Abstract
C18H16BrN3O2S, triclinic, P1̄ (no. 2), a = 11.5820(4) Å, b = 12.0492(4) Å, c = 14.3048(5) Å, α = 73.673(3)°, β = 88.190(3)°, γ = 72.686(3)°, V = 1826.02(12) Å3, Z = 4, R gt (F) = 0.0580, wRref (F 2) = 0.1565, T = 296(2) K.
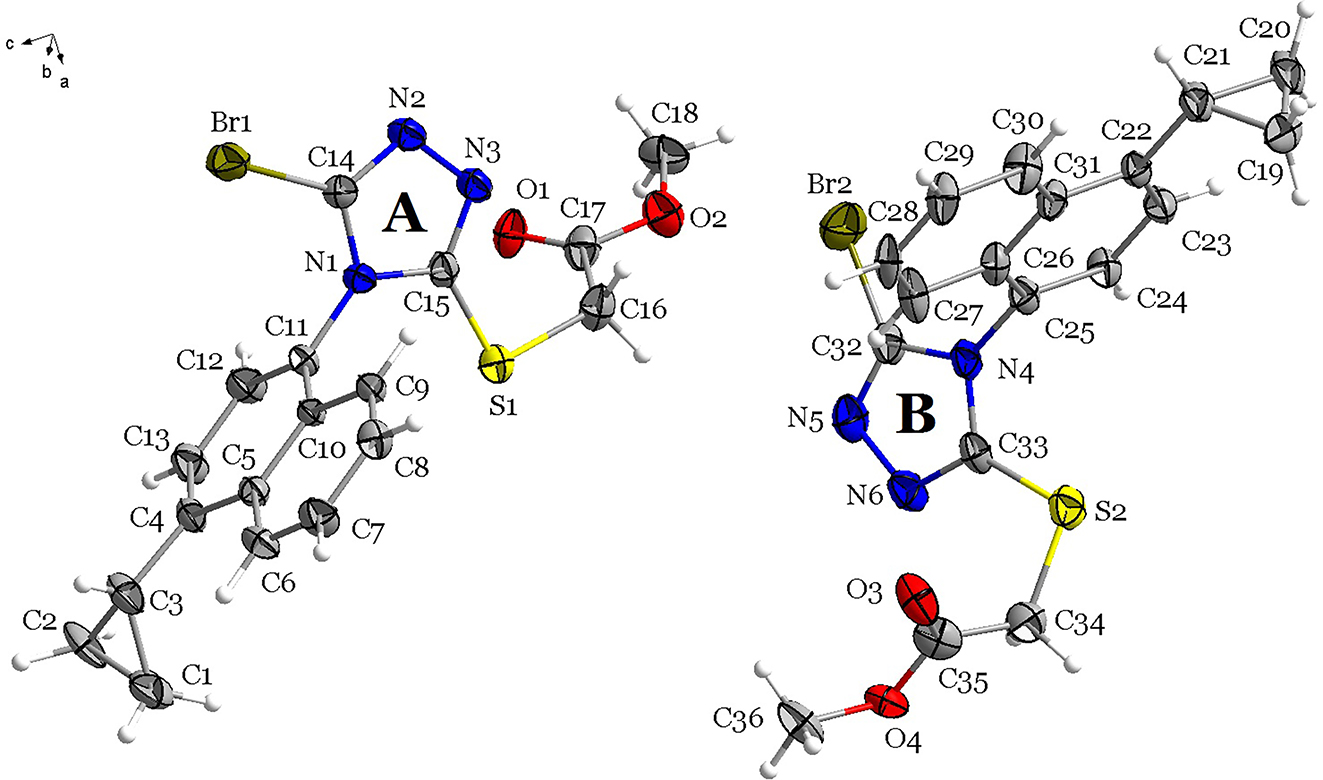
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless platelet |
| Size: | 0.22 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.38 mm−1 |
| Diffractometer, scan mode: | Multiwire proportional, φ and ω |
| θ max, completeness: | 25.0°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 19,974, 6,421, 0.038 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4,199 |
| N(param)refined: | 451 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Diamond 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Br1 | 0.85099 (5) | 0.69458 (6) | 0.47856 (5) | 0.0696 (2) |
| Br2 | 1.65220 (7) | −0.05384 (7) | 0.11585 (5) | 0.0923 (3) |
| S1 | 1.29257 (12) | 0.57124 (13) | 0.31533 (10) | 0.0578 (4) |
| S2 | 1.92178 (14) | 0.22632 (13) | −0.09661 (10) | 0.0631 (4) |
| O1 | 1.2991 (4) | 0.3082 (4) | 0.3375 (3) | 0.0839 (13) |
| O2 | 1.3269 (4) | 0.3224 (3) | 0.1791 (3) | 0.0717 (11) |
| O3 | 1.8655 (5) | 0.4044 (5) | 0.0260 (4) | 0.118 (2) |
| O4 | 2.0390 (4) | 0.3136 (4) | 0.1130 (3) | 0.0807 (12) |
| N1 | 1.0823 (3) | 0.6456 (3) | 0.4003 (3) | 0.0403 (9) |
| N2 | 0.9482 (4) | 0.6001 (4) | 0.3238 (3) | 0.0547 (11) |
| N3 | 1.0610 (4) | 0.5662 (4) | 0.2836 (3) | 0.0542 (11) |
| N4 | 1.7766 (4) | 0.0955 (4) | −0.0060 (3) | 0.0502 (10) |
| N5 | 1.8518 (4) | 0.0196 (4) | 0.1464 (3) | 0.0661 (13) |
| N6 | 1.9232 (4) | 0.0884 (4) | 0.0929 (3) | 0.0624 (12) |
| C1 | 1.3831 (7) | 0.8593 (9) | 0.6671 (6) | 0.115 (3) |
| H1A | 1.384429 | 0.931709 | 0.683571 | 0.139* |
| H1B | 1.437161 | 0.839139 | 0.617145 | 0.139* |
| C2 | 1.3639 (7) | 0.7578 (7) | 0.7465 (5) | 0.097 (2) |
| H2A | 1.405834 | 0.675732 | 0.744817 | 0.116* |
| H2B | 1.353058 | 0.768381 | 0.811298 | 0.116* |
| C3 | 1.2685 (5) | 0.8328 (5) | 0.6701 (4) | 0.0639 (15) |
| H3 | 1.202443 | 0.890637 | 0.692049 | 0.077* |
| C4 | 1.2278 (4) | 0.7821 (4) | 0.5969 (3) | 0.0448 (12) |
| C5 | 1.1714 (4) | 0.8634 (4) | 0.5055 (3) | 0.0385 (10) |
| C6 | 1.1608 (4) | 0.9878 (4) | 0.4788 (4) | 0.0497 (12) |
| H6 | 1.192726 | 1.018675 | 0.521190 | 0.060* |
| C7 | 1.1049 (5) | 1.0636 (4) | 0.3926 (4) | 0.0555 (14) |
| H7 | 1.098763 | 1.145588 | 0.376766 | 0.067* |
| C8 | 1.0564 (4) | 1.0200 (4) | 0.3273 (4) | 0.0483 (12) |
| H8 | 1.018103 | 1.072903 | 0.268479 | 0.058* |
| C9 | 1.0651 (4) | 0.9000 (4) | 0.3497 (3) | 0.0400 (11) |
| H9 | 1.032442 | 0.871681 | 0.305857 | 0.048* |
| C10 | 1.1231 (4) | 0.8182 (4) | 0.4385 (3) | 0.0348 (10) |
| C11 | 1.1339 (4) | 0.6930 (4) | 0.4660 (3) | 0.0381 (10) |
| C12 | 1.1898 (5) | 0.6169 (5) | 0.5512 (4) | 0.0542 (13) |
| H12 | 1.196759 | 0.534816 | 0.566611 | 0.065* |
| C13 | 1.2376 (5) | 0.6620 (5) | 0.6170 (4) | 0.0562 (14) |
| H13 | 1.276632 | 0.608680 | 0.675422 | 0.067* |
| C14 | 0.9651 (4) | 0.6455 (4) | 0.3928 (3) | 0.0455 (12) |
| C15 | 1.1374 (4) | 0.5938 (4) | 0.3313 (3) | 0.0434 (11) |
| C16 | 1.3093 (5) | 0.4961 (5) | 0.2216 (4) | 0.0618 (14) |
| H16A | 1.243420 | 0.540556 | 0.172520 | 0.074* |
| H16B | 1.384268 | 0.499683 | 0.190556 | 0.074* |
| C17 | 1.3104 (5) | 0.3654 (5) | 0.2556 (4) | 0.0587 (14) |
| C18 | 1.3321 (6) | 0.1966 (5) | 0.1963 (6) | 0.093 (2) |
| H18A | 1.344304 | 0.175459 | 0.136089 | 0.139* |
| H18B | 1.398074 | 0.145882 | 0.242570 | 0.139* |
| H18C | 1.257468 | 0.185218 | 0.221692 | 0.139* |
| C19 | 1.4901 (7) | 0.2985 (9) | −0.4410 (4) | 0.104 (3) |
| H19A | 1.423724 | 0.361120 | −0.482204 | 0.125* |
| H19B | 1.566146 | 0.317293 | −0.444200 | 0.125* |
| C20 | 1.4952 (6) | 0.1724 (9) | −0.4276 (5) | 0.104 (3) |
| H20A | 1.574434 | 0.113229 | −0.422357 | 0.124* |
| H20B | 1.431980 | 0.157067 | −0.460370 | 0.124* |
| C21 | 1.4611 (5) | 0.2271 (5) | −0.3463 (4) | 0.0593 (14) |
| H21 | 1.374588 | 0.247099 | −0.334202 | 0.071* |
| C22 | 1.5433 (4) | 0.1932 (4) | −0.2572 (3) | 0.0462 (12) |
| C23 | 1.6523 (4) | 0.1064 (5) | −0.2446 (4) | 0.0519 (13) |
| H23 | 1.676351 | 0.066866 | −0.292428 | 0.062* |
| C24 | 1.7294 (4) | 0.0748 (4) | −0.1619 (3) | 0.0479 (12) |
| H24 | 1.803516 | 0.014971 | −0.155392 | 0.058* |
| C25 | 1.6967 (4) | 0.1307 (4) | −0.0918 (3) | 0.0435 (11) |
| C26 | 1.5859 (5) | 0.2244 (4) | −0.1003 (3) | 0.0486 (12) |
| C27 | 1.5521 (6) | 0.2886 (5) | −0.0296 (4) | 0.0678 (16) |
| H27 | 1.603812 | 0.270458 | 0.024682 | 0.081* |
| C28 | 1.4432 (6) | 0.3775 (6) | −0.0413 (5) | 0.083 (2) |
| H28 | 1.420455 | 0.417751 | 0.006217 | 0.100* |
| C29 | 1.3670 (6) | 0.4081 (6) | −0.1222 (5) | 0.083 (2) |
| H29 | 1.293641 | 0.469059 | −0.128989 | 0.099* |
| C30 | 1.3976 (5) | 0.3503 (5) | −0.1923 (4) | 0.0660 (16) |
| H30 | 1.345147 | 0.372983 | −0.246850 | 0.079* |
| C31 | 1.5084 (4) | 0.2559 (4) | −0.1840 (3) | 0.0467 (12) |
| C32 | 1.7686 (5) | 0.0260 (5) | 0.0850 (4) | 0.0554 (13) |
| C33 | 1.8753 (5) | 0.1321 (4) | 0.0026 (3) | 0.0489 (12) |
| C34 | 2.0253 (5) | 0.2659 (5) | −0.0326 (4) | 0.0663 (15) |
| H34A | 2.085713 | 0.192396 | 0.003863 | 0.080* |
| H34B | 2.066765 | 0.313856 | −0.079473 | 0.080* |
| C35 | 1.9647 (6) | 0.3372 (5) | 0.0373 (5) | 0.0665 (16) |
| C36 | 1.9926 (7) | 0.3760 (6) | 0.1853 (5) | 0.104 (3) |
| H36A | 2.053495 | 0.352615 | 0.237062 | 0.156* |
| H36B | 1.971436 | 0.462017 | 0.155679 | 0.156* |
| H36C | 1.922060 | 0.354962 | 0.211324 | 0.156* |
1 Source of materials
The methyl 2-{[4-(4-cyclopropyl-1-naphthyl)-4H-1,2,4-triazole-3-yl]thio}acetate (40.0 g, 0.113 mol) was dissolved in 360 mL of dry dichloromethane at room temperature, and stirred evenly. Then, carbonyldiimidazole (0.92 g, 5.67 mmol) was added in batches, and the mixture was stirred for 30 min. Cooling to 0 °C, adding N-bromosuccinimide (22.2 g, 0.125 mol) in batches, and controlling the temperature to 0 °C for 40 min. The reaction was monitored by TLC. After the reaction, 300 mL of 10 % sodium sulfite aqueous solution was added and stirred for 1 h. Separate the organic layer, wash it once with 200 mL water and 200 mL saturated sodium chloride solution respectively, and dry the organic layer with anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to obtain a brownish yellow oil. Using ethyl acetate as eluent, the product was separated by rapid column chromatography, and the solvent was evaporated under reduced pressure to obtain light yellow solid product (35.9 g) with a yield of 73.5 %.
2 Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with U iso(H) = 1.5 U eq(C) for methyl H atoms and 1.2 U eq(C) for all other H atoms.
3 Comment
Lesinurad sodium was first developed by Aread Biosciences and then continued to be developed by Astra Zeneca. 5 Lesinurad sodium, whose chemical name is 2-{[5-bromo-4-(4-cyclopropylnaphthalene-1-yl)-4h-1,2,4-triazol-3-yl]thio}acetic acid, is the first inhibitor of uric acid selective reabsorption transporter-1(URAT1). Lesinurad sodium is mainly used for treating hyperuricemia related to gout in combination with xanthine oxidase inhibitors (such as allopurinol and febuxostat). 6 , 7 , 8 The optimization of the process of lesinurad sodium and its derivatives has attracted extensive attention of researchers. 9 , 10 , 11 In order to make it more suitable for industrial production, we have been devoted to its synthesis and process optimization of lesinurad sodium. Recently, we reported the synthesis and crystal structures of two intermediates of lesinurad sodium. 12 , 13 Herein we reveal the synthesis and crystal structure of the key intermediate of lesinurad sodium methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate.
In the molecules of the title structure, both bond lengths and angles are very similar to those given in the literature. 12 , 13 , 14 In the asymmetric unit of the title compound, there are two molecules, molecule A and molecule B. In molecule A, the dihedral angles formed by the naphthalene ring plane, the 1,2,4-triazole plane and the cyclopropyl group plane are 51.7(4)°, are 69.7(4)°, 79.3(2)° and 63.5(1)°, respectively. The torsion angles of N1–C15–S1–C16, C15–S1–C16–C17, S1–C16–C17–O2, C16–C17–O2–C18, N4–C33–S2–C34, C33–S2–C34–C35, S2–C34–C35–O4 and C34–C35–O4–C36 are 177.4°, −75.6°, −178.1°, 179.3°, 167.2°, −64.2°, 148.8° and 179.8°, respectively. Intermolecular C–H⋯S hydrogen bonds connect two adjacent the title structures into a dimer. Dimers are further stabilized in the stacking structure by π–π stacking.
