Abstract
5-Lipoxygenase (5-LOX) plays a key role in the pathway of leukotriene biosynthesis. To predict the inhibitory activity of flavonoid inhibitors against 5-LOX from Spatholobus suberectus Dunn, Autodock 4.2 and comparative molecular field analysis (CoMFA) were employed. For the positive inhibitors (n=7), the value of the coefficient of determination (R2) between the binding free energy, calculated using Autodock 4.2, and the experimental pIC50 is 0.838. In the training set (n=21) of inhibitors against 5-LOX, the R2 of non-cross-validated partial least squares analysis between the actual and predicted pIC50 values, using the no-validation with the optimum number of components set to 6, is 0.997 (p=0.000). For the model generated by CoMFA, the contribution of electrostatic and steric factors are 0.522 and 0.478, respectively. Among the flavonoids of S. suberectus, liquiritigenin, catechin, butin, 3′,4′,7-trihydroxyflavone, plathymenin, and gallocatechin are the more potent inhibitors of 5-LOX based on the calculated binding free energy and the predicted pIC50 value.
1 Introduction
Spatholobus suberectus Dunn (Fabaceae) [1], a traditional Chinese medicinal herb, has been investigated for its antitumor [2], skin whitening [3], and especially anti-inflammatory [4–6] activities. Li et al. [4] reported that the ethanol extract from S. suberectus showed inhibitory activity against 5-lipoxygenase (5-LOX). There are many secondary compounds in S. suberectus, but the major bioactive substances are flavonoids [7], including 3′,4′,7-trihydroxyflavone, formononetin, calycosin, prunetin, eriodictyol, butin, liquiritigenin, plathymenin, dihydroquercetin, and dihydrokaempferol (see Figure 1 for structures) [8].
![Figure 1: Chemical structures of flavonoid compounds from S. suberectus Dunn [3, 7, 8].](/document/doi/10.1515/znc-2014-4110/asset/graphic/j_znc-2014-4110_fig_001.jpg)
Chemical structures of flavonoid compounds from S. suberectus Dunn [3, 7, 8].
5-LOX, a nonheme iron dioxygenase containing approximately 673 amino acid residues [9], plays a key role in catalyzing arachidonic acid conversion to leukotriene A4, an important proinflammatory mediator, which can be further converted into different inflammatory mediators by different enzymes. 5-LOX activity is short-lived apparently because of the intrinsic instability of the enzyme. Gilbert et al. [10] reported the crystal structure at 2.4 Å resolution of human 5-LOX stabilized by the replacement of a 5-LOX-specific destabilizing sequence. The replacement of this destabilizing sequence does not affect the catalytic fidelity [10]. There are many inflammatory diseases such as allergic rhinitis, asthma, atherosclerosis, psoriasis, and atopic dermatitis [11, 12], some of which can be cured through the application of 5-LOX inhibitors. At present, the known inhibitors against 5-LOX can be classified into three types: redox-active inhibitors, iron-ligand inhibitors, and non-redox-type inhibitors [13]. Flavonoids, a group of important natural compounds with the basic structural scaffold of benzo-γ-pirone, which are widely distributed in the Plant Kingdom [14], exhibit inhibitory activity against 5-LOX. However, it is unclear which flavonoids are the key anti-inflammatory ingredients in S. suberectus.
In this study, the automated procedures to predict the binding of ligands to proteins [i.e., Autodock 4.2 and comparative molecular field analysis (CoMFA)] [15] were used to predict the inhibitory activity of S. suberectus flavonoids against 5-LOX.
2 Materials and methods
2.1 Docking trail
2.1.1 Ligand preparation:
The two-dimensional structures of the S. suberectus flavonoids (Figure 1) were constructed by ChemDraw Ultra and then converted into three-dimensional structures by Chem3D Ultra in which energy minimization was performed using the MOPAC program [16] and the AM1 semiempirical Hamiltonian. Before the docking trials, nonpolar hydrogen atoms were merged and Gasteiger charges were added. The rotatable bonds in the ligands were detected by Autodocktools 1.5.4 [17] and were all released with the active torsions that move the fewest atoms.
2.1.2 Protein preparation:
The three-dimensional structure of 5-LOX (pdb code: 3O8Y) was obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (New York, NY, USA). 5-LOX has two same chains: A-chain and B-chain [10]. This structure is used for docking by deleting B-chain and retaining A-chain. Before the docking trial, nonpolar hydrogen atoms were merged, nonintegral charges on 5-LOX were corrected, and Gasteiger charges were also added by Autodocktools 1.5.4.
2.1.3 Docking parameter setting:
The number of points in the x-, y-, and z-dimensions was 100, 100, 100, respectively. The grid spacing was 0.375 Å, and the coordinates of the grid center were 8.725, 22.431, and −1.338. A Lamarckian genetic algorithm was used to search for the optimal binding mode within the 5-LOX active site. The number of genetic algorithm runs and the maximum number of energy evaluations were 100 and 2.5 million, respectively. The translation step was 0.2, and the quaternion step and the torsion step were both set to 5.0 during the search. All other parameters not mentioned were set to default.
2.2 CoMFA experiment
The structures of all 21 inhibitors (see Table 1) in the training set against 5-LOX were constructed using the Sketcher module, and energy optimization was performed using the quasi-Newton method (BFGS) with Tripos Force Field simultaneous assignment of Gasteiger–Huckel charges. Aligning the training database, the carbon atoms at positions 4–8 of the flavone structure were chosen as the common substructure exhibited by all inhibitors, and 7-{[5-fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(furan-3-yl)-2H-chromen-2-one [22], the most potent inhibitor in the training set of 21 inhibitors, was chosen as the template molecule. The superposition is shown in Figure 2. The compounds in the test set were aligned as well using the same carbon atoms. CoMFA in which the parameters were set to default was calculated. The CoMFA Field Class was Tripos standard. Both the steric and electrostatic cut-offs were all set to default values of 30 kcal/mol, and the region of the grid was automatically created by CoMFA, for which the carbon atom with sp3 hybridization and a positive charge was used as the probe. The size of the rectilinear dimension of the lattice unit cell was 2.0. PLS analysis with the leave-one-out and no-validation was used to generate the model in the routine of quantitative structure-activity relationship. Leave-one-out of the dependent column (pIC50) and setting the column filtering to 2.0 kcal/mol were done to determine the best value of the components before no-validation was performed with the optimum number of components.
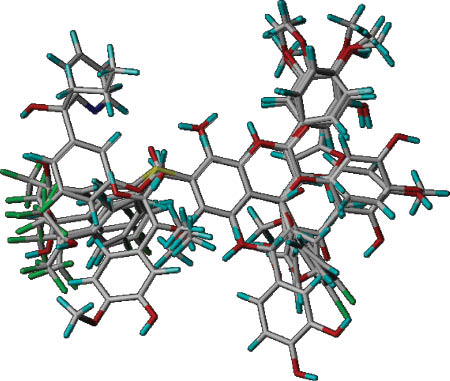
Superposition of 21 inhibitors in the training set used for the CoMFA analysis.
The compounds in the test set were aligned fairly well by using the same carbon atoms.
Inhibitors for the training set of CoMFA.
| Compound | Structure | IC50, μM | References |
|---|---|---|---|
| Centaureidin | 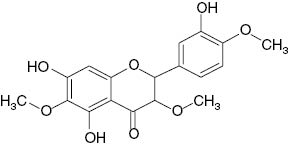 | 20 | [18] |
| 5,3′-Dihydroxy-4′-methoxy-7-carbomethoxyflavonol | 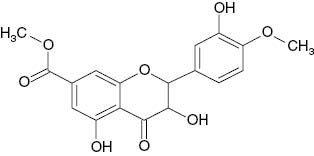 | 29 | [18] |
| Genistein | 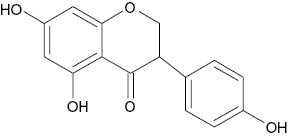 | 125 | [19] |
| Daidein | 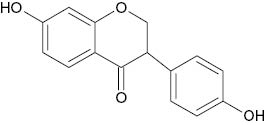 | 157 | [19] |
| Eupatilin | 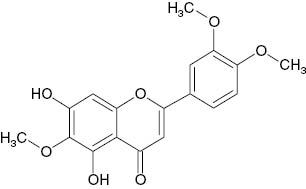 | 14 | [20] |
| 4′-Demethyleupatilin | 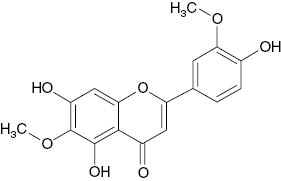 | 18 | [20] |
| 3,6-Dimethylether-6-hydroxykaempferol | 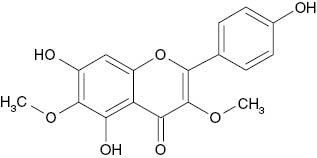 | 182 | [21] |
| 3,6,4′-Trimethylether-6-hydroxykaempferol | 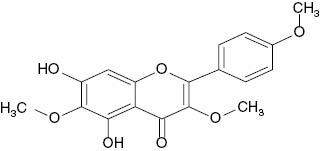 | 58 | [21] |
| 3,6,3′-Trimethylether-quercetagetin | 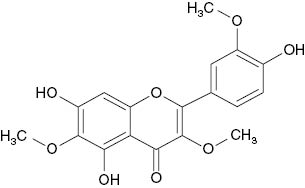 | 167 | [21] |
| 6-Methylether-6-hydroxyluteolin | 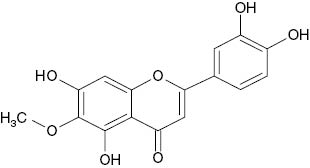 | 97 | [21] |
| 6,3′-Dimethylether-6-hydroxyluteolin | 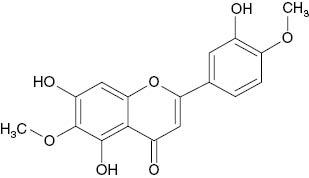 | 84 | [21] |
| 7-{[5-Fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(furan-3-yl)-2H-chromen-2-one | 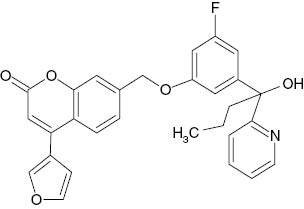 | 9×10−3 | [22] |
| Daphnetin | 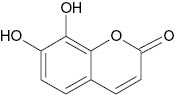 | 20 | [23] |
| 7-{[5-Fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(4-fluorophenyl)-2H-chromen-2-one | 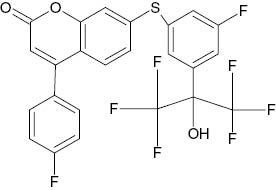 | 2.7×10−2 | [22] |
| 7-{[5-Fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(4-chlorophenyl)-2H-chromen-2-one | 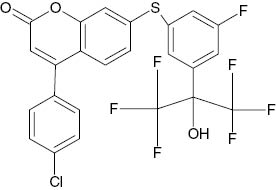 | 0.180 | [22] |
| 7-{[5-Fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-phenyl-2H-chromen-2-one | 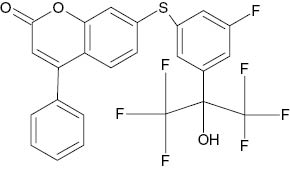 | 2.6×10−2 | [22] |
| 7-({3-Fluoro-5-[1-hydroxy-1-(pyridin-2-yl)propyl]phenoxy}methyl)-4-(furan-3-yl)-2H-chromen-2-one | 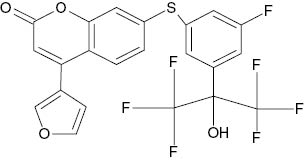 | 0.175 | [22] |
| Baicalein | 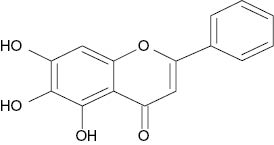 | 1.2 | [24] |
| Quercetin | 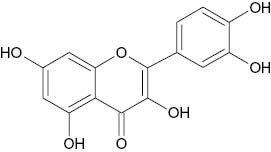 | 2.1 | [24] |
| Luteolin | 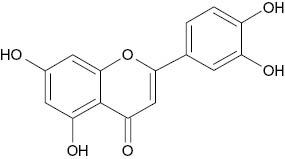 | 0.8 | [25] |
| Epicatechin |  | 22 | [26] |
3 Results
3.1 Results from the docking study
3.1.1 Results with established inhibitors of 5-LOX
Before predicting the inhibitory activity of flavonoids from S. suberectus against 5-LOX, as shown in Table 2, seven established inhibitors of 5-LOX were selected to evaluate the performance of Autodock 4.2 used in this study. There was a good relationship between the binding free energies and the pIC50 (negative decadic logarithm of IC50) values. The value of the coefficient of determination (R2) is 0.838; SD and P are 0.216 and 0.00355, respectively.
Seven established inhibitors of 5-LOX.
| Compound | Structure | IC50, μM | References |
|---|---|---|---|
| Caffeic acid | 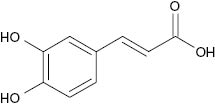 | 46 | [24] |
| Baicalein | 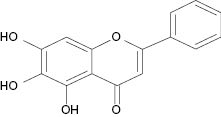 | 1.2 | [24] |
| Catechol | 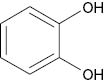 | 62 | [24] |
| Quercetin | 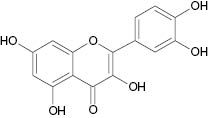 | 2.1 | [24] |
| Esculetin | 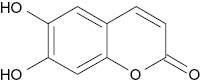 | 4.5 | [24] |
| Luteolin | 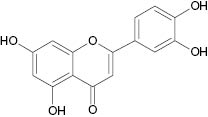 | 0.8 | [25] |
| Epicatechin | 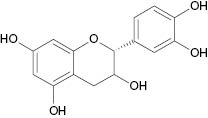 | 22 | [26] |
3.1.2 Prediction of the inhibitory activities of the S. suberectus flavonoids
The more negative the binding free energy of a ligand, the stronger is its inhibitory activity. As can be seen in Table 3, the S. suberectus flavonoids are predicted to inhibit 5-LOX to different extents, with 3′,4′,7-trihydroxyflavone, plathymenin, butin, catechin, liquiritigenin, eriodictyol, dihydroquercetin, dihydrokaempferol, and gallocatechin having stronger inhibitory activity.
Binding free energies calculated using Autodock 4.2 and pIC50 values predicted using CoMFA.
| Compound | TORSDOF | Binding free energy, kcal/mol | pIC50 predicted by CoMFA |
|---|---|---|---|
| 3′,4′,7-Trihydroxyflavone | 4 | −9.06 | 5.568 |
| Formononetin | 3 | −7.89 | 3.746 |
| Calycosin | 4 | −7.95 | 3.904 |
| Prunetin | 4 | −7.59 | 4.816 |
| Eriodictyol | 5 | −10.24 | 4.495 |
| Plathymenin | 5 | −8.57 | 5.361 |
| Dihydroquercetin | 6 | −8.68 | 4.602 |
| Butin | 4 | −9.49 | 5.362 |
| Dihydrokaempferol | 5 | −8.39 | 4.633 |
| Liquiritigenin | 3 | −8.88 | 5.461 |
| 6-Methoxyeriodictyol | 6 | −8.24 | 4.395 |
| (2S)-7-Hydroxy-6-methoxy-flavanone | 3 | −7.56 | 4.769 |
| Gallocatechin | 7 | −9.44 | 5.027 |
| Catechin | 6 | −9.03 | 5.483 |
| Epicatechin | 6 | −7.92 | 4.661 |
3.2 Results from CoMFA
3.2.1 Results for the inhibitors in the training set
Before generating the CoMFA model, 21 inhibitors of 5-LOX were chosen as the training set (see Table 1). The experimental IC50 values, in molar concentration, were converted to the pIC50 (negative decadic logarithm of IC50) values. The optimum number of components generated is 6 by one cross-validated analysis (i.e., the leave-one-out), and the R2 is 0.710. The value of R2 is 0.997 with no validated partial least square (PLS) analysis with the optimum number set to 6; the SE of estimate and the F value (n1=6, n2=14) are 0.0960 and 692.059, respectively, and the probability (R2=0) is 0.000. The contribution of steric and electrostatic factors to the generated CoMFA model is 0.478 and 0.522, respectively.
As shown in Figure 3, in the CoMFA model generated of the electrostatic map, more positive charges near C3 and C6 and more negative charges near C2 may increase binding. In the steric map, more bulky groups near C2 and C7 and less bulky groups near C3, C5, and C6 may increase binding.
![Figure 3: 7-{[5-fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(furan-3-yl)-2H-chromen-2-one, the most potent inhibitor of 5-LOX in the training set, within the contour of the CoMFA model.Based on the steric contours, the green areas mean that more bulky groups near these areas are favorable to increasing binding and the yellow areas mean that less bulky groups near these areas are favorable to increasing binding. Based on the electrostatic contours, the blue areas mean that more positive charges near these areas are favorable to increasing binding and the red areas mean that more negative charges near these areas are favorable to increasing binding.](/document/doi/10.1515/znc-2014-4110/asset/graphic/j_znc-2014-4110_fig_003.jpg)
7-{[5-fluoro-3-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]sulfanyl}-4-(furan-3-yl)-2H-chromen-2-one, the most potent inhibitor of 5-LOX in the training set, within the contour of the CoMFA model.
Based on the steric contours, the green areas mean that more bulky groups near these areas are favorable to increasing binding and the yellow areas mean that less bulky groups near these areas are favorable to increasing binding. Based on the electrostatic contours, the blue areas mean that more positive charges near these areas are favorable to increasing binding and the red areas mean that more negative charges near these areas are favorable to increasing binding.
The contour of the CoMFA model of the flavonoid inhibitor luteolin is shown in Figure 4. In the electrostatic map, more positive charges near C3, C6, C2′, and C5′ and more negative charges near C2 and C4′ may increase binding, whereas, in the steric map, more bulky groups near C2, C5′, and C7 and less bulky groups near C3, C5, C6, C4′, and C5′ may increase binding.
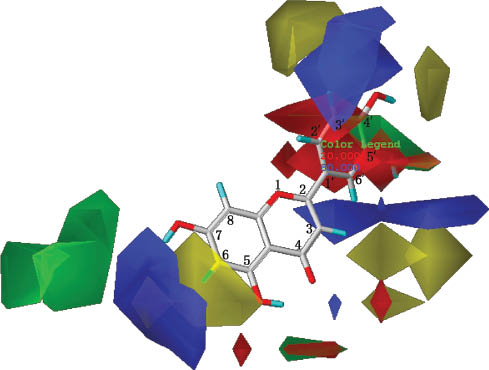
Luteolin, a flavonoid inhibitor of 5-LOX, within the contour of the CoMFA model.
Based on the steric contours, the green areas mean that more bulky groups near these areas are favorable to increasing binding and the yellow areas mean that less bulky groups near these areas are favorable to increasing binding. Based on the electrostatic contours, the blue areas mean that more positive charges near these areas are favorable to increasing binding and the red areas mean that more negative charges near these areas are favorable to increasing binding.
3.2.2 Prediction of the inhibitory activities of the compounds from S. suberectus
Before predicting the inhibitory activity of compounds from S. suberectus, the performance of the model generated by CoMFA must be evaluated. To confirm the performance of the model, the inhibitory activities of five inhibitors in the test set whose IC50 values had been reported were predicted by the model (see Table 4). The predicted pIC50 values of the five inhibitors in the test set using the generated CoMFA model are close to those obtained experimentally. The value of the R2 is 0.875; SD and P are 0.236 and 0.0195, respectively.
Five inhibitors in the test set of CoMFA.
| Compound | Structure | IC50 (μM) | References |
|---|---|---|---|
| 7-{[3-Fluoro-5-(3-hydroxypentan-3-yl)phenoxy]methyl}-4-(furan-3-yl)-2H-chromen-2-one | 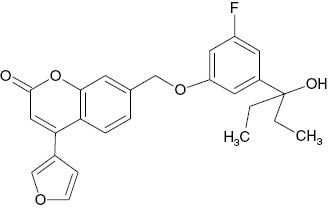 | 1.5×10−2 | [22] |
| 7-({3-Fluoro-5-[1-hydroxy-1-(thiazol-2-yl)propyl]phenoxy}methyl)-4-(4-fluorophenyl)-2H-chromen-2-one | 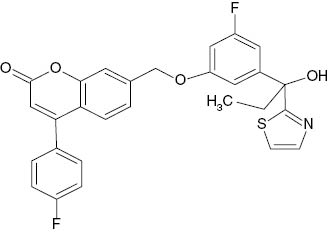 | 0.175 | [22] |
| 7-{[3-Fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenoxy]methyl}-4-(4-fluorophenyl)-2H-chromen-2-one | 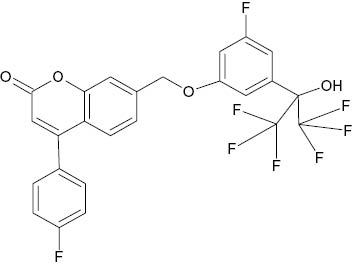 | 5.5×10−2 | [22] |
| 7-({3-Fluoro-5-[1-hydroxy-1-(6,8-dioxa-bicyclo[3.2.1]octan-1-yl)methyl]phenoxy}methyl)-4-(furan-3-yl)-2H-chromen-2-one | 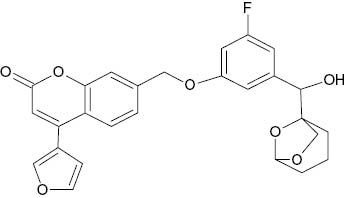 | 0.2 | [22] |
| 7-({3-Fluoro-5-[1-hydroxy-1-(6,8-dioxa-bicyclo[3.2.1]octan-1-yl)methyl]phenoxy}methyl)-4-phenyl-2H-chromen-2-one | 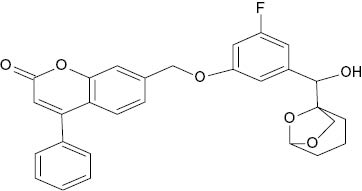 | 0.3 | [22] |
The larger the value of the pIC50 value obtained, the stronger is the inhibitory activity of the corresponding compound. As shown in Table 3, 3′,4′,7-trihydroxyflavone, liquiritigenin, catechin, gallocatechin, butin, and plathymenin are the strong inhibitors of 5-LOX.
3.2.3 Molecular binding mode
The molecular binding mode of the 5-LOX ligands is illustrated in Figure 5. The conformation of the ligands was obtained based on the lowest binding free energy scored by Autodock 4.2.
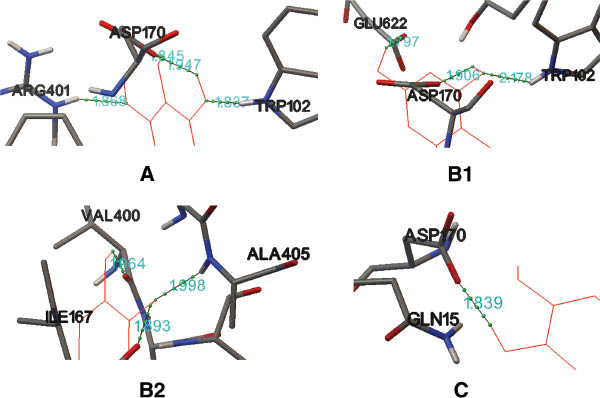
Interactions between the ligands (A) baicalein, (B1 and B2) eriodictyol, and (C) (2S)-7-hydroxy-6-methoxy-flavanone and 5-LOX.
The thin green solid lines represent the hydrogen bonds. The thin red solid lines represent the ligands. The amino acid residues in the active site are shown in bold lines. As there are six hydrogen bonds between eriodictyol and 5-LOX, it is difficult to distinguish the hydrogen bonds in one presentation, so two presentations (B1 and B2) are provided for eriodictyol.
Three compounds from S. suberectus [i.e., baicalein and eriodictyol in the conformation of their lowest calculated binding free energy, and (2S)-7-hydroxy-6-methoxy-flavanone, with the highest binding free energy] were selected as examples to define the molecular binding mode and the likely inhibitory mechanism. As shown in Figure 5A, there are four hydrogen bonds in the complex between baicalein and 5-LOX. One hydrogen bond is formed between the oxygen atom of the 5-hydroxy group in ring A and a hydrogen atom of the imino group in the chain of ARG401 (distance 1.868 Å). The other two hydrogen bonds are located between the hydrogen atoms of the 6- and 7-hydroxy groups in ring A and a carboxyl oxygen atom in the chain of ASP170, whose distances are 1.845 and 1.947 Å, respectively. The fourth hydrogen bond is formed between the oxygen atom of the 7-hydroxy group in ring A and the hydrogen atom on the nitrogen of the indole ring of TRP102 (distance 1.837 Å). Figure 5B1 and B2 shows the complex formed between eriodictyol and 5-LOX. There are six hydrogen bonds: ASP170, 5-OH of ring A (1.906 Å); TRP102, 5-OH of ring A (2.178 Å); GLU622, 7-OH of ring A (1.797 Å); ALA405, 3′-OH of ring B (1.998 Å); ILE167, 3′-OH of ring B (1.893 Å); and VAL400, 4′-OH of ring B (1.964 Å). On the contrary, there is only a single hydrogen bond in the complex of (2S)-7-hydroxy-6-methoxy-flavanone and 5-LOX (ASP170, 7-OH of ring A; 1.839 Å). Therefore, the hydrogen bonds formed could stabilize hydrophobic interactions.
4 Discussion
Of the phytochemicals from S. suberectus (Figure 1), the six strongest 5-LOX inhibitors, 3′,4′,7-trihydroxyflavone, plathymenin, butin, liquiritigenin, gallocatechin, and catechin, were screened by Autodock 4.2 and CoMFA.
The structure of flavonoids is composed of three rings: rings A, C, and B, giving rise to a C6-C3-C6 skeleton. According to the degree of saturation of the benzo-γ-pirone ring and the different substituents present on the rings [14], the flavonoids can be classified into several types, including flavone, isoflavone, flavan-3-ol, and flavanone. Both the docking simulations and the CoMFA model suggest that the 4′-hydroxy group is essential for potent 5-LOX inhibition by the compounds from S. suberectus.
The binding properties of a compound are determined by its conformation. CoMFA requires a minimum of 10 samples, better 15 and more, in the training set to generate an acceptable model that can be used to predict the inhibitory activity of experimentally untested compounds. As can be seen in Table 3 and Figure 1, the inhibitory activity of the isoflavone compounds is low based on the predicted pIC50. Among the flavanones and flavanonols, the inhibitory activities of butin (pIC50 5.362), plathymenin (pIC50 5.361), and liquiritigenin (pIC50 5.461) are higher than those of the other compounds. This may indicate that the hydroxy group at C5 lowers their inhibitory activity. For the flavan-3-ols, the binding affinity of compounds with 2R configuration is higher than that of those with 2S configuration. The predicted pIC50 value of catechin (pIC50 5.483) is higher than that of gallocatechin (pIC50 5.027); this may indicate that the 5′-hydroxy group lowers the binding affinity, as in the case of the flavanones and flavanonols.
The results of the molecular docking, modeled with Autodock 4.2, depict a common binding mode of the 5-LOX inhibitors. As shown in Table 3 and Figure 1, among the flavanones and flavan-3-ols, the binding free energies of epicatechin [with (2S)-configuration; −7.92] and (2S)-7-hydroxy-6-methoxy-flavanone (−7.56) are less negative than those of most of the other compounds. This may indicate that the hydroxy group at C5 can lower a compound’s inhibitory activity. This rule is also found for the isoflavones. Compared with formononetin (−7.89), the binding free energy of calycosin (−7.95) is more negative, which may indicate that the 3′-hydroxy group lowers the binding free energy. Among flavan-3-ols, 2R configuration seems to be more in favor of increasing the binding affinity than the 2S configuration. The binding affinity of gallocatechin (−9.44) is more negative than that of catechin (−9.03); this may indicate that the 5′-hydroxy group reduces the affinity.
5 Conclusions
In the last few decades, many compounds isolated from traditional Chinese medicinal herbs were tested for their inhibitory activity against 5-LOX. However, it is not possible to test the inhibitory activity of every compound against 5-LOX, because such studies are a great workload and are time-consuming.
In this study, the inhibitory activities of 15 compounds from S. suberectus against 5-LOX were predicted with the aid of Autodock 4.2 and CoMFA. Of these, 3′,4′,7-trihydroxyflavone, liquiritigenin, catechin, butin, gallocatechin, and plathymenin were the most potent inhibitors of 5-LOX. This finding may be of help in selecting the active principles isolated from traditional Chinese medicinal herbs as test samples and in designing new highly potent inhibitors of 5-LOX by Autodock 4.2 and CoMFA.
Acknowledgments
This work was financially supported by Guangxi Natural Science Foundation (No. 2013GXNSFAA019168).
References
1. Zheng X-L, Xing F-W. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol 2009;124:197–210.10.1016/j.jep.2009.04.042Search in Google Scholar
2. Liu B, Liu J-L, Chen J, Zhu D-M, Zhou H-J. A study on anticancer activity of Caulis spatholobi extract on human osteosarcoma saos-2 cells. Afr J Tradit Complement 2013;10:256–60.10.4314/ajtcam.v10i5.6Search in Google Scholar
3. Lee M-H, Lin Y-P, Hsu F-L, Zhan G-R, Yen K-Y. Bioactive constituents of Spatholobus suberectus in regulating tyrosinase-related proteins and mRNA in HEMn cells. Phytochemistry 2006;67:1262–70.10.1016/j.phytochem.2006.05.008Search in Google Scholar
4. Li R-W, Lin G-D, Myers S-P, Leach D-N. Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol 2003;85:61–7.10.1016/S0378-8741(02)00339-2Search in Google Scholar
5. Yang M, Xiao C-H, Wu Q-F, Niu M-C, Yao Q, Li K-Q, et al. Anti-inflammatory effect of Sanshuibaihu decoction may be associated with nuclear factor-κB and p38 MAPKα in collagen-induced arthritis in rat. J Ethnopharmacol 2010;127:264–73.10.1016/j.jep.2009.11.010Search in Google Scholar
6. Zhang L, Ravipati A-S, Koyyalamudi S-R, Jeong S-C, Reddy N, Bartlett J, et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med 2013;6:673–81.10.1016/S1995-7645(13)60117-0Search in Google Scholar
7. Su J, Sun Y-L, Lu S-J, Liu D-C. Determination of 28 elements of Spatholobus suberectus Dunn by ICP-MS. J. Guangxi Normal Univ 2013;31:76–81.Search in Google Scholar
8. Veitch N-C. Isoflavonoids of the Leguminosae. Nat Prod Rep 2013;30:988–1027.10.1039/c3np70024kSearch in Google Scholar PubMed
9. Radmark O. The molecular biology and regulation of 5-lipoxygenase. Am J Respir Crit Care 2000;161:11–5.10.1164/ajrccm.161.supplement_1.ltta-3Search in Google Scholar PubMed
10. Gilbert N-C, Bartlett S-G, Waight M-T, Neau D-B, Boeglin W-E, Brash A-R. The structure of human 5-lipoxygenase. Science 2011;331:217–9.10.1126/science.1197203Search in Google Scholar PubMed PubMed Central
11. Haeggström J-Z, Funk C-D. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev 2011;111:5866–98.10.1021/cr200246dSearch in Google Scholar PubMed
12. Tam V-C, Quehenberger O, Oshansky C-M, Suen R, Armando A-M, Treuting P-M, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell 2013;154:213–27.10.1016/j.cell.2013.05.052Search in Google Scholar
13. Steinhilber D, Hofmann B. Recent advances in the search for novel 5-lipoxygenase inhibitors. Basic Clin Pharmacol 2014;114:70–7.10.1111/bcpt.12114Search in Google Scholar
14. Carlo G-D, Mascolo N, Izzo A-A, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 1999;65:337–53.10.1016/S0024-3205(99)00120-4Search in Google Scholar
15. Cramer, R-D, Patterson D-E, Bunce J-D. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc 1988;110:5959–67.10.1021/ja00226a005Search in Google Scholar
16. Stewart J-J-P. MOPAC: a semiempirical molecular orbital program. J Comput Aided Mol Des 1990;4:1–103.10.1007/BF00128336Search in Google Scholar
17. Sanner M-F. Python: a programming language for software integration and development. J Mol Graphics Mod 1999;17: 57–61.Search in Google Scholar
18. Abad M-J, Bermejo P, Villar A. The activity of flavonoids extracted from Tanacetum microphyllum DC. (Compositae) on soybean lipoxygenase and prostaglandin synthetase. Gen Pharmacol 1995;26:815–9.10.1016/0306-3623(94)00242-FSearch in Google Scholar
19. Mahesha H-G, Singh S-A, Appu-Rao A-G. Inhibition of lipoxygenase by soy isoflavones: evidence of isoflavones as redox inhibitors. Arch Biochem Biophys 2007;461:176–85.10.1016/j.abb.2007.02.013Search in Google Scholar
20. Koshihara Y, Neichi T, Murota S-I, Lao A-N, Fujimoto Y, Tatsuno T. Selective inhibition of 5-lipoxygenase by natural compounds isolated from Chinese plants, Artemisia rubripes Nakai. FEBS Lett 1983;158:41–4.10.1016/0014-5793(83)80672-3Search in Google Scholar
21. Williams C-A, Harborne J-B, Geiger H, Hoult J-R-S. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry 1999;51:417–23.10.1016/S0031-9422(99)00021-7Search in Google Scholar
22. Grimm E-L, Brideau C, Chauret N, Chan C-C, Delorme D, Ducharme Y, et al. Substituted coumarins as potent 5-lipoxygenase inhibitors. Bioorg Med Chem Lett 2006;16:2528–31.10.1016/j.bmcl.2006.01.085Search in Google Scholar PubMed
23. Hoult J-R, Forder R-A, Heras B, Lobo I-B, Paya M. Inhibitory activity of a series of coumarins on leukocyte eicosanoid generation. Agents Actions 1994;42:44–9.10.1007/BF02014299Search in Google Scholar
24. Masayuki F, Tanihiro Y, Kenkichi O, Shozo Y. Studies on arachidonate 5-lipoxygenase of rat basophilic leukemia cells. Biochim Biophys Acta Lipids Lipid Metabol 1984;795:458–65.10.1016/0005-2760(84)90173-5Search in Google Scholar
25. Lee E-J, Kim J-S, Kim H-P, Lee J-H, Kang S-S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem 2010;120:134–9.10.1016/j.foodchem.2009.09.088Search in Google Scholar
26. Schewe T, Kühn H, Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J Nutr 2001;132:1825–9.10.1093/jn/132.7.1825Search in Google Scholar PubMed
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn

