Abstract
Liquid fermentations of the fungus Stereum rameale (N° 2511) yielded extracts with antibacterial activity. The antibacterial activity reached its peak after 216 h of stirring. Bioassay-guided fractionation methods were employed for the isolation of the bioactive metabolites. Three known compounds were identified: MS-3 (1), vibralactone (2) and vibralactone B (3). The three compounds showed antibacterial activity as a function of their concentration. Minimal bactericidal concentrations (MBC) of compound 1 against Gram-positive bacteria were as follows: Bacillus cereus (50 μg/mL), Bacillus subtilis (10 μg/mL) and Staphylococcus aureus (100 μg/mL). Compounds 2 and 3 were active only against Gram-negative bacteria. The MBC of compound 2 against Escherichia coli was 200 μg/mL. Compound 3 inhibited significantly the growth of E. coli and Pseudomonas aeruginosa, with MBC values of 50 and 100 μg/mL, respectively.
1 Introduction
The higher fungi, Basidiomycetes, offer an exciting field to obtain new structures with high potential for medical and agricultural applications. Higher fungi have an important advantage as producers of bioactive secondary metabolites, as they release them to liquid media. This ability has allowed the development of a powerful line of research and production, with mycelial fermentations reaching industrial volume and valuable bioactive compounds being isolated with antibacterial, antifungal, nematicidic, phytotoxic, antiviral, insecticidal, cytotoxic, anticancer and other activities [1–3].
Chile is abundant in plant species with a wide variety of symbiotic, saprophytic and parasitic fungi, many of which are still unknown. According to Palfner [4], there are approximately 3000 species of fungi reported from Chile, and around 50% of them are higher fungi. Continuing with our research program on biologically active fungal metabolites, we have discovered an unsuspected reservoir of new and potentially useful molecules produced by Chilean Basidiomycetes [5, 6], and recently we have detected that extracts obtained from Stereum rameale (Pers.: Fr.) Burt, [synonym: Stereum ochraceoflavum (Schwein.) Ellis] showed a potent inhibitory capacity against pathogenic bacteria.
Stereum rameale is predominantly distributed in tropical or subtropical regions and often found associated with woody debris, rotting trunks and sometimes on buried dead wood. The fruiting bodies are thin, elastic and tough when moist, becoming hard and brittle when dry. This crust persists all through the year but releases spores only during autumn [7].
In Chile, the first record of S. rameale was published by Guillén et al. [8], who collected the fruiting bodies from branches of Aetoxicum punctatum, a Chilean native shrub. They also investigated S. rameale as a producer of lignocellulolytic enzymes and its tolerance to metal ions.
In previous investigations, a series of interesting new compounds have been isolated from the Stereum genus, including acetylenic aromatics [9] sesquiterpenoids, such as hirsutanes [10], sterpuranes [11], cadinanes [12], stereumanes [13], isolactaranes [14] and illudalanes [15].
In general, in the scientific literature, reports on secondary metabolites and biological activities of S. rameale are quite limited. However, Mantle and Mellows [16] and Mellows et al. [17] reported from Stereum complicatum (Fr.) Fr. (also used as synonym of S. rameale) two new antibacterial sesquiterpenoids: complicatic acid and hirsutic acid C. Thus, the aims of the current study were to isolate and identify the active compounds produced by S. rameale, including submerged cultures, as well as to characterize and quantify their antibacterial effects.
2 Materials and methods
2.1 General
1H and 13C NMR spectral data (CDCl3, δ/ppm 7.27/77.0 and DMSO-d6, δ/ppm 2.50/39.5) were recorded on a Bruker DRX 500 spectrometer at 500 MHz for 1H and 125 MHz for 13C (Avance III-500 MHz, Germany). Optical rotations were measured in CHCl3 at 20 °C on a Perkin-Elmer Model 341 polarimeter (Waltham, MA, USA). Fragmentation m/z data were recorded on a Waters Q-TOF Micro system mass spectrometer, using H3PO4 as an internal standard for calibration.
