Abstract
Among secondary metabolites, alkylresorcinols are considered particularly important for the antimicrobial defense system in cereal grains. Dry rye caryopses and young seedlings contain detectable quantities of resorcinolic lipids. Overall, 11 distinct alkylresorcinol homologues were identified, which showed variable profiles during rye germination and early seedling development, especially with reference to the production of very long homologues and to side chain saturation. Additionally, changes in the alkylresorcinol composition during rye seedling growth are presented for the first time.
1 Introduction
Saturated alkylresorcinols (ARs) and their unsaturated counterparts constitute a large family of essential non-isoprenoid phenolic compounds widely distributed in cereals and other plants [1, 2]. In particular, ARs accumulate to high levels in rye grains (Secale cereale L.) with levels ranging from 0.36 up to 3.2 g kg–1 [3]. Resorcinolic lipids occur in the testa and pericarp of cereal caryopses [4], whereas the epicuticular wax layer contains only a small amount of these compounds. Due to their unique biological activities, ARs are often considered natural biofungicides [5, 6]. In fact, alkylresorcinols are potent antimicrobials significantly contributing to a critical defensive chemical barrier in cereal grains against numerous bacterial and fungal pathogens [1]. For example, ARs isolated from rye grains were found to inhibit growth of the plant pathogenic fungi Fusarium sp. and Rhizoctonia sp. [7]. Besides cereal grains, ARs have been shown to occur in developing coleoptiles [8] and in intracuticular waxes of rye leaves [9]. There is still substantial lack of an understanding of the biosynthesis process of these intriguing secondary metabolites. In order to fill this serious knowledge gap, in this study we explored the dynamics of AR metabolism during the germination of caryopses and early seedling growth of rye.
2 Materials and methods
2.1 Treatments of rye grains and growth conditions
Grains of rye (Secale cereale L.) cv. Dankowskie Zlote were provided by the “Danko” Plant Breeding Farm (Choryn, Poland). They were surface-disinfected in batches of 50 g by immersing in 0.1% (v/v) aqueous Tween 80 for 15 min, followed by a 15-min incubation in 5% (w/v) chloramine and washes in sterile distilled water. The caryopses were then placed between layers of a moistened sterile filter paper in petri dishes and incubated at 22 °C in the dark for various periods of time. One-day-old germinating caryopses and 3- and 5-day-old coleoptiles, cut off from the residual grains with a sterile scalpel blade, were collected and further investigated. Each experiment was carried out in triplicate.
2.2 AR isolation and purification
Alkylresorcinols were extracted from both whole caryopses and rye seedling samples that were dried prior to analysis (48±2°C for 12 h). All samples were treated with equal volumes of acetone three times for 24 h. Each acetone fraction was filtered through filter paper to remove any solid particles. All three acetone filtrates were combined, and the solvent was removed by vacuum evaporation in a rotavapor (IKA-Werke GmbH & Co.KG, Staufen, Germany) at 40 °C. The oily residue was diluted in n-propanol, and the organic fraction was concentrated in vacuo. The obtained residue was dissolved in 0.5 mL chloroform and then applied to a 20×20 cm preparative silica gel Si60 TLC plate (Merck, Darmstadt, Germany). Separation was carried out in n-hexane/ethyl ether/formic acid (70:30:1, v/v/v). Afterward, a 1-cm-wide section of the plate was sprayed with aqueous 0.05% (w/v) Fast Blue B Zn salt for color development. The corresponding unsprayed part of the gel was scrapped off the plate and re-extracted overnight with a mixture of acetone/methanol (4:1, v/v). After centrifugation (7500 g, 10 min), the supernatant was concentrated in vacuo. The AR fraction was redissolved in n-propanol and used for further analysis. Each procedure was performed in triplicate. The reagents were from Polskie Odczynniki Chemiczne (Gliwice, Poland) and from Chempur (Piekary Slaskie, Poland). Diazonic dye Fast Blue B Zn was obtained from Sigma-Aldrich (Saint-Louis, USA).
