Abstract
C48H35Fe2NO4P2Se2, monoclinic, P21/c, a = 20.3289(18) Å, b = 12.6909(11) Å, c = 17.6743(16) Å, β = 109.767(4)∘, V = 4291.1(7) Å3, Z = 4, Rgt(F) = 0.0638, wRref(F2) = 0.1439, T = 298(2) K.
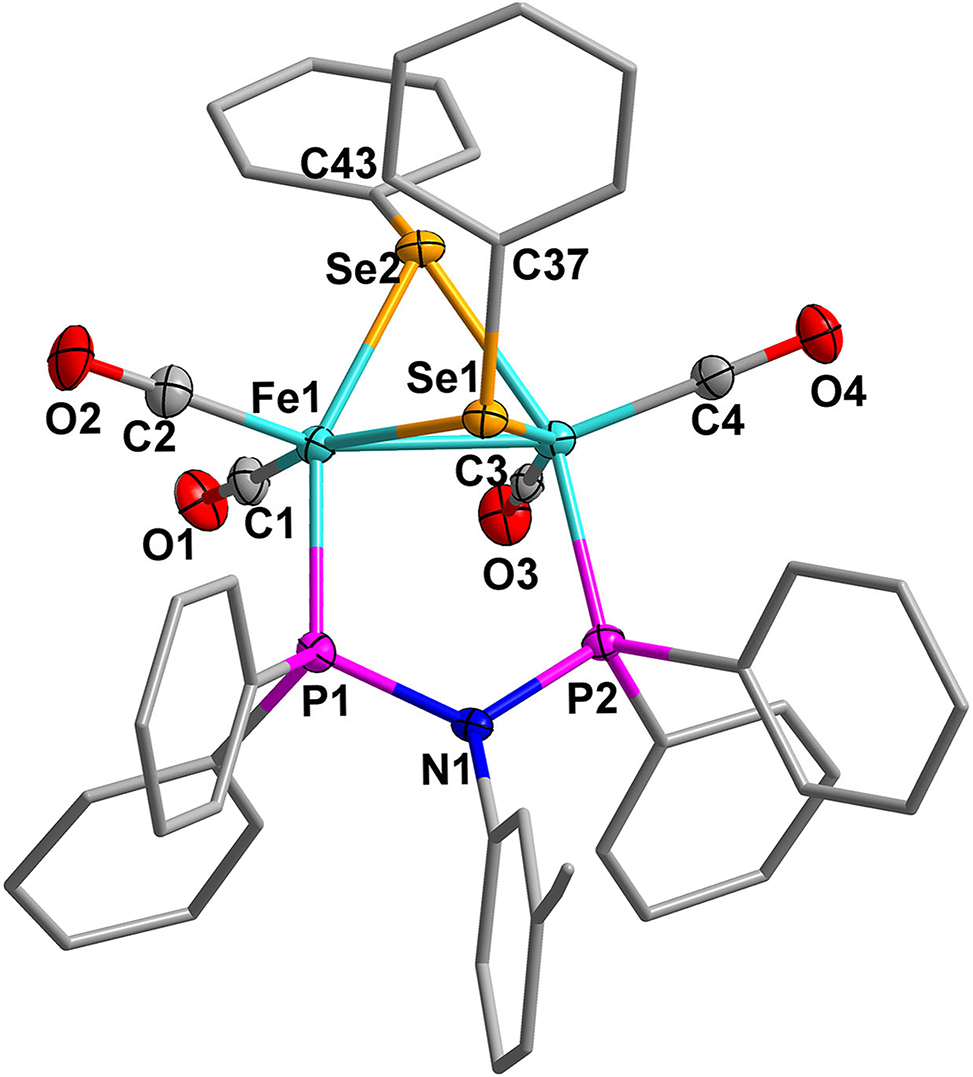
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.38 × 0.26 × 0.20 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 2.49 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker SMART-1000, φ and ω 25.0°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 19908, 7498, 0.100 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4106 |
| N(param)refined: | 532 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1165 (4) | 0.6876 (6) | 0.4179 (5) | 0.0353 (19) |
| C2 | 0.1956 (4) | 0.7142 (6) | 0.5663 (5) | 0.040 (2) |
| C3 | 0.1732 (4) | 0.6913 (6) | 0.2873 (5) | 0.0315 (18) |
| C4 | 0.3058 (4) | 0.7366 (6) | 0.3108 (4) | 0.0320 (18) |
| C5 | 0.1252 (4) | 0.4480 (6) | 0.4607 (4) | 0.045 (2) |
| C6 | 0.0846 (4) | 0.4204 (6) | 0.3840 (5) | 0.079 (3) |
| H6 | 0.102708 | 0.438966 | 0.344036 | 0.095* |
| C7 | 0.0210 (4) | 0.3694 (6) | 0.3572 (7) | 0.098 (3) |
| H7 | −0.002887 | 0.355185 | 0.303202 | 0.118* |
| C8 | −0.0038 (6) | 0.3414 (8) | 0.4179 (6) | 0.110 (4) |
| H8 | −0.045451 | 0.303837 | 0.404926 | 0.133* |
| C9 | 0.0306 (5) | 0.3670 (8) | 0.4968 (7) | 0.120 (4) |
| H9 | 0.011824 | 0.350485 | 0.536543 | 0.144* |
| C10 | 0.0945 (5) | 0.4182 (7) | 0.5155 (6) | 0.095 (3) |
| H10 | 0.118191 | 0.433590 | 0.569271 | 0.114* |
| C11 | 0.2524 (4) | 0.4551 (6) | 0.5840 (4) | 0.0311 (17) |
| C12 | 0.2440 (5) | 0.3531 (7) | 0.6030 (5) | 0.052 (2) |
| H12 | 0.216850 | 0.308764 | 0.562565 | 0.062* |
| C13 | 0.2733 (5) | 0.3128 (7) | 0.6784 (5) | 0.064 (3) |
| H13 | 0.265575 | 0.243135 | 0.689391 | 0.077* |
| C14 | 0.3149 (5) | 0.3780 (7) | 0.7385 (5) | 0.063 (3) |
| H14 | 0.335647 | 0.352283 | 0.790374 | 0.076* |
| C15 | 0.3253 (4) | 0.4791 (6) | 0.7214 (5) | 0.048 (2) |
| H15 | 0.353902 | 0.522761 | 0.761276 | 0.057* |
| C16 | 0.2932 (4) | 0.5177 (6) | 0.6439 (4) | 0.0382 (19) |
| H16 | 0.299817 | 0.587748 | 0.632916 | 0.046* |
| C17 | 0.2179 (4) | 0.4713 (6) | 0.2521 (4) | 0.0355 (18) |
| C18 | 0.2374 (5) | 0.5057 (7) | 0.1889 (4) | 0.054 (2) |
| H18 | 0.275255 | 0.551170 | 0.198565 | 0.065* |
| C19 | 0.2016 (5) | 0.4735 (7) | 0.1116 (5) | 0.066 (3) |
| H19 | 0.216276 | 0.495419 | 0.069710 | 0.079* |
| C20 | 0.1444 (5) | 0.4092 (7) | 0.0961 (5) | 0.067 (3) |
| H20 | 0.119054 | 0.390888 | 0.043312 | 0.081* |
| C21 | 0.1243 (5) | 0.3718 (7) | 0.1572 (5) | 0.069 (3) |
| H21 | 0.085449 | 0.328349 | 0.147498 | 0.083* |
| C22 | 0.1640 (4) | 0.4012 (6) | 0.2337 (5) | 0.058 (2) |
| H22 | 0.153343 | 0.371112 | 0.276106 | 0.070* |
| C23 | 0.3553 (4) | 0.4770 (6) | 0.3568 (4) | 0.0319 (17) |
| C24 | 0.4129 (4) | 0.5374 (6) | 0.3864 (5) | 0.040 (2) |
| H24 | 0.408910 | 0.603806 | 0.406719 | 0.048* |
| C25 | 0.4771 (4) | 0.5033 (7) | 0.3873 (5) | 0.050 (2) |
| H25 | 0.516176 | 0.545769 | 0.409562 | 0.060* |
| C26 | 0.4843 (5) | 0.4082 (7) | 0.3559 (5) | 0.053 (2) |
| H26 | 0.527529 | 0.386090 | 0.354574 | 0.063* |
| C27 | 0.4265 (5) | 0.3456 (7) | 0.3263 (5) | 0.055 (2) |
| H27 | 0.430425 | 0.279480 | 0.305513 | 0.067* |
| C28 | 0.3643 (4) | 0.3791 (6) | 0.3271 (5) | 0.0412 (19) |
| H28 | 0.325707 | 0.335090 | 0.306901 | 0.049* |
| C29 | 0.2761 (4) | 0.3450 (6) | 0.4435 (5) | 0.0344 (17) |
| C30 | 0.2384 (4) | 0.2564 (6) | 0.4117 (5) | 0.050 (2) |
| H30 | 0.194048 | 0.261394 | 0.373323 | 0.060* |
| C31 | 0.2682 (5) | 0.1591 (7) | 0.4382 (6) | 0.066 (3) |
| H31 | 0.242963 | 0.098775 | 0.416301 | 0.079* |
| C32 | 0.3320 (5) | 0.1479 (7) | 0.4944 (6) | 0.058 (2) |
| H32 | 0.350374 | 0.081200 | 0.510669 | 0.070* |
| C33 | 0.3699 (4) | 0.2379 (6) | 0.5277 (5) | 0.044 (2) |
| C34 | 0.3413 (4) | 0.3358 (6) | 0.5008 (5) | 0.0375 (18) |
| H34 | 0.366635 | 0.396373 | 0.521858 | 0.045* |
| C35 | 0.4349 (5) | 0.2299 (7) | 0.5912 (6) | 0.056 (2) |
| C36 | 0.4864 (6) | 0.2256 (8) | 0.6440 (7) | 0.083 (4) |
| H36 | 0.528225 | 0.222154 | 0.686987 | 0.100* |
| C37 | 0.3810 (4) | 0.7880 (6) | 0.5485 (5) | 0.0354 (19) |
| C38 | 0.4333 (5) | 0.8220 (7) | 0.5236 (6) | 0.057 (2) |
| H38 | 0.438641 | 0.793831 | 0.477481 | 0.069* |
| C39 | 0.4791 (5) | 0.8991 (7) | 0.5670 (6) | 0.069 (3) |
| H39 | 0.515798 | 0.921552 | 0.550741 | 0.083* |
| C40 | 0.4700 (5) | 0.9410 (8) | 0.6323 (7) | 0.071 (3) |
| H40 | 0.500915 | 0.992409 | 0.661325 | 0.085* |
| C41 | 0.4175 (5) | 0.9107 (7) | 0.6568 (6) | 0.068 (3) |
| H41 | 0.411496 | 0.941638 | 0.701714 | 0.082* |
| C42 | 0.3724 (5) | 0.8330 (7) | 0.6147 (5) | 0.053 (2) |
| H42 | 0.335869 | 0.811147 | 0.631546 | 0.063* |
| C43 | 0.1496 (4) | 0.9239 (6) | 0.3592 (5) | 0.040 (2) |
| C44 | 0.0989 (5) | 0.9523 (7) | 0.3887 (6) | 0.061 (2) |
| H44 | 0.098280 | 0.921984 | 0.436397 | 0.074* |
| C45 | 0.0482 (5) | 1.0249 (8) | 0.3502 (7) | 0.077 (3) |
| H45 | 0.012487 | 1.041049 | 0.370073 | 0.092* |
| C46 | 0.0514 (6) | 1.0718 (9) | 0.2833 (7) | 0.089 (3) |
| H46 | 0.018603 | 1.123116 | 0.258168 | 0.106* |
| C47 | 0.1012 (6) | 1.0459 (9) | 0.2518 (6) | 0.090 (3) |
| H47 | 0.102424 | 1.079195 | 0.205449 | 0.108* |
| C48 | 0.1511 (5) | 0.9689 (8) | 0.2889 (6) | 0.068 (3) |
| H48 | 0.184497 | 0.948631 | 0.266482 | 0.082* |
| Fe1 | 0.20562 (5) | 0.68237 (8) | 0.47512 (6) | 0.0255 (3) |
| Fe2 | 0.25452 (5) | 0.69126 (8) | 0.36364 (6) | 0.0251 (3) |
| N1 | 0.2471 (3) | 0.4492 (4) | 0.4220 (3) | 0.0220 (14) |
| O1 | 0.0579 (3) | 0.6945 (5) | 0.3821 (3) | 0.0553 (17) |
| O2 | 0.1879 (3) | 0.7343 (5) | 0.6260 (3) | 0.0643 (19) |
| O3 | 0.1188 (3) | 0.6887 (5) | 0.2390 (3) | 0.0532 (17) |
| O4 | 0.3394 (3) | 0.7691 (5) | 0.2734 (3) | 0.0552 (17) |
| P1 | 0.20776 (10) | 0.51040 (15) | 0.48374 (11) | 0.0262 (5) |
| P2 | 0.26847 (10) | 0.52271 (15) | 0.35160 (11) | 0.0266 (5) |
| Se1 | 0.32723 (4) | 0.66590 (6) | 0.49919 (4) | 0.0279 (2) |
| Se2 | 0.22984 (4) | 0.84541 (6) | 0.42415 (5) | 0.0304 (2) |
1 Source of material
A mixture of [Fe2(CO)6(μ–SeC6H5)2] (118 mg, 0.2 mmol) and (Ph2P)2NC6H4-3–CCH (97 mg, 0.2 mmol) was dissolved in 15 mL of xylene. The reaction mixture was stirred at reflux for 1 h. After removing the solvent under vacuum, the residue was subjected to preparative TLC separation using dichloromethane/pentane (1:1, v/v) as eluent. The title complex was obtained as a deep-red solid (yield 38 %) from the main band. Single crystals were obtained by diffusing the dichloromethane solution into a hexane solution at room temperature.
2 Experimental details
The structure was solved by Direct Methods with the SHELXS program. Hydrogen atoms were positioned geometrically (C–H = 0.93–0.98 Å). Their Uiso values were set to 1.2 Ueq or 1.5 Ueq of the parent atoms.
3 Comment
Hydrogenase is considered a highly promising catalyst capable of catalyzing hydrogen production under mild conditions. 5 In recent years, hydrogenase model compounds containing the 2Fe2Se unit have garnered considerable attention as synthetic catalysts. The unique structure and activity of these model compounds make them powerful tools for understanding the catalytic mechanisms of natural hydrogenase and for designing novel catalysts. 6 , 7 They share similarities in structure with the active center of natural hydrogenase but offer greater flexibility in synthesis and regulation. A series of mono- and di-phosphine substituted Fe/Se model compounds have been reported. 8 – 12 Herein, we carried out the CO substitution reaction of [Fe2(CO)6(μ–SeC6H5)2] with a disphosphine ligand (Ph2P)2NC6H4-3–CCH, and obtained the title complex. The title complex comprises a 2Fe2Se cluster with four carbonyls and one diphosphine ligand. The Se1 and Se2 atoms are linked to the two phenyl substituents through axial Se1–C37 and Se2–C43 equatorial bonds. The two phosphorus atoms of diphosphine ligand are positioned at the cisoid dibasal site, symmetrically connecting the two Fe atoms, resembling the previously reported analogues. 13 , 14 The bond length Fe1–Fe2 [2.4916(14) Å] is slightly longer than that of sulfur analogues, and the average Se–Fe–Se angles in the title complex are more open than those in sulfur analogues. 15 – 19
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was supported by Shandong Provincial Natural Science Foundation under Grant ZR2020MB019.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
5. Frey, M. Hydrogenases: Hydrogen-Activating Enzymes. Chembiochem 2002, 3, 153–160; https://doi.org/10.1002/1439-7633(20020301)3:2/3<153::aid-cbic153>3.0.co;2-b.10.1002/1439-7633(20020301)3:2/3<153::AID-CBIC153>3.0.CO;2-BSuche in Google Scholar
6. Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 3, 4081–4148; https://doi.org/10.1021/cr4005814.Suche in Google Scholar
7. Li, Y.; Rauchfuss, T. B. Synthesis of Diiron(I) Dithiolato Carbonyl Complexes. Chem. Rev. 2016, 116, 7043–7077; https://doi.org/10.1021/acs.chemrev.5b00669.Suche in Google Scholar
8. Bai, S.-F.; Du, X.-M.; Tian, W.-J.; Xu, H.; Zhang, R.-F.; Ma, C.-L.; Wang, Y.-L.; Lü, S.; Li, Q.-L.; Li, Y.-L. Di-tri- and Tetraphosphine-Substituted Fe/Se Carbonyls: Synthesis, Characterization and Electrochemical Properties. Dalton Trans. 2023, 51, 11125–11134; https://doi.org/10.1039/d2dt01376b.Suche in Google Scholar
9. Bai, S.-F.; Ma, J.-W.; Guo, Y.-N.; Du, X.-M.; Wang, Y.-L.; Li, Q.-L.; Lü, S. Aminophosphine-substituted Fe/E (E = S, Se) Carbonyls Related to [FeFe]-Hydrogenases: Synthesis, Protonation, and Electrocatalytic Proton Reduction. J. Mol. Struct. 2023, 1283, 135287; https://doi.org/10.1016/j.molstruc.2023.135287.Suche in Google Scholar
10. Abul-Futouh, H.; Abaalkhail, S. J.; Harb, M. K.; Görls, H.; Weigand, W. Structural Studies and Electrochemical Catalysis Investigation of [FeFe]-Hydrogenase H-Cluster Mimics Mediated by Monophosphane Ligands. Polyhedron 2021, 207, 115382; https://doi.org/10.1016/j.poly.2021.115382.Suche in Google Scholar
11. El-khateeb, M.; Abul-Futouh, H.; Alshurafa, H.; G?rls, H.; Weigand, W. Influence of Bidentate Phosphine Ligands on the Chemistry of [FeFe]-Hydrogenase Model: Insight into Molecular Structures and Electrochemical Characteristics. Appl. Organomet. Chem. 2020, 34, e5940; https://doi.org/10.1002/aoc.5940.Suche in Google Scholar
12. Song, L.-C.; Gai, B.; Wang, H.-T.; Hu, Q.-M. Synthesis, Characterization and Electrocatalysis of Diiron Propanediselenolate Derivatives as the Active Site Models of FeFe -hydrogenases. J. Inorg. Biochem. 2009, 103, 805–812; https://doi.org/10.1016/j.jinorgbio.2009.02.002.Suche in Google Scholar PubMed
13. Lü, S.; Bai, S.-F.; Gao, X.-P.; Wang, Y.-L.; Li, Q.-L. Aminodiphosphine Substituted 2Fe2Se Complex as New Precursor to Single and Double Butterfly Fe/Se Models Related to FeFe Hydrogenase Models. J. Mol. Struct. 2023, 1290, 135939; https://doi.org/10.1016/j.molstruc.2023.135939.Suche in Google Scholar
14. Lü, S.; Gong, S.; Qin, C.-R.; Li, Q.-L. PNP Bridged Diiron Carbonyls Containing Fe/E (E = S and Se) Cluster Core Related to the Active Site of [FeFe]–H2 Ases. J. Organomet. Chem. 2020, 929, 121581; https://doi.org/10.1016/j.jorganchem.2020.121581.Suche in Google Scholar
15. Ghosh, S.; Hogarth, G.; Hollingsworth, N.; Holt, K. B.; Richards, I.; Richmond, M. G.; Sanchez, B. E.; Unwin, D. Models of the Iron-Only Hydrogenase: a Comparison of Chelate and Bridge Isomers of Fe2(CO)4{Ph2PN(R)PPh2}-(μ-pdt) as Proton-Reduction Catalysts. Dalton Trans. 2013, 42, 6775–6792; https://doi.org/10.1039/c3dt50147g.Suche in Google Scholar PubMed
16. Liu, X.-F.; Xu, B.; Xu, H.; Li, Y.-L. Synthesis, Characterization, and Electrocatalytic Hydrogen Evolution of Diiron Dithiolato Pentacarbonyl Complexes Bearing Phosphine Ligand. Chinese J. Inorg. Chem. 2023, 39, 1619–1627.Suche in Google Scholar
17. Liu, X.-F.; Li, Y.-L.; Liu, X.-H. Synthesis, Characterization, Electrocatalytic Properties, and Antifungal Activity of Isoxazole?containing di-iron Complexes. Chinese J. Inorg. Chem. 2023, 39, 2367–2376.Suche in Google Scholar
18. Zhao, P.-H.; Ma, Z.-Y.; Hu, M.-Y.; He, J.; Wang, Y.-Z.; Jing, X.-B.; Chen, H.-Y.; Wang, Z.; Li, Y.-L. PNP–Chelated and -bridged diiron Dithiolate Complexes Fe2(μ-pdt)(CO)4{(Ph2P)2NR} Together with Related Monophosphine Complexes for the 2Fe(H) Subsite of FeFe – Hydrogenases: Preparation, Structure, and Electrocatalysis. Organometallics 2018, 37, 1280–1290; https://doi.org/10.1021/acs.organomet.8b00030.Suche in Google Scholar
19. Bai, S.-F.; Lü, S.; Li, Q.-L. Crystal Structure of bis(μ-Benzeneselenolato)-(μ-[N-Benzyl-N-(diphenylphosphanyl)-P,P-diphenylphosphinous Amide])-Tetracarbonyl diiron (Fe–Fe), C47H37Fe2NO4P2Se2. Z. Kristallogr. N. Cryst. Struct. 2023, 238, 619–621; https://doi.org/10.1515/ncrs-2023-0119.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Artikel in diesem Heft
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