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People’s Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was supported by the National Natural Science Foundation of China (22168018), the Science and Technology Plan Project of Ji’an (20233–043995).
References
1. Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Hoy, S. M. Lesinurad: First Global Approval. Drugs 2016, 76, 509–516. https://doi.org/10.1007/s40265–016–0550-y.10.1007/s40265-016-0550-ySearch in Google Scholar PubMed
6. Sattui, S. E.; Gaffo, A. L. Treatment of Hyperuricemia in Gout: Current Therapeutic Options, Latest Developments and Clinical Implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 145–159. https://doi.org/10.1177/1759720X16646703.Search in Google Scholar PubMed PubMed Central
7. Shen, Z.; Yeh, L. T.; Wallach, K.; Zhu, N.; Kerr, B.; Gillen, M. In Vitro and In Vivo Interaction Studies between Lesinurad, a Selective Urate Reabsorption Inhibitor, and Major Liver or Kidney Transporters. Clin. Drug Investig. 2016, 36, 443–452. https://doi.org/10.1007/s40261–016–0386-y.10.1007/s40261-016-0386-ySearch in Google Scholar PubMed PubMed Central
8. Miner, J. N.; Tan, P. K.; Hyndman, D.; Liu, S.; Iverson, C.; Nanavati, P.; Hagerty, D. T.; Manhard, K.; Shen, Z.; Girardet, J. L.; Yeh, L. T.; Terkeltaub, R.; Quart, B. Lesinurad, a Novel, Oral Compound for Gout, Acts to Decrease Serum Uric Acid through Inhibition of Urate Transporters in the Kidney. Arthritis Res. Ther. 2016, 18, 214–236. https://doi.org/10.1186/s13075–016–1107-x.10.1186/s13075-016-1150-7Search in Google Scholar PubMed PubMed Central
9. Zou, L.; Liu, Y.; Yao, K.; Li, J. Q.; Zhang, Z. X. Synthesis of Lesinurad. Chinese J. Pharm. 2017, 48, 488–491. https://doi.org/10.16522/j.cnki.cjph.2017.04.003.Search in Google Scholar
10. Pan, W.; Wan, T.; Jiang, N.; Gong, P.; Zhai, X. Improved Synthetic Process of Lesinurad Sodium. Chinese J. Med. Chem. 2019, 29, 363–367. https://doi.org/10.14142/j.cnki.cn21–1313/r.2019.05.005.Search in Google Scholar
11. Chen, J.; Su, W. Q.; Chen, N. G.; Wang, Y. M. Study on Synthesis Process of Lesinurad. Chin. J. Guangzhou Chem. Ind. 2023, 51, 102–104.Search in Google Scholar
12. Drumright, R. E.; Mas, R. H.; Merola, J. S.; Tanko, J. M. Interplay between Conjugative and Steric Effects in Cyclopropylarenes. J. Org. Chem. 1990, 55, 4098–4102. https://doi.org/10.1021/jo00300a029.Search in Google Scholar
13. Zeng, H. L.; He, Y.; Zhang, D. W.; Yi, X. G.; Yi, Z. Q. Synthesis and Crystal Structure of 4-(4-Cyclopropylnaphthalen-1-Yl)-2,4-Dihydro-3h-1,2,4-Triazole-3-Thione, C15H13N3S. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 415–417. https://doi.org/10.1515/NCRS-2024–0025.10.1515/ncrs-2024-0025Search in Google Scholar
14. Yuan, X. Z.; Zhang, D. W.; Yi, X. G.; Zeng, H. L.; Yi, Z. Q. Synthesis and Crystal Structure of Methyl 2-{[4-(4-Cyclopropyl-1-Naphthyl)-4h-1,2,4-Triazole-3-Yl]thio}Acetate, C18H17N3O2S. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 483–485. https://doi.org/10.1515/NCRS-2024–0056.10.1515/ncrs-2024-0056Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