2.2 Producing organism and fermentation
Fruiting bodies of S. rameale (strain 2511) were collected from the bark of a dead tree in a native forest of Nothofagus species (Nothofagaceae), near Ñuble National Reserve, Ñuble Province, Chile. The identification was done on the basis of macro- and micromorphological features, size and color of colonies. Mycelial cultures were derived from spore prints of a fruiting body. The strain 2511 is kept on YMG (yeast extract, malt extract, glucose) agar containing the following (g/L): glucose 4, malt extract 10, yeast extract 4 and agar 20, at pH 5.5. A voucher specimen of the fungus is deposited in the herbarium of the Laboratory of Applied Microbiology and Mycology, Department of Agroindustries, Faculty of Agricultural Engineering, University of Concepción. All culture media were purchased from Merck-Chile and Difco-Chile, Santiago de Chile.
Fermentation on a larger scale was carried out in a 10-L glass bottle (DURAN®) containing 7.5 L of YMG medium with stirring (120 rpm) and aeration (3 L/min) at 20 °C. To prevent foaming, silicone antifoam (Merck, Darmstadt, Germany) was added. The fermentor was inoculated with 100 mL of a well-grown culture in the same medium. Daily samples were withdrawn and assayed for pH, glucose and maltose content, biomass production and antibacterial activity. These aliquots were filtered and then extracted with EtOAc. The combined extracts were dried with Na2SO4 and concentrated in vacuo (45 °C). Extracts were dissolved in MeOH (100 μg/mL) and then used for the antibacterial tests. The fermentation was stopped when the glucose was exhausted and the antibacterial (Bacillus cereus, Salmonella) activity of the extract had reached its peak [18].
2.3 Isolation of bioactive compounds
The culture was filtered to separate the mycelium from the broth. The mycelium was extracted with methanol (MeOH-extract). The broth culture was extracted with EtOAc (3×7 L) and the organic phase concentrated under reduced pressure (EtOAc-extract). This extract was further applied onto a silica gel column (Merck 60, 0.063∼0.2 mm; column 3×30 cm) eluted with n-hexane and with a gradient of hexane-EtOAc mixtures up to 100% EtOAc to enhance polarity. Fractions and purified compounds were analyzed by analytical and preparative thin-layer chromatography (TLC) and eluted with mixtures of solvents (hexane-EtOAc, chloroform-MeOH). The separated components were visualized under ultraviolet light (254 and 366 nm), and spraying with 20% H2SO4 followed by heating. The bioactive bands of the preparative TLC were separately scraped from the air-dried plate and eluted with MeOH. This suspension was filtered and the organic phase evaporated. Active compounds were isolated by bioautography-guided fractionation according to Thines et al. [19]. All solvents were purchased from Merck-Chile, Santiago de Chile.
2.4 Bacterial strains
Six bacterial isolates were included in this study: B. cereus (LMM-876), Bacillus subtilis (LMM-013), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (LMM-213), Salmonella sp. (LMM-352) and Staphylococcus aureus (LMM-292). All strains, except E. coli, had been isolated from different food products. Bacterial strains were grown in Mueller-Hinton Broth at 37 °C.
2.5 Antimicrobial assays
Antimicrobial activity was determined in the serial dilution assay or the plate diffusion assay as described by Anke et al. [20].
Total extracts, fractions and isolated compounds 1, 2 and 3 dissolved in MeOH were assayed in the disc diffusion test at 100 μg/6 mm disc. Bacterial cultures were diluted with sterile water to obtain a microbial suspension of 106–108 CFU/mL. Petri plates containing 20 mL of culture medium with 2% (w/v) of molten agar were inoculated with 200 μL of microbial suspension and allowed to solidify in a sterile chamber. After solidification, the discs were placed on the inoculated culture medium. The positive control was penicillin G and streptomycin (streptomycin sulphate) 100 μg/6 mm disc. Methanol was used as negative control. The plates were incubated at 37 °C, and the inhibition zones (diameters) around the discs were measured (mm) after 24 h.
Compounds that showed the highest activity against one or more test microorganisms in the primary assay were considered potentially active and selected to determine their minimal inhibitory concentration (MIC) value in serial dilution assays. The MIC of compounds 1, 2 and 3 was determined for Gram-positive and Gram-negative bacteria, using the serial agar dilution assay in concentrations ranging from 1 μg/mL to 200 μg/mL. The tested compounds were placed in wells, and the methanol was evaporated. Suspensions of bacteria were dispensed at 0.2 mL/well in 96-well microtiter plates. Bacterial suspensions without additions were used as a negative control and streptomycin as a positive control. The MIC values were taken as the lowest concentrations of the compounds in the wells that did not allow visible bacterial growth after 24 h of incubation at 37 °C, as evidenced by the turbidity of the cultures. The contents of the wells in which a MIC had been observed were streaked, using a sterile wire loop, on a sterile agar plate and incubated at 37 °C for 24 h. The lowest concentration of the compounds that did not allow bacterial growth was assigned as the minimal bactericidal concentration (MBC).
3 Results and discussion
As shown in Figure 1, after 216 h of fermentation, the mycelial biomass had markedly increased (Figure 1A), while the content of maltose decreased after 72 h at a rather continuous rate; glucose increased gradually over the first 120 h and then decreased continually. At 240 h, the medium was depleted of both maltose and glucose, and the fermentation was stopped (Figure 1B).
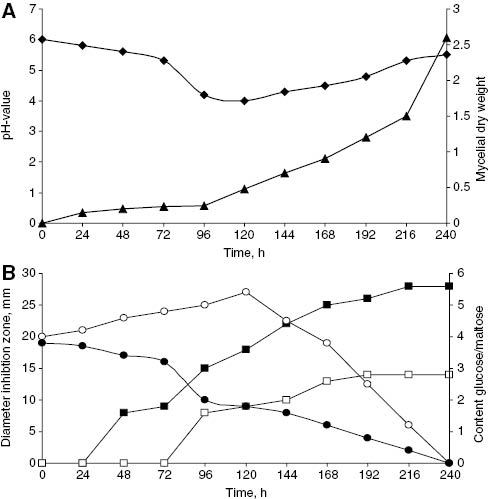
Fermentation of S. rameale (2511). (A) ♦, pH; ▴, mycelial dry weight (g/L). (B) ▪, inhibition zone B. cereus (mm); □, inhibition zone Salmonella (mm); ○, glucose (g/L); •, maltose (g/L).
The antimicrobial activities of the extracts obtained from the daily aliquots increased during this period, against both B. cereus and Salmonella sp. (Figure 1B). The fermentation was terminated after 240 h. Evaporation of the organic phase yielded 954 mg of EtOAc-extract. Silica gel column chromatography yielded seven fractions. The crude extract exhibited strong antibacterial activity against the three Gram-positive bacteria, B. cereus, B. subtilis and S. aureus, while it was weak (E. coli, P. aeruginosa) or negligible (Salmonella sp.) against the three Gram-negative bacteria. Mycelial extracts did not contain antibiotic activity.
Table 1 shows the results obtained by the in vitro plate diffusion method. Following the bioassay-guided fractionation, the MeOH-extract and fractions 1, 3, 4, 6 and 7 exhibited a weak antibacterial activity, while the EtOAc-extract and fractions 2 and 5 exhibited distinct antibacterial activities. Elution with n-hexane/EtOAc (7:3 and 4:6) yielded fraction 2 (112 mg) and fraction 5 (123 mg), respectively. Fraction 2 was active against B. cereus, B. subtilis and S. aureus, while fraction 5 was active against E. coli and P. aeruginosa. Both fractions were further purified by preparative TLC. Through a direct bioautographic assay, compounds 1 (21.2 mg), 2 (27.1 mg) and 3 (17.5 mg) were isolated and their structures identified (Figure 2). All NMR data (1H–13C) were assigned by comparison with previously reported data [21, 22].
