 3 h amidinization temperature. The new flocculant was employed in coalmine wastewater treatment, and the most suitable dosage was 60 mg/L. Compared with the conventional flocculant (PAM), CMC-g-PAMD presented an overwhelming high turbidity removal performance with a transmittance of 96.1%, which is bound to have broad prospects for wastewater treatment in the future.
Acknowledgments
This work was financially supported by the Local service fund of education department of Shaanxi province (18JF031), Xi’an science and technology innovation guidance project (201805041YD19CG25(4)), and Open Foundation of Shaanxi Key Laboratory of Lacustrine Shale Gas Accumulation and Exploitation (YJSYZX18SKF0003) was greatly acknowledged.
References
1 Yang Y.P., Yang Z.P., Xu G., Wang N.L., Situation and prospect of energy consumption for china’s thermal power generation. Chin Soc Electr Eng, 2013, 33(23), 1-11.Search in Google Scholar
2 Duan N., Guo J.P., Xie B.C., Is there a difference between the energy and CO2 emission performance for china’s thermal power industry? A bootstrapped directional distance function approach. Appl Energy, 2016, 162, 1552-1563.10.1016/j.apenergy.2015.02.066Search in Google Scholar
3 National Bureau of Statistics of the People’s Republic of China, China statistical yearbook China. Statistical Publishing House, Beijing, 2017.Search in Google Scholar
4 Zeng Q., Li G.S., Dong J.X., Pu Y., Typical ecological and environmental issues and countermeasures in coal mining in Xinjiang Region. Min Saf Environ Prot, 2017, 44(1), 106-110.Search in Google Scholar
5 He B., Li S.R., Effluent sewage recycling project in wangjiashan coal mine. Coal, 2018, 27(2), 0018-02.Search in Google Scholar
6 Bian Z.F., Inyang H.I., Daniels J.L., Otto F., Environmental issues from coal mining and their solutions. Int J Min Sci Technol, 2010, 20(2), 215-223.10.1016/S1674-5264(09)60187-3Search in Google Scholar
7 Chabukdhara M., Singh O.P., Coal mining in Northeast India: an overview of environmental issues and treatment approaches. Int J Coal Sci & Technol, 2016, 3(2), 1-10.10.1007/s40789-016-0126-1Search in Google Scholar
8 Chai S.L., Robinson J., Mei F.C., A review on application of flocculants in wastewater treatment. Proc Saf Environ Prot, 2014, 92(6), 489-508.10.1016/j.psep.2014.04.010Search in Google Scholar
9 Yan M.Q., Wang D.S., Yu J.F., Ni J.R., Edwards M., Qu J.H., Enhanced coagulation with polyaluminum chlorides: role of ph/alkalinity and speciation. Chemosphere, 2008, 71(9), 1665-1673.10.1016/j.chemosphere.2008.01.019Search in Google Scholar PubMed
10 Song L., Application of flocculants to water treatment and its forecast. Ind Water Treat, 2010, 30(6), 4-7.Search in Google Scholar
11 Wang Q., Zhao L., Research progress of new inorganic polymer flocculants. Liaoning Chem Ind, 2017, 46(2), 0185-02.Search in Google Scholar
12 Hou M.L., Wei Y.X., Wu Z., Liu H., Cao F.M., A study on the preparation and application of polymer ferric salt flocculants. Chem Propellants Polym Mater 2003, 1829(1), 151-157.Search in Google Scholar
13 Hu F., Research progress of species of aluminum salt flocculants. Pap Sci Technol, 2007, 26(3), 0036-05.Search in Google Scholar
14 Zhang P.Y., Wu Z., Zhang G.M., Zeng G.M., Zhang H., Li J., et al., Coagulation characteristics of polyaluminum chlorides pac-al 30, on humic acid removal from water. Sep Purif Technol, 2008, 63(3), 642-647.10.1016/j.seppur.2008.07.008Search in Google Scholar
15 Zhang M., Xiao F., Wang D.S., Xu X.Z., Zhou Q., Comparison of novel magnetic polyaluminum chlorides involved coagulation with traditional magnetic seeding coagulation: coagulant characteristics, treating effects, magnetic sedimentation efficiency and floc properties. Sep Purif Technol, 2017, 182, 118-127.10.1016/j.seppur.2017.03.028Search in Google Scholar
16 Kirwan L.J., Investigating bauxite residue flocculation by hydroxamate and polyacrylate flocculants utilising the focussed beam reflectance measurement probe. Int J Miner Process, 2009, 90(1), 74-80.10.1016/j.minpro.2008.10.010Search in Google Scholar
17 Gerchman Y., Vasker B., Tavasi M., Mishael Y., Kinel-Tahan Y., Yehoshua Y., Effective harvesting of microalgae: Comparison of different polymeric flocculants. Bioresour Technol, 2017, 228, 141-146.10.1016/j.biortech.2016.12.040Search in Google Scholar PubMed
18 Liu C., Hong B., Xu K., Zhang M.Y., An H.Y., Tan Y., et al., Synthesis and application of salt tolerance amphoteric hydrophobic associative flocculants. Polym Bull, 2014, 71(12), 3051-3065.10.1007/s00289-014-1237-8Search in Google Scholar
19 Zou Y.Q., Li S.S., Wang Y.Q., et al., Flocculation behavior of cationic pea starch prepared by the graft copolymerization of acrylamide for wastewater treatment. J Appl Polym Sci, 2016, 133(37), 43922-43911.10.1002/app.43922Search in Google Scholar
20 Li S.S., Zheng L., Wang Y.Q., Han X.L., Sun W., Yue Y.J., et al., Polyacrylamide-grafted legume starch for wastewater treatment: synthesis and performance comparison. Polym Bull, 2017, 74(11), 4371-4392.10.1007/s00289-017-1959-5Search in Google Scholar
21 Ochoa G.J.R., Muñoz H.M., Reinoso D., et al., Stability of inverse microemulsions of acrylamide-based anionic flocculants: evidence about the need of unsaturated surfactants. e-Polymers, 2008, 8(1), 320-329.10.1515/epoly.2008.8.1.320Search in Google Scholar
22 Ochoa G.J.R., Río F., Sasia P.M., et al., Synthesis of cationic flocculants based on acrylamide and (2-(acryloyloxy)ethyl) trimethylammonium chloride co polymers by semicontinuous inverse microemulsion co polymerization. Part I: Criteria for selection of comonomer formulation. e-Polymers, 2006, 6(1), 327-340.10.1515/epoly.2006.6.1.327Search in Google Scholar
23 Zhou H.J., Zhou L., Yang X.Y., Optimization of preparing a high yield and high cationic degree starch graft copolymer as environmentally friendly flocculant: Through response surface methodology. Int J Biol Macromol, 2018, 10(15), 1431-1437.10.1016/j.ijbiomac.2018.06.155Search in Google Scholar PubMed
24 Huang M., Liu Z.Z., Li A.M., et al., Dual functionality of a graft starch flocculant: Flocculation and antibacterial performance. J Environ Manage, 2017, 196, 63-71.10.1016/j.jenvman.2017.02.078Search in Google Scholar PubMed
25 Furusho Y., Endo T., Supramolecular polymer gels formed from carboxy-terminated telechelic polybutadiene and polyamidine through amidinium-carboxylate salt bridge. J Polym Sci, Part A: Polym Chem, 2014, 52(13), 1815-1824.10.1002/pola.27187Search in Google Scholar
26 Furusho Y., Endo T., Capture and release of CO2 by polyamidine. J Polym Sci, Part A: Polym Chem, 2013, 51(16), 3404-3411.10.1002/pola.26737Search in Google Scholar
27 Aleksandrova E.L., Dudkina M.M., Ten’Kovtsev A.V., Polyamidine supramolecular structures—a new class of photosensitive polymeric semiconductors. Semiconductors, 2003, 37(3), 266-270.10.1134/1.1561516Search in Google Scholar
28 Guo B., Gao B.Y., Rong H.Y., et al., Coagulation performance and floc properties of compound bioflocculant-aluminum sulfate dual-coagulant in treating kaolin-humic acid solution. Chem Eng J, 2011, 173(2), 400-406.10.1016/j.cej.2011.07.077Search in Google Scholar
29 Wang Y.Q., Yue Y.J., Ren J.H., et al., A polyamidine-based flocculant: synthesis and characterization. China Invention Patent: 201810434161.X.Search in Google Scholar
30 Cai T., Yang Z., Yang H., Li A.M., Cheng R.S., Preparation and flocculation properties of carboxymethyl cellulose-graft-polyacrylamide. J Nanjing University, 2013, 49(4), 500-505.Search in Google Scholar
31 Siqueira E.J., Salon M.B., Belgacem M.N., et al., Carboxymethyl-cellulose (CMC) as a model compound of cellulose fibers and polyamideamine epichlorohydrin (PAE)–CMC interactions as a model of PAE–fibers interactions of PAE‐based wet strength papers. J Appl Polym Sci, 2015, 132(26), 42144 (1 of 10).10.1002/app.42144Search in Google Scholar
32 Sabaa M.W., Elzanaty A.M., Abdelgawad O.F., et al., Synthesis, characterization and antimicrobial activity of Schiff bases modified chitosan-graft-poly(acrylonitrile). Int J Biol Macromol, 2017, 109, 1280-1291.10.1016/j.ijbiomac.2017.11.129Search in Google Scholar PubMed
33 Guo B., Yu H., Gao B.Y., et al., Novel cationic polyamidine: Synthesis, characterization, and sludge dewatering performance. J Environ Sci, 2017, 51(1), 305-314.10.1016/j.jes.2016.08.002Search in Google Scholar PubMed
34 Zhao Y.X., Wang Z.T., The study on the synthesis of the starch grafted polyamidine. 2008.Search in Google Scholar
35 Ghaemy M., Pour Z.S., Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). Rsc Adv, 2015, 5(79), 64106-64118.10.1039/C5RA08025HSearch in Google Scholar
36 Reddy K.R., Hassan M., Gomes V.G., Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl Catal A Gen, 2015, 489, 1-16.10.1016/j.apcata.2014.10.001Search in Google Scholar
© 2019 Yue et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die
Articles in the same Issue
- Special Issue: Polymers and Composite Materials / Guest Editor: Esteban Broitman
- A novel chemical-consolidation sand control composition: Foam amino resin system
- Bottom fire behaviour of thermally thick natural rubber latex foam
- Preparation of polymer–rare earth complexes based on Schiff-base-containing salicylic aldehyde groups attached to the polymer and their fluorescence emission properties
- Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56
- Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene
- Effect of surface modifications on the properties of UHMWPE fibres and their composites
- Thermal degradation kinetics investigation on Nano-ZnO/IFR synergetic flame retarded polypropylene/ethylene-propylene-diene monomer composites processed via different fields
- Properties of carbon black-PEDOT composite prepared via in-situ chemical oxidative polymerization
- Regular articles
- Polyarylene ether nitrile and boron nitride composites: coating with sulfonated polyarylene ether nitrile
- Influence of boric acid on radial structure of oxidized polyacrylonitrile fibers
- Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment
- Application of calcium montmorillonite on flame resistance, thermal stability and interfacial adhesion in polystyrene nanocomposites
- Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications
- Polycation-globular protein complex: Ionic strength and chain length effects on the structure and properties
- Improving the flame retardancy of ethylene vinyl acetate composites by incorporating layered double hydroxides based on Bayer red mud
- N, N’-sebacic bis(hydrocinnamic acid) dihydrazide: A crystallization accelerator for poly(L-lactic acid)
- The fabrication and characterization of casein/PEO nanofibrous yarn via electrospinning
- Waterborne poly(urethane-urea)s films as a sustained release system for ketoconazole
- Polyimide/mica hybrid films with low coefficient of thermal expansion and low dielectric constant
- Effects of cylindrical-electrode-assisted solution blowing spinning process parameters on polymer nanofiber morphology and microstructure
- Stimuli-responsive DOX release behavior of cross-linked poly(acrylic acid) nanoparticles
- Continuous fabrication of near-infrared light responsive bilayer hydrogel fibers based on microfluidic spinning
- A novel polyamidine-grafted carboxymethylcellulose: Synthesis, characterization and flocculation performance test
- Synthesis of a DOPO-triazine additive and its flame-retardant effect in rigid polyurethane foam
- Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution
- Enhanced thermal oxidative stability of silicone rubber by using cerium-ferric complex oxide as thermal oxidative stabilizer
- Long-term durability antibacterial microcapsules with plant-derived Chinese nutgall and their applications in wound dressing
- Fully water-blown polyisocyanurate-polyurethane foams with improved mechanical properties prepared from aqueous solution of gelling/ blowing and trimerization catalysts
- Preparation of rosin-based polymer microspheres as a stationary phase in high-performance liquid chromatography to separate polycyclic aromatic hydrocarbons and alkaloids
- Effects of chemical modifications on the rheological and the expansion behavior of polylactide (PLA) in foam extrusion
- Enhanced thermal conductivity of flexible h-BN/polyimide composites films with ethyl cellulose
- Maize-like ionic liquid@polyaniline nanocomposites for high performance supercapacitor
- γ-valerolactone (GVL) as a bio-based green solvent and ligand for iron-mediated AGET ATRP
- Revealing key parameters to minimize the diameter of polypropylene fibers produced in the melt electrospinning process
- Preliminary market analysis of PEEK in South America: opportunities and challenges
- Influence of mid-stress on the dynamic fatigue of a light weight EPS bead foam
- Manipulating the thermal and dynamic mechanical properties of polydicyclopentadiene via tuning the stiffness of the incorporated monomers
- Voigt-based swelling water model for super water absorbency of expanded perlite and sodium polyacrylate resin composite materials
- Simplified optimal modeling of resin injection molding process
- Synthesis and characterization of a polyisocyanide with thioether pendant caused an oxidation-triggered helix-to-helix transition
- A glimpse of biodegradable polymers and their biomedical applications
- Development of vegetable oil-based conducting rigid PU foam
- Conetworks on the base of polystyrene with poly(methyl methacrylate) paired polymers
- Effect of coupling agent on the morphological characteristics of natural rubber/silica composites foams
- Impact and shear properties of carbon fabric/ poly-dicyclopentadiene composites manufactured by vacuum‐assisted resin transfer molding
- Effect of resins on the salt spray resistance and wet adhesion of two component waterborne polyurethane coating
- Modifying potato starch by glutaraldehyde and MgCl2 for developing an economical and environment-friendly electrolyte system
- Effect of curing degree on mechanical and thermal properties of 2.5D quartz fiber reinforced boron phenolic composites
- Preparation and performance of polypropylene separator modified by SiO2/PVA layer for lithium batteries
- A simple method for the production of low molecular weight hyaluronan by in situ degradation in fermentation broth
- Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber
- Developing an epoxy resin with high toughness for grouting material via co-polymerization method
- Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation
- Study on the synthesis of hexene-1 catalyzed by Ziegler-Natta catalyst and polyhexene-1 applications
- Fabrication and characterization of conductive microcapsule containing phase change material
- Desorption of hydrolyzed poly(AM/DMDAAC) from bentonite and its decomposition in saltwater under high temperatures
- Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives
- The application of a phosphorus nitrogen flame retardant curing agent in epoxy resin
- High performance polyimide films containing benzimidazole moieties for thin film solar cells
- Rigid polyurethane/expanded vermiculite/ melamine phenylphosphate composite foams with good flame retardant and mechanical properties
- A novel film-forming silicone polymer as shale inhibitor for water-based drilling fluids
- Facile droplet microfluidics preparation of larger PAM-based particles and investigation of their swelling gelation behavior
- Effect of salt and temperature on molecular aggregation behavior of acrylamide polymer
- Dynamics of asymmetric star polymers under coarse grain simulations
- Experimental and numerical analysis of an improved melt-blowing slot-die