2.3 Quantitative and qualitative analyses of ARs
Resorcinolic lipids were analyzed by gas chromatography using an HP 5890 Series II gas chromatograph coupled with a JEOL SX-102A mass spectrometer according to the method described elsewhere [10]. The identification of the individual AR homologues was achieved based on both the molecular ion and the common base peak ion at m/z 268, which is characteristic of those molecules. The retention times of the respective homologues were 8.8 min (M+ 462, C15:1), 9.3 min (M+ 464, C15:0), 9.8 min (M+ 490, C17:1), 10.4 min (M+ 492, C17:0), 11.1 min (M+ 518, C19:1), 11.6 min (M+ 520, C19:0), 12.2 min (M+ 546, C21:1), 12.7 min (M+ 548, C21:0), 13.3 min (M+ 574, C23:1), 13.8 min (M+ 576, C23:0), 14.3 min (M+ 602, C25:1) and 14.9 min (M+ 604, C25:0), respectively. The relative composition and total amount of the homologues were estimated on the basis of the area of the peak ion at m/z 268. In addition, the microcolorimetric method described by Tluscik et al. [11] was used for quantitative AR analysis. All determinations were carried out at least in triplicate.
3 Results and discussion
Rye caryopses contain large quantities of alkylresorcinols [1, 3]. In the present study, the total concentration of ARs in the tested intact rye caryopses was 581.9 mg kg–1, which is within the lower content range previously reported in Swedish cereal cultivars (549–1022 mg kg–1) [12]. Saturated homologues, such as 1,3-dihydroxy-5-n-heptadecylbenzene (AR C17:0) and 1,3-dihydroxy-5-n-nonadecylbenzene (AR C19:0) were the most predominant ARs in all samples tested in this study (Table 1). Similar results were reported in the earlier studies of Deszcz and Kozubek [8] and Kulawinek et al. [2] who showed that these constituents are the major secondary compounds in rye grains and seedlings, respectively. Existing differences in AR concentrations between various cultivars of the same cereal species have been well documented [3]. In fact, both quantitative and qualitative levels of ARs in plant organs and tissues can be affected by various, often hard-to-define, biotic and abiotic factors not only within distinct cultivars but also between individual plants of the same cultivar, even within the same ecological niche [10, 13–16]. During the germination of rye caryopses, significant changes in the abundance and/or composition of the ARs were observed. Compared to the AR content of whole ungerminated grains (about 582 mg kg–1), the content in 1-day-old germinating caryopses was reduced to about 505 mg kg–1, while the respective homologue profiles remained almost identical, indicating that the ARs present in the germinating grain originated from the original storage pool; yet it remains unclear whether the seedlings are able to utilize ARs accumulated in the seeds. Since light conditions favor the energy-dependent uptake of exogenous ARs, while limiting their accumulation in green seedlings [8], our experiments were performed in darkness, which not only accurately mimicked basic conditions of rye germination but also appeared to stimulate de novo production of resorcinolic lipids. Table 1 summarizes both the AR content and composition during germination and early seedling growth. The AR content in the coleoptiles of 3-day-old seedlings was 2.03 mg kg–1 and increased during further seedling growth. Interestingly, AR homologue composition in the developing seedlings was clearly distinct from that observed in the grains. There was a slight, about 3%, decrease in the content of unsaturated AR homologues, but significant alterations were detected in the pattern of the saturated ones. In fact, the ratios of C19:0/C21:0 and C21:0/C23:0 changed from initial 2.7 and 4.1 to 1.9 and 1.8, respectively, during seedling development. Interestingly, the proportion of saturated AR homologues increased from 82% to about 92% during seedling growth, while that of the monounsaturated counterparts decreased. In addition, the proportions of short (≤C17), long (C19–C21) and very long (≥C23) AR homologues varied dynamically during germination and seedling development. The group of very long side chain homologues was minor in all analyzed samples, but their content significantly increased from 4.4% to 8.9% during seedling development. An accumulation mainly of these homologues in cuticular waxes of the leaves of 3-week-old rye plants was also noted by Ji and Jetter [9].
Modification in content and homologue composition of ARs during germination of rye caryopses and early seedling development.
| Sample | Content*, mgkg–1 DW | R#: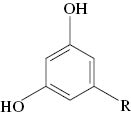 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C15:0 | C17:1 | C17:0 | C19:1 | C19:0 | C21:1 | C21:0 | C23:1 | C23:0 | C25:1 | C25:0 | ||
| Ungerminated caryopses | 581.90±5.32a | 3.50 | 7.40 | 39.60 | 7.40 | 26.30 | 2.30 | 9.10 | 0.60 | 2.10 | 0.30 | 1.40 |
| 1-day-old germinating caryopses | 505.27±6.24b | 3.00 | 6.61 | 37.14 | 7.61 | 27.63 | 2.70 | 10.31 | 0.40 | 2.50 | 0.20 | 1.90 |
| Coleoptiles of 3-day-old seedlings | 2.03±0.03c | 7.43 | 5.45 | 37.13 | 6.44 | 20.79 | 2.97 | 10.89 | 0.00 | 5.94 | 0.00 | 2.97 |
| Coleoptiles of 5-day-old seedlings | 6.25±0.09d | 9.62 | 1.76 | 21.64 | 3.85 | 29.33 | 1.92 | 24.04 | 0.64 | 5.93 | 0.00 | 1.28 |
DW, dry weight. *Mean values±SD of alkylresorcinol contents from n=3 experiments. Values followed by different letters (a-d) are significantly different at p=0.05 according to Duncan’s multiple range test. #R, C15–C25 saturated or monounsaturated side-chain.
Although the process of AR biosynthesis in plants still remains largely undescribed, some encouraging results have been published. It has been discovered that aliphatic carbon chains of fatty acids serve as precursors in the biosynthesis of ARs [17], which takes place in plastids [8], i.e., the same organelle in which fatty acids are synthesized in plant cells. Furthermore, the production of cuticular wax components also begins in plastids, from where they are exported to the plant cuticle via the endoplasmic reticulum and the plasma membrane [18]. Thus, any disorder or induced alterations in the functionality of these organelles leads to significant modifications of AR content and homologue patterns [19]. Our current study provides novel insight into the AR dynamics during rye caryopsis germination and early seedling growth.
Acknowledgments
The authors are grateful to Dr. Yoshikatsu Suzuki, our long-term collaborator at the Polymer Chemistry Lab - RIKEN (Japan), for the GC-MS analyses of alkylresorcinols.
References
1. Kozubek A, Tyman JH. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem Rev 1999;99:1–26.10.1021/cr970464oSearch in Google Scholar
2. Kulawinek M, Jaromin A, Kozubek A, Zarnowski R. Alkylresorcinols in selected polish rye and wheat cereals and whole-grain cereal products. J Agric Food Chem 2008;56:7236–42.10.1021/jf801707gSearch in Google Scholar
3. Ross AB, Kamal-Eldin A, Aman P. Dietary alkylresorcinols: absorption, bioactivities, and possible use as biomarkers of whole-grain wheat- and rye-rich foods. Nutr Rev 2004;3:81–95.10.1111/j.1753-4887.2004.tb00029.xSearch in Google Scholar
4. Landberg R, Kamal-Eldin A, Salmenkallio-Marttila M, Rouau X, Aman P. Localization of alkylresorcinols in wheat, rye and barley kernels. J Cereal Sci 2008;48:401–6.10.1016/j.jcs.2007.09.013Search in Google Scholar
5. Kozubek A, Tyman JH. Bioactive phenolic lipids. Stud Nat Prod Chem 2005;30:111–90.10.1016/S1572-5995(05)80032-8Search in Google Scholar
6. Zarnowski R, Kozubek A. Resorcinolic lipids as natural biofungicides. In: Dehne HW, Gisi U, Kuck KH, Russel PE, Lyr H, editors. Modern fungicides and antifungal compounds III. Th. Mann Verlag, Bonn: AgroConcept GmbH, 2002:337–47.Search in Google Scholar
7. Zarnowski R, Kozubek A, Pietr SJ. Effect of rye 5-n-alkylresorcinols on in vitro growth of phytopathogenic Fusarium and Rhizoctonia fungi. Bull Pol Acad Sci: Biol Sci 1999;47:231–5.Search in Google Scholar
8. Deszcz L, Kozubek A. Higher cardol homologs (5-alkylresorcinols) in rye seedlings. Biochim Biophys Acta 2000;1483:241–50.10.1016/S1388-1981(99)00187-0Search in Google Scholar
9. Ji X, Jetter R. Very long alkylresorcinols accumulate in the intracuticular wax of rye (Secale cereale L.) leaves near the tissue surface. Phytochemistry 2008;69:1197–207.10.1016/j.phytochem.2007.12.008Search in Google Scholar PubMed
10. Magnucka EG, Suzuki Y, Pietr SJ, Kozubek A, Zarnowski R. Action of benzimidazole fungicides on resorcinolic lipid metabolism in rye seedlings depends on thermal and light growth conditions. Pest Biochem Physiol 2007;88:219–25.10.1016/j.pestbp.2006.11.008Search in Google Scholar
11. Tluscik F, Kozubek A, Mejbaum-Katzenellenbogen W. Alkylresorcinols in rye (Secale cereale L.) grains. VI. Colorimetric micromethod for the determination of alkylresorcinols with the use of diazonium salt, Fast Blue B. Acta Soc Bot Polon 1981;50:645–51.10.5586/asbp.1981.086Search in Google Scholar
12. Ross AB, Kamal-Eldin A, Jung C, Shepherd MJ, Aman P. Gas chromatographic analysis of alkylresorcinols in rye (Secale cereale L.) grains. J Sci Food Agric 2001;81:1405–11.10.1002/jsfa.956Search in Google Scholar
13. Magnucka EG, Suzuki Y, Pietr SJ, Kozubek A, Zarnowski R. Cycloate, an inhibitor of fatty acid elongase, modulates metabolism of very-long-side-chain alkylresorcinols in rye seedlings. Pest Manag Sci 2009;65:1065–70.10.1002/ps.1792Search in Google Scholar PubMed
14. Pietr SJ, Kita W, Sowiński J, Nowak W, Biliński Z, Nadziak J, et al. The influence of Cedomon on yield and fungal infection of spring barley in field conditions in Poland. IOBC WPRS Bull 2002;25:333–6.Search in Google Scholar
15. Zarnowski R, Suzuki Y. 5-n-alkylresorcinols from grains of winter barley (Hordeum vulgare L.). Z Naturforsch 2004;59c:315–7.10.1515/znc-2004-5-603Search in Google Scholar PubMed
16. Zarnowski R, Suzuki Y, Pietr SJ. Alkyl- and alkenylresorcinols of wheat grains and their chemotaxonomic significance. Z Naturforsch 2004;59c:190–6.10.1515/znc-2004-3-411Search in Google Scholar PubMed
17. Suzuki Y, Kurano M, Esami Y, Yamaguchi I, Doi Y. Biosynthesis of 5-alkylresorcinol in rice: incorporation of a putative fatty acid unit in the 5-alkylresorcinol carbon chain. Bioorg Chem 2003;31:437–52.10.1016/j.bioorg.2003.08.003Search in Google Scholar PubMed
18. Kunst L, Samuels L. Plant cuticles shine:advances in wax biosynthesis and export. Curr Opin Plant Biol 2009;12:721–7.10.1016/j.pbi.2009.09.009Search in Google Scholar PubMed
19. Magnucka EG, Pietr SJ, Kozubek A, Zarnowski R. Various effects of the photosystem II – inhibiting herbicides on 5-n-alkylresorcinol accumulation in rye seedlings. Pest Biochem Physiol 2014;116:56–62.10.1016/j.pestbp.2014.09.015Search in Google Scholar PubMed
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn