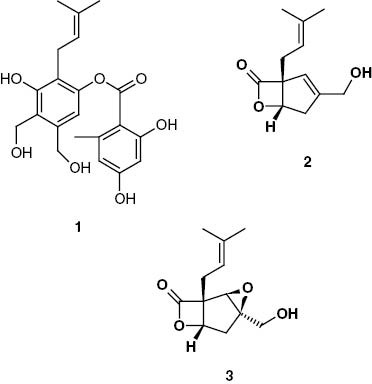
Compounds isolated from S. rameale (2511). 1, MS-3; 2, vibralactone; and 3, vibralactone B.
Antibacterial activities of extracts, fractions and pure compounds isolated from S. rameale.
| Sample | Bc | Bs | Sa | Ec | Pa | Sal |
|---|---|---|---|---|---|---|
| EtOAc-extract | 28 | 27 | 14 | 10 | 10 | – |
| MeOH-extract | – | 8 | – | – | – | 9 |
| Fr.1 | – | – | – | – | – | – |
| Fr.2 | 29 | 28 | 32 | – | – | – |
| Fr.3 | 8 | 8 | – | – | – | – |
| Fr.4 | – | 9 | – | – | – | – |
| Fr.5 | – | – | – | 14 | 10 | – |
| Fr.6 | – | – | – | 9 | – | – |
| Fr.7 | – | – | – | – | – | – |
| MS-3 (1) | 25 | 25 | 28 | – | – | – |
| Vibralactone (2) | – | – | – | 12 | 9 | – |
| Vibralactone B (3) | – | – | – | 14 | 13 | – |
| Streptomycin | 32 | 28 | 30 | 25 | 14 | 22 |
| Penicillin G | 30 | 28 | 27 | 25 | 24 | 14 |
Numbers indicate diameter of the inhibition zone (mm). Active=15 or more; moderate=10–15; weak=<10 or diffuse; –, no inhibition zone. Bc, B. cereus; Bs, B. subtilis; Ec, E. coli; Pa, P. aeruginosa; Sal, Salmonella spp.; Sa, S. aureus; EtOAc-extract, ethylacetate extract; MeOH-extract, methanolic extract; Fr., fraction.
Compound 1 was obtained as colorless crystals (soluble in MeOH). The molecular formula was found to be C21H24O7. The HRMS-ESI calculated for the dimer C42H48O14 calcd. for [M+H]+m/z 777.3122. Found: 777.3154. calcd. for [M+Na]+m/z 799.2942. Found: 799.2968 [M+Na]+. From the NMR data, this compound was identified as 3-hydroxy-4, 5-bis (hydroxymethyl)-2-(3″′-methyl-2″′-butenyl)-phenyl 2′, 4′-dihydroxy-6′-methyl-benzoate (MS-3). 1H NMR (500 MHz, DMSO-d6) δ/ppm: 1.53 (s, 3H,5″′), 1.54 (s, 3H,4″′), 2.38 (s, 3H,6′), 3.21 (d, 2H, J=6.7 Hz), 4.48 (d, 2H, J=5.4 Hz), 4.68 (d, 2H, J=3.4 Hz), 5.03 (t, 1H, J=6.9 Hz), 5.15 (t, 1H, J=5.4 Hz), 5.69 (t, 1H, J=3.4 Hz), 6.22 (s, 1H), 6.23 (s, 1H), 6.70 (s, 1H), 9.27 (s, 1H), 10.09 (s, 1H), 10.60 (s, 1H). 13C NMR (125 MHz, DMSO-d6) δ/ppm: 168.4, 161.3, 161.0, 154.8, 148.1, 140.7, 139.1, 130.5, 122.5, 121.6, 120.0, 112.4, 110.2, 107.8, 100.5, 60.5, 56.8, 25.5, 22.9, 21.8, 17.5.
Compound 2 was obtained as colorless oil (soluble in MeOH). [α]D20: – 126.5° (c=0.10, CHCl3). Molecular formula: C12H16O3. HRMS-ESI calc. for [M+Na]+m/z 231.0997. Found: 231.0917 [M+Na]+. Based on NMR data, compound 2 was identified as vibralactone. 1H NMR (500 MHz, CDCl3) δ/ppm: 1.64 (s, 3H), 1.73 (s, 3H), 2.43 (dd, 1H, J=15.1, 7.4 Hz), 2.62 (dd, 1H, J=15.1, 7.4 Hz), 2.76 (m, 2H), 4.25 (s, 2H), 4.81 (dd, 1H, J=1.3, 4.7 Hz), 5.13 (t, 1H, J=7.4 Hz), 5.62 (s, 1H). 13C NMR (125 MHz, CDCl3) δ/ppm: 173.0 (C-7), 146.5 (C-3), 136.0 (C-10), 122.5 (C-2), 117.2 (C-9), 78.5 (C-5), 75.1 (C-1), 61.4 (C-13), 37.3 (C-4), 27.6 (C-8), 25.8 (C-12), 18.0 (C-11).
Compound 3 was obtained as colorless crystals (soluble in MeOH). [α]D20: – 56.5° (c=0.14, CHCl3). The molecular formula was found to be C12H16O4. HRMS-ESI calc. for [M+Na]+m/z 247.0946. Found: 247.0976 [M+Na]+. From the NMR data, compound 3 was identified as vibralactone B. 1H NMR (500 MHz, CDCl3) δ/ppm: 1.66 (s, 3H), 1.74 (s, 3H), 2.09 (dd, 1H, J=16.5, 6.6 Hz), 2.44 (d, 1H, J=16.5 Hz), 2.51 (dd, 1H, J=15.1, 7.5 Hz), 2.65 (dd, 1H, J=15.1, 7.2 Hz), 3.54 (s, 1H), 3.82 (d, 1H, J=12.7 Hz), 3.91 (d, 1H, J=12.7 Hz), 4.81 (d, 1H, J=6.5 Hz), 5.15 (t, 1H, J=7.4 Hz). 13C NMR (125 MHz, CDCl3) δ/ppm: 168.5 (C-7), 137.0 (C-10), 116.2 (C-9), 82.2 (C-5), 77.1 (C-3), 69.0 (C-1), 61.2 (C-2), 60.8 (C-13), 31.7 (C-4), 25.9 (C-8), 25.8 (C-12), 18.0 (C-11).
Compound 1 exhibited the highest antibacterial activity with inhibition zones of 25, 25 and 28 mm against B. cereus, B. subtilis and S. aureus, respectively. This compound showed no antibacterial activity against E. coli, P. aeruginosa and Salmonella sp. Compound 2 was not active against most of the bacteria tested, except E. coli, which was moderately inhibited with inhibition zones of 12 mm. Compound 3 had a moderate activity against E. coli and P. aeruginosa, generating inhibition zones of 14 and 13 mm, respectively.
The MIC and MBC values are shown in Table 2. These results showed that compound 1 exhibited a potent bactericidal effect, and its MBC value was 10 μg/mL against B. subtilis and 50 μg/mL against B. cereus and S. aureus.
MIC and MBC of compounds isolated from S. rameale.
| Bacteria | (μg/mL) | ||
|---|---|---|---|
| MS-3 (1) | Vibralactone (2) | Vibralactone B (3) | |
| B. cereus | 10a 50b | nt | nt |
| B. subtilis | 10b | nt | nt |
| E. coli | nt | 100a 200b | 20a 50b |
| P. aeruginosa | nt | – | 100b |
| S. aureus | 10a 50b | nt | nt |
aBacteriostatic (MIC); bbactericidal (MBC); –, no effects up 200 μg/mL; nt, not tested.
Compound 2 showed a MBC value of 200 μg/mL against E. coli. Compound 3 inhibited significantly the growth of E. coli and P. aeruginosa, with an MBC value of 50 and 100 μg/mL, respectively.
In conclusion, the antibacterial activity of S. rameale seems to be related mainly to the compounds 1, 2 and 3. While compound 1 was able to inhibit only Gram-positive bacteria, compound 2 and 3 were exclusively active against Gram-negative bacteria. Compound 3 differs from 2 in that it has an epoxide group in the pentacyclic ring, which replaces the original double bond.
This difference apparently confers activity against a selective and reduced spectrum of sensitive bacterial strains. We have reported that the presence of an epoxide group in fungal metabolites can decisively affect antimicrobial activity, such as the compounds favolon and favolon B. Hence, in the absence of this group weak or null activity was observed [23].
These molecules have already been reported from several species of the genera Stereum and Boreostereum. Thus, compound 1 was previously isolated from an unidentified mushroom by Kurasawa et al. [24] and characterized as inhibitor of glyoxalase I and of the proliferation of Yoshida sarcoma cells. Other benzoates related to compound 1 and isolated from S. hirsutum exhibited antibacterial, anti-inflammatory and anti-tumor effects [25]. Vibralactone (2) and vibralactone derivatives (B-M) were isolated from cultures of Boreostereum vibrans [26–29], of which compound 2 potently inhibited pancreatic lipase with an IC50 value of 0.4 mg/mL [22], while vibralactones D-F were active against human and mouse 11β-HSD1 and 11β-HSD2 cell lines [30]. Recently, the unusual fused β-lactone bicyclic system of compound 2 has been established as a potent and selective inhibitor of diverse disease-associated classes of enzymes, such as the caseinolytic peptidase (ClpP1P2) complex in Listeria monocytogenes [31] and acyl-protein-thioesterases (APT1, APT2) in HeLa cells [32]. Even though compounds 1–3 have previously been identified, this is the first time that these metabolites and their antibacterial properties are reported from S. rameale.
Based on the above information, we might infer that the antibacterial activities exhibited by compounds 2 and 3 inhibit one or more pathways involved in the reproduction and probably the virulence of the Gram-negative bacteria assayed (E. coli, P. aeruginosa), in which the presence of the β-lactone system and the epoxide group would be key in the observed activity.
Our investigation has revealed that extracts and pure compounds from S. rameale have considerable antibacterial activity toward important pathogenic bacteria, making them a good alternative as potential antimicrobials agents. Further in vivo studies are necessary to confirm their safety and efficacy, which may lead to its widespread use in the preservation of raw and processed food.
Acknowledgments
The authors are grateful for the support provided by CONICYT-Chile, through Fondecyt Program: P.A. for Grant N° 11100331, N° 1120924 and IFS/No. F/3972-1; C.L.C. for Fondecyt N° 1130242 and DIUBB 019090 R/01; and J.B. for Fondecyt N° 1110656. We are grateful to Dr. Götz Palfner (mycologist) for the identification of the fungal samples.
References
1. Lorenzen K, Anke T. Basidiomycetes as a source for new bioactive natural products. Curr Org Chem 1998;2:329–64.10.2174/1385272802666220128213627Suche in Google Scholar
2. Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, Díez MT, et al. Screening of basidiomycetes for antimicrobial activities. Antonie Van Leeuwenhoek 2000;78:129–39.10.1023/A:1026552024021Suche in Google Scholar
3. Liermann JC, Thines E, Opatz T, Anke H. Drimane sesquiterpenoids from Marasmius sp. inhibiting the conidial germination of plant-pathogenic fungi. J Nat Prod 2012;75:1983–6.10.1021/np300337wSuche in Google Scholar
4. Palfner G. Taxonomische Studien an Ektomykorrhizen aus den Nothofagus-Wäldern Mittelsüdchiles. In: J Cramer, editor. Bibliotheca mycologica, Vol. 190, Berlin, Stuttgart, 2001;243.Suche in Google Scholar
5. Aqueveque P, Anke T, Sterner O. The himanimides, new bioactive compounds from Serpula himantioides (Fr.) Karst. Z Naturforsch 2002;57c:257–62.10.1515/znc-2002-3-410Suche in Google Scholar
6. Aqueveque P, Anke T, Saez K, Silva M, Becerra J. Antimicrobial activity of submerged cultures of Chilean Basidiomycetes. Planta Med 2010;76:1–5.10.1055/s-0030-1249853Suche in Google Scholar
7. Lazo W. Hongos de Chile. Atlas Micológico, Universidad de Chile, Santiago: Chile, 2001;231.Suche in Google Scholar
8. Guillén Y, Palfner G, Machuca Á. Screening for lignocellulolytic enzymes and metal tolerance in isolates of wood-rot fungi from Chile. Interciencia 2011;36:860–8.Suche in Google Scholar
9. Dubin GM, Fkyerat A, Tabacchi R. Acetylenic aromatic compounds from Stereum hirsutum. Phytochemistry 2000;53:571–4.10.1016/S0031-9422(99)00565-8Suche in Google Scholar
10. Liermann JC, Schüffler A, Wollinsky B, Birnbacher J, Kolshorn H, Anke T, et al. Hirsutane-type sesquiterpenes with uncommon modifications from three basidiomycetes. J Org Chem 2010;75:2955–61.10.1021/jo100202bSuche in Google Scholar PubMed
11. Xie JL, Li LP, Dai ZQ. Isolation and identification of two new metabolites from silver leaf fungus Stereum purpureum. J Org Chem 1992;57:2313–6.10.1021/jo00034a023Suche in Google Scholar
12. Li GH, Duan M, Yu ZF, Li L, Dong JY, Wang XB, et al. Stereumin A-E, sesquiterpenoids from the fungus Stereum sp. CCTCC AF 207024. Phytochemistry 2008;69:1439–45.10.1016/j.phytochem.2008.01.012Suche in Google Scholar
13. Li G, Liu F, Shen L, Zhu H, Zhang K. Stereumins H-J, stereumane-type sesquiterpenes from the fungus Stereum sp. J Nat Prod 2011;74:296–9.10.1021/np100813fSuche in Google Scholar PubMed
14. Opatz T, Kolshorn H, Anke H. Sterelactones: new isolactarane type sesquiterpenoids with antifungal activity from Stereum sp. IBWF 01060. J Antibiot 2008;61:563–7.10.1038/ja.2008.75Suche in Google Scholar PubMed
15. Isaka M, Srisanoh U, Choowong W, Boopratuang T. Sterostreins A-E, new terpenoids from cultures of the basidiomycete Stereum ostrea BCC 22955. Org Lett 2011;13:4886–9.10.1021/ol2019778Suche in Google Scholar
16. Mantle PG, Mellows G. Production of hirsutanes by Stereum complicatum. T Brit Mycol Soc 1973;61:513–9.10.1016/S0007-1536(73)80120-2Suche in Google Scholar
17. Mellows G, Manthe PG, Feline TC. Sesquiterpenoid metabolites from Stereum complicatum. Phytochemistry 1973;12:2717–20.10.1016/0031-9422(73)85086-1Suche in Google Scholar
18. Rojas de la Parra V, Mierau V, Anke T, Sterner O. Niveulone, a heterocyclic spiro terpenoid from the ascomycete Dasyscyphus niveus. J Antibiot 2006;59:57–60.10.1038/ja.2006.9Suche in Google Scholar
19. Thines E, Eilbert F, Sterner O, Anke H. Glisoprenin A, an inhibitor of the signal transduction pathway leading to appressorium formation in germinating conidia of Magnaporthe grisea on hydrophobic surfaces. FEMS Microbiol Lett 1997;151:219–24.10.1111/j.1574-6968.1997.tb12573.xSuche in Google Scholar
20. Anke H, Bergendorff O, Sterner O. Assays of the biological activities of guaiane sesquiterpenoids isolated from the fruit bodies of edible Lactarius species. Food Chem Toxicol 1989;27:393–7.10.1016/0278-6915(89)90145-2Suche in Google Scholar
21. Kurasawa S, Naganawa H, Takeuchi T, Umezawa H. The structure of MS-3: a glyoxalase inhibitor produced by a mushroom. Agric Biol Chem Tokyo 1975;39:2009–14.10.1271/bbb1961.39.2009Suche in Google Scholar
22. Liu DZ, Wang F, Liao TG, Tang JG, Steglich W, Zhu HJ, et al. Vibralactone: a lipase inhibitor with an unusual fused β-lactone produced by cultures of the basidiomycete Boreostereum vibrans. Org Lett 2006;8:5749–52.10.1021/ol062307uSuche in Google Scholar PubMed
23. Aqueveque P, Anke T, Anke H, Sterner O, Becerra J, Silva M. Favolon B, a new triterpenoid isolated from the Chilean Mycena sp. strain 96180. J Antibiot 2005;58:61–4.10.1038/ja.2005.7Suche in Google Scholar PubMed
24. Kurasawa S, Takeuchi T, Umezawa H. Studies on glyoxalase inhibitor isolation of a new active agent, MS-3, from a mushroom culture. Agri Biol Chem Tokio 1975;39:2003–8.10.1080/00021369.1975.10861901Suche in Google Scholar
25. Ma K, Bao L, Han J, Jin T, Yang X, Zhao F, et al. New benzoate derivatives and hirsutane type sesquiterpenoids with antimicrobial activity and cytotoxicity from the solid-state fermented rice by the medicinal mushroom Stereum hirsutum. Food Chem 2014;143:239–45.10.1016/j.foodchem.2013.07.124Suche in Google Scholar PubMed
26. Jiang MY, Wang F, Yang XL, Fang LZ, Dong ZJ, Zhu HJ, et al. Derivatives of vibralactone from cultures of the Basidiomycete Boreostereum vibrans. Chem Pharm Bull 2008;56:1286–8.10.1248/cpb.56.1286Suche in Google Scholar PubMed
27. Wang GQ, Wei K, Feng T, Li ZH, Zhang L, Wang QA, et al. Vibralactones G-J from cultures of the basidiomycete Boreostereum vibrans. J Asian Nat Prod Res 2012;14:115–20.10.1080/10286020.2011.636037Suche in Google Scholar PubMed
28. Wang GQ, Wei K, Li ZH, Feng T, Ding JH, Wang QA, et al. Three new compounds from the cultures of basidiomycete Boreostereum vibrans. J Asian Nat Prod Res 201315:950–5.10.1080/10286020.2013.824429Suche in Google Scholar PubMed
29. Wang GQ, Wei K, Zhang L, Li ZH, Wang QA, Liu JK. Three new vibralactone-related compounds from cultures of Basidiomycete Boreostereum vibrans. J Asian Nat Prod Res 2014;16:447–52.10.1080/10286020.2014.901312Suche in Google Scholar PubMed
30. Jiang MY, Zhang L, Dong ZJ, Yang ZL, Leng Y, Liu JK. Vibralactones D-F from cultures of the Basidiomycete Boreostereum vibrans. Chem Pharm Bull 2010;58:113–6.10.1248/cpb.58.113Suche in Google Scholar PubMed
31. Zeiler E, Braun N, Böttcher T, Kastenmller A, Weinkauf S, Sieber SA. Vibralactone as a tool to study the activity and structure of the ClpP1P2 complex from Listeria monocytogenes. Angew Chem Int Ed 2011;50:11001–4.10.1002/anie.201104391Suche in Google Scholar PubMed
32. List A, Zeiler E, Gallastegui N, Rusch M, Hedberg C, Sieber SA, et al. Omuralide and vibralactone: differences in the proteasome-β-lactone-γ-lactam binding scaffold alter target preferences. Angew Chem Int Ed 2014;53:571–4.10.1002/anie.201308567Suche in Google Scholar PubMed
©2015 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn
Artikel in diesem Heft
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn

