Abstract
C24H20BrN3O3, triclinic, P
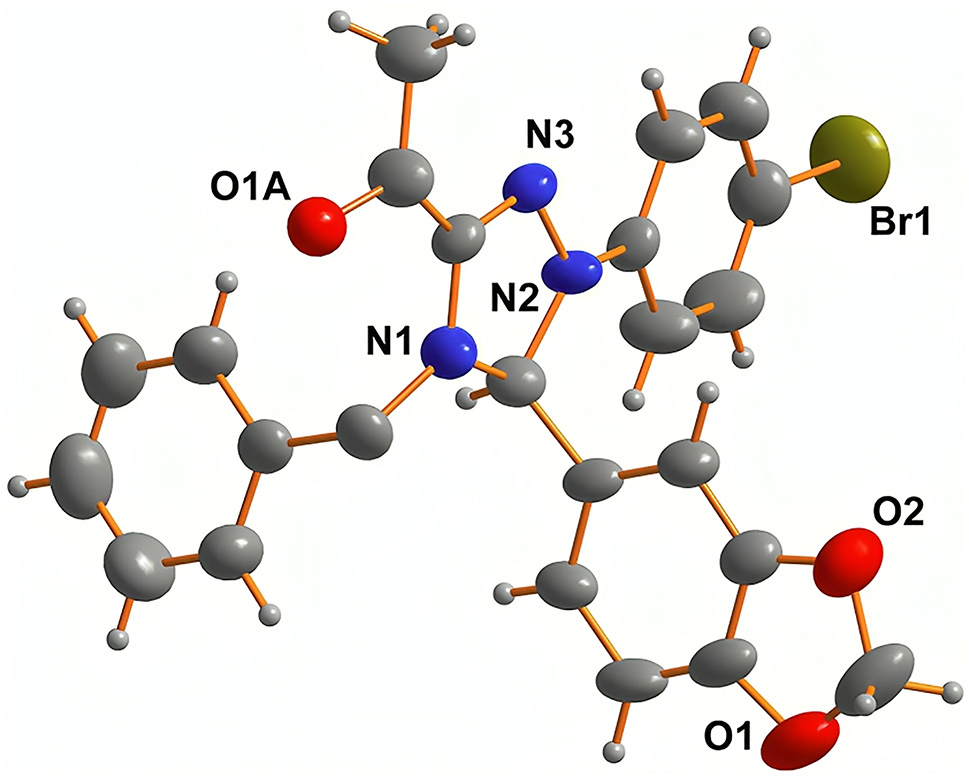
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.18 × 0.18 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.94 mm−1 |
| Diffractometer, scan mode: | STOE IPDS II, ω |
| θmax, completeness: | 25.7°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 9,356, 4,041, 0.072 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,368 |
| N(param)refined: | 281 |
| Programs: | X-Area, 1 X-RED, 2 SHELX, 3 WinGX/ORTEP-3, 4 Mercury 5 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.7147 (6) | 0.4279 (6) | 0.6198 (6) | 0.0402 (13) |
| C2 | 0.5452 (7) | 0.3057 (6) | 0.6832 (6) | 0.0446 (13) |
| H2 | 0.594763 | 0.309714 | 0.778455 | 0.053* |
| C3 | 0.3783 (7) | 0.1801 (6) | 0.5945 (6) | 0.0426 (13) |
| C4 | 0.3347 (7) | 0.0712 (6) | 0.6399 (7) | 0.0485 (14) |
| H4 | 0.405515 | 0.078891 | 0.727407 | 0.058* |
| C5 | 0.1854 (8) | −0.0510 (7) | 0.5564 (8) | 0.0575 (16) |
| H5 | 0.155767 | −0.125535 | 0.585803 | 0.069* |
| C6 | 0.0865 (8) | −0.0549 (6) | 0.4309 (8) | 0.0550 (16) |
| C7 | −0.1127 (9) | −0.1163 (8) | 0.2164 (9) | 0.082 (2) |
| H7A | −0.122230 | −0.176076 | 0.141066 | 0.098* |
| H7B | −0.215049 | −0.122365 | 0.185388 | 0.098* |
| C8 | 0.1284 (7) | 0.0550 (7) | 0.3872 (7) | 0.0501 (14) |
| C9 | 0.2737 (7) | 0.1751 (6) | 0.4653 (6) | 0.0478 (14) |
| H9 | 0.301277 | 0.248735 | 0.434320 | 0.057* |
| C10 | 0.5082 (6) | 0.5129 (6) | 0.7379 (6) | 0.0411 (13) |
| C11 | 0.4187 (8) | 0.4459 (7) | 0.7892 (7) | 0.0641 (18) |
| H11 | 0.395977 | 0.354746 | 0.785918 | 0.077* |
| C12 | 0.3630 (9) | 0.5132 (8) | 0.8453 (8) | 0.070 (2) |
| H12 | 0.300873 | 0.466707 | 0.877196 | 0.084* |
| C13 | 0.4001 (8) | 0.6482 (7) | 0.8532 (6) | 0.0553 (16) |
| C14 | 0.4883 (8) | 0.7154 (7) | 0.8040 (7) | 0.0624 (17) |
| H14 | 0.511585 | 0.806901 | 0.808485 | 0.075* |
| C15 | 0.5435 (8) | 0.6489 (7) | 0.7474 (7) | 0.0559 (16) |
| H15 | 0.605104 | 0.696358 | 0.715397 | 0.067* |
| C16 | 0.8261 (8) | 0.4646 (7) | 0.5740 (7) | 0.0559 (16) |
| C17 | 0.9037 (8) | 0.6083 (7) | 0.5764 (8) | 0.0649 (18) |
| H17A | 0.896132 | 0.670508 | 0.634891 | 0.097* |
| H17B | 1.013703 | 0.639024 | 0.610765 | 0.097* |
| H17C | 0.851969 | 0.608948 | 0.484947 | 0.097* |
| C18 | 0.6856 (7) | 0.1896 (6) | 0.6341 (6) | 0.0486 (14) |
| C19 | 0.7971 (7) | 0.2025 (6) | 0.7786 (6) | 0.0445 (13) |
| C20 | 0.9383 (8) | 0.3262 (7) | 0.8729 (7) | 0.0625 (17) |
| H20 | 0.965055 | 0.405140 | 0.848550 | 0.075* |
| C21 | 1.0391 (9) | 0.3336 (9) | 1.0016 (8) | 0.076 (2) |
| H21 | 1.133157 | 0.417343 | 1.063705 | 0.091* |
| C22 | 1.0024 (10) | 0.2195 (10) | 1.0390 (8) | 0.078 (2) |
| H22 | 1.071798 | 0.224624 | 1.125893 | 0.094* |
| C23 | 0.8643 (10) | 0.0979 (9) | 0.9491 (8) | 0.079 (2) |
| H23 | 0.838071 | 0.020161 | 0.975159 | 0.095* |
| C24 | 0.7624 (8) | 0.0890 (7) | 0.8194 (7) | 0.0614 (17) |
| H24 | 0.668569 | 0.004721 | 0.758500 | 0.074* |
| N1 | 0.6436 (5) | 0.3003 (5) | 0.6297 (5) | 0.0465 (12) |
| N2 | 0.5582 (5) | 0.4420 (5) | 0.6781 (5) | 0.0432 (11) |
| N3 | 0.6683 (5) | 0.5144 (5) | 0.6464 (5) | 0.0400 (11) |
| O1 | −0.0654 (6) | −0.1612 (5) | 0.3314 (6) | 0.0773 (15) |
| O2 | 0.0054 (6) | 0.0257 (5) | 0.2589 (5) | 0.0739 (14) |
| O1Aa | 0.8895 (18) | 0.3870 (11) | 0.5745 (17) | 0.054 (4)* |
| O1Bb | 0.8221 (15) | 0.3648 (10) | 0.5027 (15) | 0.056 (4)* |
| Br1 | 0.32084 (11) | 0.73907 (10) | 0.92824 (9) | 0.0899 (4) |
-
aOccupancy: 0.46(2), bOccupancy: 0.54(2).
1 Source of materials
The title compound was synthesized by 1,3-dipolar cycloaddition reaction (1,3–DPCA) of N-(3,4–methylenedioxybenzylidene) benzylamine and N-(4-bromophenyl)-2-oxopropanehydrazonoyl chloride during our research in the construction of chiral 1,2,4-triazoline derivatives. The reaction mixture was refluxed in ethanol with an excess amount of triethylamine according to the procedure reported earlier. 6 Colorless blocks for the compound, suitable for the X-ray structure analysis, were obtained after slow evaporation of ethanolic solution of the crude compound.
2 Experimental details
All the non-hydrogen atoms were refined as anisotropic except oxygen atom O1 which splits into two positions as O1A and O1B. The hydrogen atoms were generated geometrically with Uiso(H) = 1.2.
3 Comment
1,2,4–Triazolines stand as an intriguing yet underexplored category of heterocycles, holding considerable promise for diverse biological activities. 7 Their adaptability in organic synthesis and pivotal role as intermediates in the synthesis of various compounds, spanning pharmaceuticals to agrochemicals, 8 have attracted medicinal chemists over the last decade. In our endeavour to synthesize enantio-pure derivatives of 1,2,4-triazolines using carbohydrate moiety as a chiral scaffold, 6 we synthesized the target triazoline via 1,3–DPCA from achiral precursors to investigate the stereo selectivity output of this reaction. The crystallographic analysis provided evidence supporting the proposed structure and confirmed the absolute configuration of the newly formed chiral centre (C2). The unit cell contained two molecules of the compound in a racemic mixture, each exhibiting a distinct absolute configuration at C2. The observed length of the C(1)=N(3) bond is 1.300(7) Å and confirms its double-bond character by falling within the typical range reported in the literature (1.27–1.32 Å) for triazolines and triazoles. 9 , 10 , 11 As expected, the adjacent single C–N bond C(2)–N(2) equals 1.467(7) Å, and is long due to its σ-bonding nature. Bromine is the heaviest atom in the molecule. Its covalent bond C(13)–Br(1) is the longest, 1.903(6) Å, and is comparable to related compounds. 9 , 11
Interestingly, the oxygen atom, O1, of the acetyl unit splits into two positions as O1A and O1B. The C16=O bond length is in average 1.27 Å, confirming its bonding character. 12 , 13 The bromophenyl and 1,2,4-triazoline define the equatorial plane of the compound. In fact, the dioxybenzoyl unit is about 130.7° above this plane. The arm of the benzyl unit is hanging below the plane as the N1–C18–19 is twisted about 113.7°.
Funding source: Sultan Qaboos University
Award Identifier / Grant number: IG/SCI/CHEM/24/03
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was funded by Sultan Qaboos University for their generous financial support through the internal grant number IG/SCI/CHEM/24/03.
References
1. Stoe & Cie. X–AREA, Version 1.30: Program for the Acquisition and Analysis Data; Stoe & Cie GmbH: Darmatadt, Germany, 2005.Search in Google Scholar
2. Stoe & Cie. X–RED, Version 1.28b: Program for Data Reduction and Absorption Correction; Stoe &Cie GmbH: Darmstadt, Germany, 2005.Search in Google Scholar
3. Sheldrick, G. M. SHELX97. Program for Crystal Structure Solution and Refinement; University of Göttingen: Germany, 1997.Search in Google Scholar
4. Farrugia, L. J. WinGX Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Crystallogr. 1999, 32, 837–838; https://doi.org/10.1107/s0021889899006020.Search in Google Scholar
5. Macrae, C. F.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Shields, G. P.; Taylor, R.; van der Streek, T. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 39, 453–457; https://doi.org/10.1107/s002188980600731x.Search in Google Scholar
6. Al Maqbali, A. S.; Al Rasbi, N. K.; Zoghaib, W. M.; Sivakumar, N.; Robertson, C. C.; Shongwe, M. S.; Grzegorzek, N.; Abdel-Jalil, R. J. Stereoselective Asymmetric Syntheses of Molecules with a 4,5–Dihydro-1H-[1,2,4]–Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy and Pharmacology. Molecules 2024, 29, 12; https://doi.org/10.3390/molecules29122839.Search in Google Scholar PubMed PubMed Central
7. Monge, D.; Jensen, K. L.; Marin, I.; Jørgensen, K. A. Synthesis of 1, 2, 4-Triazolines: Base-Catalyzed Hydrazination/Cyclization Cascade of α-Isocyano Esters and Amides. Org. Lett. 2011, 13, 328–331; https://doi.org/10.1021/ol102812c.Search in Google Scholar PubMed
8. Sathyanarayana, R.; Poojary, B. Exploring Recent Developments on 1, 2, 4-Triazole: Synthesis and Biological Applications. J. Chin. Chem. Soc. 2020, 67, 459–477; https://doi.org/10.1002/jccs.201900304.Search in Google Scholar
9. Saleem, R. S. Z.; Tepe, J. J. Synthesis of 1,2,4-Triazolines and Triazoles Utilizing Oxazolones. J. Org. Chem. 2010, 75, 4330–4332; https://doi.org/10.1021/jo100716m.Search in Google Scholar PubMed
10. Karczmarzyk, Z.; Pitucha, M.; Wysocki, W.; Fruzinski, A.; Olender, E. Ethyl 2-(3-Methyl-5-Sulfanylidene-4,5-Dihydro-1H-1,2,4-Triazol-4-yl) Acetate. Acta Crystallogr. 2012, E68, 3264–3265; https://doi.org/10.1107/s1600536812044716.Search in Google Scholar
11. Altowyan, M. S.; Haukka, M.; Soliman, S. M.; Barakat, A.; Boraei, A. T.; Aboelmagd, A. Stereoselective Synthesis of New 4-Aryl-5-Indolyl-1,2,4-Triazole S-and N-β-Galactosides: Characterizations, X-Ray Crystal Structure and Hirshfeld Surface Analysis. Crystals 2023, 13, 797; https://doi.org/10.3390/cryst13050797.Search in Google Scholar
12. Altowyan, M. S.; Haukka, M.; Soliman, S. M.; Barakat, A.; Boraei, A. T.; Aboelmagd, A. Stereoselective Synthesis of New 4–Aryl-5-Indolyl-1, 2, 4-Triazole S-and N-β–Galactosides: Characterizations, X-Ray Crystal Structure and Hirshfeld Surface Analysis. Crystals 2023, 13, 797; https://doi.org/10.3390/cryst13050797.Search in Google Scholar
13. Liu, J.-Q.; Ji, L. Crystal Structure of 4-(Acetoxymethyl)-6-(3-Acetyl-3-(4-Fluorophenyl) Thioureido) Cyclohex-4-ene-1, 2, 3-Triyl Triacetate, C24H26FN2O9S. Z. Kristallogr. N. Cryst. Struct. 2018, 234, 189–190; https://doi.org/10.1515/ncrs-2018-0326.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]


