Abstract
A series of novel arylmethylamine derivatives of honokiol (5a-m) was prepared. Their insecticidal activity was tested against the pre-third-instar larvae of the oriental armyworm (Mythimna separata Walker), a typical lepidopteran pest. Compounds 5a, 5b, 5e, 5h, and 5k exhibited insecticidal activity equal to, or higher than, that of the positive control toosendanin.
1 Introduction
Honokiol (1; Figure 1) was isolated as a bioactive biphenyl-neolignan from the root and stem bark of Magnolia officinalis and Magnolia obovata [1]. Additionally, compound 1 exhibited a variety of biological activities, including anticancer [2], anxiolytic [3], and neuroprotective [4] activities. Conversely, the increasing use of synthetic agrochemicals over the years has resulted in the development of resistance in insect pest populations and in environmental problems. Therefore, development of new agrochemicals originated from plant secondary metabolites has received much research attention [5–7]. To the best of our knowledge, little attention has been paid to structural modifications of 1 as insecticidal agents. Moreover, arylmethylamine moieties are found in some molecules showing various types of biological properties such as herbicidal, insecticidal, fungicidal, and antitumor activities [8]. In continuation of our program on the development of novel natural-product-based pesticidal agents [9–11], herein we semisynthesized a series of novel arylmethylamine derivatives of 1 (5a-m; Scheme 1) by introduction of the arylmethylamine fragment to its skeleton. The oriental armyworm (Mythimna separata Walker) is an important and typical insect pest of crops, and sometimes its outbreaks can spread widely and result in complete crop loss. Development of new effective, selective, and safe pesticides for controlling M. separata is still a challenging task. Consequently, the insecticidal activities of the newly synthesized compounds 5a-m were tested against the pre-third-instar larvae of M. separata in vivo.
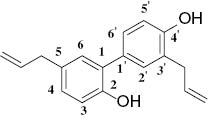
Chemical structure of honokiol (1).

Synthesis of arylmethylamine derivatives of honokiol (5a-m). Reagents and conditions: (A) conc. HNO3, CH2Cl2/glacial acetic acid (1:1, v/v), 0 °C to room temperature, 4 h, yield 33%; (B) SnCl2.2H2O, ethanol, room temperature, 36 h, yield 51%; (C) RCHO, ethanol, 0 °C to room temperature, 4–12 h; (D) NaBH4, ethanol, 0 °C to room temperature, 5–8 h, yield 27%–86%.
2 Experimental
2.1 Reagents and instrumentation
All reagents and solvents were of reagent grade or purified according to standard methods before use. Analytical thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC) were performed with silica gel plates using silica gel 60 GF254 (Qingdao Haiyang Chemical Co., Qingdao, China). Silica gel column chromatography was performed with silica gel 200–300 mesh (Qingdao Haiyang Chemical Co.). Melting points (m.p.) were determined on a XT-4 digital melting point apparatus (Beijing Tech Instrument Co., Beijing, China) and were uncorrected. Proton nuclear magnetic resonance spectra (1H NMR) were recorded in DMSO-d6 or CDCl3 on a Bruker Avance 400 or 500 MHz instrument (Fällanden, Switzerland), and tetramethylsilane (TMS) was used as the internal standard. High-resolution mass spectra (HR-MS) were obtained on an IonSpec 4.7 Tesla FTMS instrument (Tesla, Lake Forest, CA, USA).
2.2 Synthesis of 3, 5′-dinitrohonokiol (2)
To a mixture of 1 (532.7 mg, 2 mmol), glacial acetic acid (10 mL) and CH2Cl2 (10 mL), a solution of concentrated nitric acid (4.2 mmol) in CH2Cl2 (20 mL) was added dropwise below 0 °C. After addition, the solution was allowed to warm slowly from 0 °C to room temperature. The reaction progress was monitored by TLC analysis. When the reaction was complete after 4 h, the mixture was poured into ice water and neutralized by addition of saturated aqueous NaHCO3. The mixture was then extracted with CH2Cl2 (3×50 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated in vacuo and purified by silica gel column chromatography eluting with petroleum ether/ethyl acetate (6:1, v/v) to give compound 2 (235 mg) in 33% yield as orange lamellar solid. – m.p. 80–82 °C. –1H NMR (500 MHz, CDCl3): δ 11.05 (s, 1H, -OH), 11.03 (s, 1H, -OH), 8.19 (s, 1H), 7.97 (s, 1H), 7.70 (s, 1H), 7.45 (s, 1H), 5.93–6.02 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 5.13–5.19 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 3.55 (s, 2H, -CH2CH), 3.42 (s, 2H, -CH2CH). –HRMS m/z calcd for C18H16N2O6 ([M]+), 356.1003, found, 356.1003.
2.3 Synthesis of 3, 5′-diaminohonokiol (3)
SnCl2.2H2O (3384.5 mg, 15 mmol) was added to a solution of 2 (356.8 mg, 1 mmol) in ethanol (20 mL) and the reaction mixture was then stirred at room temperature. When the reaction was complete after 36 h, the mixture was concentrated under reduced pressure, diluted with EtOAc (50 mL) and neutralized by addition of saturated aqueous NaHCO3. The mixture was then extracted with EtOAc (3×50 mL). The combined organic phase was dried over anhydrous Na2SO4, concentrated in vacuo and purified by silica gel column chromatography eluting with petroleum ether/ethyl acetate (2:1, v/v) to give compound 3 (151 mg) in 51% yield as white powder. – m.p. 142–144 °C. –1H NMR (400 MHz, DMSO-d6): δ 6.62 (d, J=2.4 Hz, 1H), 6.42 (d, J=2.0 Hz, 1H), 6.37 (d, J=2.0 Hz, 1H), 6.17 (d, J=2.4 Hz, 1H), 5.83–5.98 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 4.95–5.08 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.58 (s, 4H, 2×-NH2), 3.29 (d, J=6.8 Hz, 2H, -CH2CH), 3.14 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C18H21N2O2 ([M+H]+), 297.1599, found, 297.1598.
2.4 General procedure for synthesis of arylmethylamine derivatives of honokiol (5a-m)
To a stirred solution of the corresponding aromatic aldehydes (0.59 mmol) in absolute ethanol (5 mL) at 0 °C, a solution of 3 (0.27 mmol) in absolute ethanol (5 mL) was added slowly. After addition, the solution was allowed to warm slowly from 0 °C to room temperature, and the reaction progress was monitored by TLC analysis. When the reaction was complete after 4–12 h, the mixture was cooled at 0 °C, and an excess of NaBH4 (2.16 mmol) was added. After addition, the solution was allowed to warm slowly from 0 °C to room temperature and the reaction progress was checked by TLC analysis. When the reaction was complete after 5–8 h, the solvent of the mixture was removed by rotary evaporation to give a solid, to which 10 mL water were added. The pH value of the mixture was adjusted to >7 by saturated aqueous NaHCO3. Then the mixture was extracted with EtOAc (3×30 mL). Finally, the combined organic phase was dried over anhydrous Na2SO4, concentrated in vacuo, and purified by PTLC to afford 5a-m in 27%–86% yield.
5a: – Yield 40%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.39–7.41 (m, 2H), 7.31–7.37 (m, 6H), 7.26–7.28 (m, 2H), 6.62 (s, 1H), 6.56 (s, 1H), 6.43 (s, 1H), 6.39 (s, 1H), 5.87–6.08 (m, 2H, 5-CH=CH2 and 33′-CH=CH2), 5.22–5.30 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.98–5.07 (m, 2H, 2×-NH), 4.50–4.53 (m, 2H, 2×-OH), 4.36 (s, 2H, -NHCH2), 4.33 (s, 2H, -NHCH2), 3.41 (d, J=6.0 Hz, 2H, -CH2CH), 3.25 (d, J=6.4 Hz, 2H, -CH2CH). –HRMS m/z calcd for C32H33N2O2 ([M+H]+), 477.2540, found, 477.2537.
5b: – Yield 58%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.33 (s, 1H), 7.31 (s, 1H), 7.29 (s, 1H), 7.26 (s, 1H), 6.85–6.89 (m, 4H), 6.63 (d, J=1.6 Hz, 1H), 6.55 (s, 1H), 6.45 (s, 1H), 6.40 (s, 1H), 5.89–6.07 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 5.21–5.29 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.99–5.09 (m, 4H, 2×-NH and 2×-OH), 4.28 (s, 2H, -NHCH2), 4.25 (s, 2H, -NHCH2), 3.80 (s, 3H, -OCH3), 3.78 (s, 3H, -OCH3), 3.40 (d, J=6.4 Hz, 2H, -CH2CH), 3.26 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C34H37N2O4 ([M+H]+), 537.2759, found, 537.2748.
5c: – Yield 50%. Colorless viscous liquid. 1H NMR (400 MHz, CDCl3) δ: 7.30 (s, 1H), 7.28 (s, 1H), 7.26 (s, 1H), 7.24 (s, 1H), 7.13–7.16 (m, 4H), 6.63 (d, J=1.6 Hz, 1H), 6.56 (s, 1H), 6.45 (s, 1H), 6.40 (s, 1H), 5.89–6.08 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 5.16–5.29 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.96–5.14 (m, 4H, 2×-NH and 2×-OH), 4.31 (s, 2H, -NHCH2), 4.28 (s, 2H, -NHCH2), 3.41 (d, J=6.4 Hz, 2H, -CH2CH), 3.26 (d, J=6.4 Hz, 2H, -CH2CH), 2.34 (s, 3H, -CH3), 2.33 (s, 3H, -CH3). –HRMS m/z calcd for C34H37N2O2 ([M+H]+), 505.2855, found, 505.2850.
5d: – Yield 42%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 8.01 (d, J=1.6 Hz, 2H), 7.99 (d, J=2.0 Hz, 2H), 7.46 (s, 1H), 7.44 (s, 1H), 7.43 (s, 1H), 7.41 (s, 1H), 6.56 (s, 1H), 6.53 (s, 1H), 6.34 (s, 1H), 6.32 (s, 1H), 5.99–6.09 (m, 1H, -CH=CH2), 5.82–5.92 (m, 1H, -CH=CH2), 5.23–5.31 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.95–5.15 (m, 4H, 2×-NH and 2×-OH), 4.42 (s, 4H, 2×-NHCH2), 4.35–4.37 (m, 4H, 2×-COOCH2CH3), 3.42 (d, J=6.0 Hz, 2H, -CH2CH), 3.19 (d, J=6.8 Hz, 2H, -CH2CH), 1.36–1.40 (m, 6H, 2×-COOCH2CH3). –HRMS m/z calcd for C38H41N2O6 ([M+H]+), 621.2961, found, 621.2959.
5e: – Yield 86%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.42–7.45 (m, 1H), 7.36–7.39 (m, 3H), 7.19–7.22 (m, 4H), 6.58 (s, 1H), 6.57 (s, 1H), 6.37 (s, 1H), 6.34 (s, 1H), 5.99–6.09 (m, 1H, -CH=CH2), 5.84–5.94 (m, 1H, -CH=CH2), 5.23–5.42 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.89–5.13 (m, 4H, 2×-NH and 2×-OH), 4.46 (s, 2H, -NHCH2), 4.44 (s, 2H, -NHCH2), 3.42 (d, J=6.4 Hz, 2H, -CH2CH), 3.22 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C32H31N2O2Cl2 ([M+H]+), 545.1755, found, 545.1757.
5f: – Yield 55%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.29–7.33 (m, 8H), 6.55 (s, 1H), 6.54 (s, 1H), 6.37 (s, 1H), 6.36 (s, 1H), 5.98–6.08 (m, 1H, -CH=CH2), 5.85–5.95 (m, 1H, -CH=CH2), 5.18–5.31 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.98–5.11 (m, 4H, 2×-NH and 2×-OH), 4.33 (s, 2H, -NHCH2), 4.31 (s, 2H, -NHCH2), 3.41 (d, J=6.0 Hz, 2H, -CH2CH), 3.48 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C32H31N2O2Cl2 ([M+H]+), 545.1755, found, 545.1757.
5g: – Yield 62%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.45–7.46 (m, 2H), 7.43–7.44 (m, 2H), 7.25–7.27 (m, 2H), 7.21–7.24 (m, 2H), 6.55 (s, 1H), 6.54 (s, 1H), 6.36 (s, 1H), 6.35 (s, 1H), 5.98–6.08 (m, 1H, -CH=CH2), 5.84–5.95 (m, 1H, -CH=CH2), 5.14–5.31 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.94–5.11 (m, 4H, 2×-NH and 2×-OH), 4.31 (s, 2H, -NHCH2), 4.30 (s, 2H, -NHCH2), 3.41 (d, J=6.4 Hz, 2H, -CH2CH), 3.22 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C32H31N2O2Br2 ([M+H]+), 633.0749, found, 633.0747.
5h: – Yield 53%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.30–7.37 (m, 4H), 6.99–7.04 (m, 4H), 6.57 (s, 1H), 6.56 (s, 1H), 6.39 (s, 2H), 5.98–6.08 (m, 1H, -CH=CH2), 5.86–5.96 (m, 1H, -CH=CH2), 5.21–5.42 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.98–5.10 (m, 4H, 2×-NH and 2×-OH), 4.32 (s, 2H, -NHCH2), 4.30 (s, 2H, -NHCH2), 3.41 (d, J=6.4 Hz, 2H, -CH2CH), 3.24 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C32H31N2O2F2 ([M+H]+), 513.2357, found, 513.2348.
5i: – Yield 63%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.89–7.91 (m, 2H), 7.82–7.84 (m, 2H), 7.64–7.68 (m, 1H), 7.47–7.54 (m, 4H), 7.41–7.45 (m, 4H), 7.19–7.23 (m, 3H), 7.05–7.11 (m, 2H), 6.54 (d, J=1.6 Hz, 1H), 6.33 (d, J=1.6 Hz, 1H), 6.22 (s, 2H), 5.98–6.06 (m, 1H, -CH=CH2), 5.84–5.94 (m, 1H, -CH=CH2), 5.22–5.30 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.96–5.05 (m, 4H, 2×-NH and 2×-OH), 4.21 (s, 2H, -NHCH2), 4.14 (s, 2H, -NHCH2), 3.41 (d, J=6.4 Hz, 2H, -CH2CH), 3.21 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C44H41N2O8S2 ([M+H]+), 789.2312, found, 789.2299.
5j: – Yield 27%. White solid. –m.p. 58–60 °C. –1H NMR (400 MHz, CDCl3): δ 7.80–7.84 (m, 4H), 7.60–7.68 (m, 2H), 7.45–7.53 (m, 5H), 7.28–7.30 (m, 3H), 6.91–6.93 (m, 4H), 6.54 (d, J=1.6 Hz, 1H), 6.48 (d, J=1.6 Hz, 1H), 6.37 (d, J=1.6 Hz, 1H), 6.31 (d, J=2.0 Hz, 1H), 5.98–6.09 (m, 1H, -CH=CH2), 5.83–5.93 (m, 1H, -CH=CH2), 5.24–5.31 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.96–5.04 (m, 4H, 2×-NH and 2×-OH), 4.32 (s, 4H, 2×-NHCH2), 3.42 (d, J=6.4 Hz, 2H, -CH2CH), 3.21 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C44H41N2O8S2 ([M+H]+), 789.2306, found, 789.2299.
5k: – Yield 60%. White lamellar crystal. –m.p. 62–64 °C. –1H NMR (400 MHz, CDCl3): δ 7.84–7.88 (m, 4H), 7.58–7.66 (m, 2H), 7.43–7.52 (m, 4H), 7.08–7.10 (m, 2H), 6.83–6.91 (m, 4H), 6.55 (d, J=1.6 Hz, 1H), 6.51 (d, J=1.6 Hz, 1H), 6.39 (d, J=1.6 Hz, 1H), 6.35 (d, J=1.6 Hz, 1H), 5.99–6.09 (m, 1H, -CH=CH2), 5.84–5.94 (m, 1H, -CH=CH2), 5.24–5.31 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.97–5.06 (m, 4H, 2×-NH and 2×-OH), 4.29 (s, 4H, 2×-NHCH2), 3.48 (s, 6H, 2×-OCH3), 3.42 (d, J=6.4 Hz, 2H, -CH2CH), 3.23 (d, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C46H45N2O10S2 ([M+H]+), 849.2539, found, 849.2510.
5l: – Yield 80%. Colorless viscous liquid. –1H NMR (400 MHz, CDCl3): δ 7.37–7.37 (m, 1H), 7.35–7.36 (m, 1H), 6.70 (d, J=2.0 Hz, 1H), 6.58 (d, J=1.6 Hz, 1H), 6.51 (d, J=1.6 Hz, 1H), 6.44 (d, J=1.6 Hz, 1H), 6.30–6.33 (m, 2H), 6.26 (d, J=3.2 Hz, 1H), 6.23 (d, J=3.2 Hz, 1H), 5.91–6.07 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 5.15–5.40 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 4.91–5.11 (m, 4H, 2×-NH and 2×-OH), 4.36 (s, 2H, -NHCH2), 4.31 (s, 2H, -NHCH2), 3.40 (d, J=6.0 Hz, 2H, -CH2CH), 3.50 (s, J=6.8 Hz, 2H, -CH2CH). –HRMS m/z calcd for C28H29N2O4 ([M+H]+), 457.2115, found, 457.2112.
5m: – Yield 69%. Colorless viscous liquid. –1H NMR (500 MHz, CDCl3): δ 7.19–7.21 (m, 2H), 7.03–7.04 (m, 1H), 6.99–7.00 (m, 1H), 6.94–6.97 (m, 2H), 6.69 (d, J=1.5 Hz, 2H), 6.58 (d, J=2.0 Hz, 1H), 6.51 (d, J=1.5 Hz, 1H), 6.44 (d, J=1.5 Hz, 1H), 5.92–6.06 (m, 2H, 5-CH=CH2 and 3′-CH=CH2), 5.21–5.28 (m, 4H, 5-CH=CH2 and 3′-CH=CH2), 5.00–5.15 (m, 4H, 2×-NH and 2×-OH), 4.54 (s, 2H, -NHCH2), 4.51 (s, 2H, -NHCH2), 3.40 (d, J=6.0 Hz, 2H, -CH2CH), 3.28 (d, J=7.0 Hz, 2H, -CH2CH). –HRMS m/z calcd for C28H29N2O2S2 ([M+H]+), 489.1671, found, 489.1665.
2.5 Insecticidal assay of 1–3 and 5a-m
The insecticidal activity of compounds 1–3 and 5a-m against the pre-third-instar larvae of M. separata was assessed by the leaf-dipping method as described previously [10]. For each compound, 30 larvae (ten larvae per group) were used. Acetone solutions of 1–3, 5a-m and toosendanin (a positive control, supplied by Research and Development Center of Biorational Pesticide, Northwest A and F University, Shaanxi Province, China) were prepared at the concentration of 1 mg/mL. Fresh wheat leaves were dipped into the corresponding solution for 3 s, then taken out and dried in a room at room temperature. Leaves treated with acetone alone were used as a control group. Several treated leaves were kept in each dish, to which ten larvae were added. When the leaves in a dish had been consumed, appropriately treated fresh ones were added. After 48 h, untreated fresh leaves were added to all dishes until the adults emerged. The experiment was carried out at 25±2 °C and a relative humidity of 65%–80% in a 12/12 h (light/dark) photoperiod. The insecticidal activity of the tested compounds against the pre-third-instar larvae of M. separata was calculated by the following formula:
where T is the mortality rate in the group treated with the tested compounds, and C is the mortality rate in the blank control group (T and C were all expressed as the percentage).
3 Results and discussion
As illustrated in Scheme 1, first, 3, 5′-dinitrohonokiol (2) was prepared by nitration of 1 with concentrated nitric acid [12]. Second, 3, 5′-diaminohonokiol (3) was obtained by reduction of 2 with stannous chloride. Finally, compound 3 reacted with aromatic aldehydes to afford intermediates 4a-m, which directly further reacted with NaBH4 to smoothly give the target compounds 5a-m in 27%–86% yields. The structures of all target compounds were thoroughly characterized by 1H NMR, HRMS, and mp.
The insecticidal activity of compounds 1–3 and 5a-m against the pre-third-instar larvae of M. separata was tested at the concentration of 1 mg/mL by the leaf dipping method. Toosendanin, isolated from Melia azedarach, was used as a positive control at 1 mg/mL. Leaves treated with acetone alone were used as a blank control group. As shown in Table 1, the corresponding mortality rates were low after 10 days, but then greatly increased up to 20 days and continued to increase during the next 10 days. Obviously, these honokiol derivatives, in a time-dependent manner, different from other conventional neurotoxic insecticides such as organophosphates, carbamates, and pyrethroids, exhibited delayed insecticidal activity. For example, the corrected mortality rate of 5b against M. separata after 10 days was only 3.3%, after 20 days it had increased to 34.5%, and after 36 days to 55.2%. Meanwhile, the symptoms of M. separata treated by the tested compounds were also observed in the same way as in our previous works for podophyllotoxin derivatives [9–11]. The pupation of the larvae and the adult emergence of M. separata were inhibited by these compounds, therefore, the stage from the larvae to adulthood of M. separata was prolonged as compared with the control group. Moreover, some larvae died with the slim and wrinkled bodies during the larval period; during the stage of pupation, many larvae of the treated groups moulted to malformed pupae; some malformed moths with imperfect wings also appeared during the adult emergence period.
Insecticidal activity of compounds 1–3 and 5a-m against Mythimna separata on leaves treated with a concentration of 1 mg/mL.
| Compounds | Corrected mortality rate (%) | ||
|---|---|---|---|
| 10 days | 20 days | 36 days | |
| 1 | 3.3±3.3 | 10.3±3.3 | 27.6±0 |
| 2 | 3.3±3.3 | 27.6±5.8 | 41.4±3.3 |
| 3 | 3.3±3.3 | 44.8±3.3 | 51.7±3.3 |
| 5a | 6.7±3.3 | 31.0±6.7 | 48.3±5.8 |
| 5b | 3.3±3.3 | 34.5±3.3 | 55.2±3.3 |
| 5c | 3.3±3.3 | 13.8±3.3 | 27.6±5.8 |
| 5d | 3.3±3.3 | 20.7±3.3 | 37.9±0 |
| 5e | 6.7±3.3 | 41.4±6.7 | 58.6±0 |
| 5f | 6.7±3.3 | 27.6±0 | 44.8±3.3 |
| 5g | 0±0 | 10.3±3.3 | 20.7±3.3 |
| 5h | 6.7±3.3 | 41.4±3.3 | 51.7±3.3 |
| 5i | 3.3±3.3 | 10.3±6.7 | 13.8±3.3 |
| 5j | 0±0 | 27.6±5.8 | 34.5±3.3 |
| 5k | 0±0 | 24.1±6.7 | 48.3±0 |
| 5l | 6.7±3.3 | 17.2±5.8 | 41.4±3.3 |
| 5m | 0±0 | 20.7±3.3 | 24.1±3.3 |
| Toosendanin | 10.0±5.8 | 24.1±3.3 | 48.3±5.8 |
| Blank control | 0±0 | 3.3±3.3 | 3.3±3.3 |
Among the derivatives, compounds 3, 5a, 5b, 5e, 5h, and 5k, exhibited insecticidal activity equal to or higher than that of toosendanin. Introduction of nitro or amino groups on the phenyl ring of 1 provided the more potent derivatives 2 and 3 as compared to 1. To the arylmethylamine derivatives of honokiol (5a-m), introduction of the methoxy group at the C4 position on the phenyl ring of 5a led to the more promising compound 5b. Introduction of a chlorine atom at the C2 position, or a fluorine atom at the C4 position of the phenyl ring of 5a gave the potent compounds 5e and 5h. However, when a chlorine atom was introduced at the C4 position on the phenyl ring of 5a, the insecticidal activity of the corresponding compound 5f was less than that of 5e, which contains a chlorine atom at the C2 position. All in all, when the phenyl ring of 5a was substituted by a fur-2-yl or thien-2-yl moiety, the insecticidal activity of the corresponding compounds 5l and 5m was not improved as compared to 5a.
In conclusion, we have prepared a series of novel arylmethylamine derivatives of honokiol. Their insecticidal activity was tested against the pre-third-instar larvae of M. separata in vivo. Compounds 3,5a, 5b, 5e, 5h and 5k exhibited equal or higher insecticidal activity than toosendanin (a positive control). This will encourage us to further exploit new honokiol derivatives as insecticidal agents in the future.
Acknowledgments
This work has been supported by Special Funds of Central Colleges Basic Scientific Research Operating Expenses (YQ2013008), Northwest A and F University, Yangling, P.R. China.
References
1. Fukuyama Y, Otoshi Y, Miyoshi K, Nakamura K, Kodama M, Nagasawa M, et al. Neurotrophic sesquiterpene-neolignans from Magnolia obovata: structure and neurotrophic activity. Tetrahedron 1992;48:377–92.10.1016/S0040-4020(01)89002-5Search in Google Scholar
2. Kong ZL, Tzeng SC, Liu YC. Cytotoxic neolignans: an SAR study. Bioorg Med Chem Lett 2005;15:163–6.10.1016/j.bmcl.2004.10.011Search in Google Scholar PubMed
3. Maruyama Y, Kuribara H, Morita M, Yuzurihara M, Weintraub ST. Identification of magnolol and honokiol as anxiolytic agents in extracts of Saiboku-to, an oriental herbal medicine. J Nat Prod 1998;61:135–8.10.1021/np9702446Search in Google Scholar PubMed
4. Tripathi S, Chan MH, Chen CP. An expedient synthesis of honokiol and its analogues as potential neuropreventive agents. Bioorg Med Chem Lett 2012;22:216–21.10.1016/j.bmcl.2011.11.030Search in Google Scholar PubMed
5. Porte LF, Santin SM, Chiavelli LU, Silva CC, Faria TJ, Faria RT, et al. Bioguided identification of antifungal and antiproliferative compounds from the Brazilian orchid Miltonia flavescens Lindl. Z Naturforsch 2014;69c:46–52.10.5560/znc.2012-0192Search in Google Scholar PubMed
6. Bai PH, Bai CQ, Liu QZ, Du SS, Liu ZL. Nematicidal activity of the essential oil of Rhododendron anthopogonoides aerial parts and its constituent compounds against Meloidogyne incognita. Z Naturforsch 2013;68c:307–12.10.1515/znc-2013-7-808Search in Google Scholar
7. Acikgoz B, Karalti I, Ersoz M, Coskun ZM, Cobanoglu G, Sesal C, et al. Screening of antimicrobial activity and cytotoxic effects of two Cladonia species. Z Naturforsch 2013;68c:191–7.10.5560/ZNC.2013.68c0191Search in Google Scholar
8. Song HJ, Liu YX, Xiong LX, Li YQ, Yang N, Wang QM, et al. Design, synthesis, and insecticidal activity of novel pyrazole derivatives containing α-hydroxymethyl-N-benzyl carboxamide, α-chloromethyl-N-benzyl carboxamide, and 4,5-dihydrooxazole moieties. J Agric Food Chem 2012;60:1470–9.10.1021/jf204778vSearch in Google Scholar PubMed
9. Xu H, Wang QT, Guo Y. Stereoselective synthesis of 4α-alkyloxy-2-α/β-bromopodophyllotoxin derivatives as insecticidal agents. Chem Eur J 2011;17:8299–303.10.1002/chem.201100855Search in Google Scholar PubMed
10. Wang Y, Shao YH, Wang YY, Fan LL, Yu X, Zhi XY, et al. Synthesis and quantitative structure-activity relationship (QSAR) study of novel isoxazoline and oxime derivatives of podophyllotoxin as insecticidal agents. J Agric Food Chem 2012;60:8435–43.10.1021/jf303069vSearch in Google Scholar PubMed
11. Che ZP, Yu X, Zhi XY, Fan LL, Yao XJ, Xu H, et al. Synthesis of novel 4α-(acyloxy)-2′(2′,6′)-(di)halogenopodophyllotoxin derivatives as insecticidal agents. J Agric Food Chem 2013;61:8148–55.10.1021/jf4025079Search in Google Scholar PubMed
12. Taferner B, Schuehly W, Huefner A, Baburin I, Wiesner K, Ecker GF, et al. Modulation of GABAA-receptors by honokiol and derivatives: subtype selectivity and structure–activity relationship. J Med Chem 2011;54:5349–61.10.1021/jm200186nSearch in Google Scholar PubMed
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn
Articles in the same Issue
- Frontmatter
- Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages
- Characterization of a mineral coating of the plant Dyerophytum indicum
- Semisynthesis and insecticidal activity of arylmethylamine derivatives of the neolignan honokiol against Mythimna separata Walker
- Dynamics of alkylresorcinols during rye caryopsis germination and early seedling growth
- New nitrogenous compounds from a Red Sea sponge from the Gulf of Aqaba
- Synthesis and antiproliferative evaluation of novel 5-(4-methylpiperazin-1-yl)-2-phenyl- 1H-benzimidazole derivatives
- Differential cytotoxic activity of the petroleum ether extract and its furanosesquiterpenoid constituents from Commiphora molmol resin
- A novel C25 sterol peroxide from the endophytic fungus Phoma sp. EA-122
- Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale
- Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn

