Abstract
Objective
Deltamethrin, synthetic pyrethroid, is a suspected endocrine disruptor contaminating ecosystems as toxic pollutant via agricultural activities and vector controls. The objective of the study is to determine the possible effects on human by evaluating antioxidant enzyme levels and total antioxidant status (TAS) of invertebrate model organism crayfish exposure to sublethal deltamethrin.
Materials and methods
Crayfish were exposed to 0.05 μg/L deltamethrin for 48 h and 7 days. Hemolymph samples were taken for TAS and total haemocyte counts (THCs). Gill, hepatopancreas and muscle tissues were examined for superoxide dismutase (SOD), glutathion peroxidase (GPx) and catalase (CAT) enzyme activities.
Results
THCs were decreased (p < 0.05) and hemolymph TAS levels were increased according to control groups. Gill SOD, CAT and GPx enzyme activities were significantly rised. Hepatopancreas SOD activities unchanged. Hepatopancreas CAT activities were increased significantly after 48 h (p < 0.05), but returned back to controls after 7 days. Hepatopancreas GPx and muscle SOD activities were rised (p < 0.05), while muscle CAT and GPx values did not affect from deltametrin.
Conclusion
Deterioration of ecosystems are directly affect the humans. The toxic effects of deltamethrin for different stages of organisms on the food web will provide basic data to understand and estimate the effects on the human beings.
Öz
Amaç
Sentetik piretroid olan deltametrin tarımsal aktivite ve vektör kontrolü sonucu ekosisteme bulaşan ve endokrin bozucu etkisi olduğu şüphelenilen bir toksik kirlticidir. Bu çalışmanın amacı, omurgasız model organizma tatlı su istakozlarının subletal deltametrine maruziyetlerinin antioksidan enzim düzeyleri ve toplam antioksidan durumuna (TAS) etkileri belirlenerek insan üzerindeki olası etkileri değerlendirilecektir.
Gereç ve Yöntem
Tatlı su istakozları 48 saat ve seven gün süreyle 0.05 μg/L deltametrine maruz bırakıldı. TAS ve toplam hemosit sayıları (THS) için hemolenf örnekleri alındı. Solungaç, hepatopankreas ve kas dokuları süperoksit dismutaz (SOD), glutatyon peroksidaz (GPx) ve katalaz (CAT) enzim aktiviteleri açısından incelendi.
Bulgular
Kontrol gruplarına göre THC'ler azalırken (p < 0.05) ve hemolenf TAS düzeyleri arttı. Solungaç SOD, CAT ve GPx enzim aktiviteleri istatistik olarak önemli ölçüde artmıştır (p < 0.05). Hepatopankreas SOD aktiviteleri değişmedi. Hepatopankreas CAT aktiviteleri 48 saat sonra önemli ölçüde artarken (p < 0.05), seven gün sonra kontrol değerlerine geri dönmüştür. Hepatopankreas GPx ve kas SOD aktiviteleri artarken (p < 0.05), kas CAT ve GPx değerleri deltametrinden etkilenmemiştir.
Sonuçlar
Ekosistemlerin bozulması insanları doğrudan etkilemektedir. Besin ağında farklı trofik düzeyde organizmalarda deltametrinin toksik etkileri, insanlar üzerindeki etkilerini anlamak ve tahmin etmek için temel veriler sağlayacaktır.
Introduction
Water bodies are frequently subjected to endocrine disruptors related to anthropogenic activities like agriculture, inducing a contamination by rain water and surface runoff containing various chemicals such as pesticides [1]. Pyrethroids, one of the widely used pesticides, have demonstrated viable representative for organochlorined, organophosphated and carbamate ones owing to being high bio efficiency, biodegradability and relatively less toxic for higher vertebrates [2]. The synthetic pyrethroid, deltamethrin ((S)α-cyano-3-phenoxybenzyl-(1R)-cis-3-(2.2-dibromovinyl)-2,2-imethylcyclopropanecarboxylate) has widely applied for crop protection, control of malaria and other vector borne diseases [3], [4], [5].
The toxic activity of deltamethrin is primarily at the voltage dependent sodium channels of the nerve cell membranes and inducing the neurotoxicity [6]. In addition to neurotoxic effects, the oxidative stress related to deltamethrin also induces toxic effects like other pesticides [7], [8], [9], [10], [11], [12], [13]. Oral application of 5 mg/kg of deltamethrin to mice induced oxidative stress and activated the mitochondrial pathway of apoptosis [14].
Freshwater crayfish, an alternative model organism to vertebrates for the assessment of environmental contaminants as a nontarget species, are important bioindicator organisms for water quality of freshwaters bodies [15]. The narrow-clawed crayfish, Astacus leptodactylus, is an ecologically important species in Eurasian freshwater bodies; has important role at the aquatic food webs in diverse water bodies and evaluating ecotoxicological risks [16].
In their study, although Meng et al. [17] determined motor axon activity and neuromuscular transmission effects of deltamethrin, no data was available about the effects of deltamethrin on the antioxidant enzyme levels and total antioxidant status (TAS) of crayfish. However, the effects of deltamethrin were studied in vertebrate organisms, very limited data were found for invertebrate organisms. The present study aimed to assess the antioxidant enzyme levels (SOD, CAT and GPx) on different vital organs with TAS and total haemocyte counts (THCs) for the haemolymph of crayfish (A. leptodactylus Eschscholtz 1823) after exposure to deltamethrin, a synthetic pyrethroid pesticides, contaminating aquatic environments as toxic contaminant of agricultural activities. The recent study will give an insight to the literature about the antioxidant and oxidant levels of related invertebrate species.
Materials and methods
Experimental organism
The narrow clawed freshwater crayfish (A. leptodactylus Eschscholtz, 1823), invertebrate model organism, was used as representative organism of fresh water ecosystems. Live organisms (N = 128) were obtained from a fisherman during the fishing season. The mean weight and the length of crayfish were 30.57 ± 5.26 g and 10.64 ± 0.48 cm, respectively.
Test chemical
Deltamethrin (China, Changzhou Kangmei Chem. Ind.Co. Ltd.), technical grade (98%) was used in the experiment and obtained from the Hacettepe University Insecticide Test Lab. The stock solution was dissolved in DMSO and stored at +4 °C till the dosing time.
Acclimatisation period and experimental design
Crayfish were acclimatised to laboratory medium for fifteen days before the trials and were fed with fresh trout during that period. Cleaning of the tanks were done twice in a day and the tanks were aerated constantly. All experiments were carried out according to the rules of “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication No. 85–23, revised 1996). After adaptation period, crayfish were stocked to experimental aquariums containing with 100 L of dechlorinated tap water. Animals were selected randomly for control and experiment groups, each stocked as 16 crayfish/8 aquaria's. The water composition was: DO2 6.96 ± 0.10 mg/L; pH = 7.74 ± 0.03; conductivity 0.298 ± 0.01 mS/cm. Sublethal deltamethrin concentration was selected as the 1/10 of 96 h LC50 values, 0.05 μg/L. The stability of deltamethrin concentrations in the aquarium water was provided by renewing every 48 h. Exposure lasted for one week. Haemolymph and tissue samples were taken after 48 h and 7 days.
Haemolymph sampling and analysis
Following the exposure to deltamethrin, haemolymph was taken on the base of the 2nd walking leg under ice anaesthesia using disposable syringe. Hemolymph samples were used for counting the total haemocytes and TAS determinations. THCs were performed with Thoma counting chamber according to Benli [18].
After haemolymph samples were centrifuged at 3,500 rpm, 10 min at 4 °C, TAS (mmol/L) were determined by a Randox commercial kits (Randox Laboratories Ltd., Crumlin, UK). The principle of the assay is incubating 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate) (ABTS) with ferrylmyoglobin radical, formed by the activation of a peroxidase (metmyoglobin) with hydrogen peroxide (H2O2), to produce the radical cation ABTS+ [19].
Tissue sampling and analysis
Crayfish were sacrificed under ice anaesthesia and tissues were dissected; gill, muscle and hepatopancreas tissue samples were taken and stored for antioxidant enzyme analysis at −80 °C.
Superoxide Dismutase Activity (SOD)
SOD activity was done by the method of Sun et al. [20] with the Superoxide Dismutase Assay Kit (Cayman Chemical Company). Tissue samples were homogenized in Tris-HCl containing EDTA (1/10; w/v). SOD activity was inhibited by nitro blue tetrazolium reduction, with xanthine-xanthine oxidase used as a superoxide generator, and 1 international unit (IU) is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. Tissue SOD activity was stated as U/mg protein.
Catalase Activity (CAT)
Catalase activity was determined by Catalase Assay Kit (Cayman Chemical Company). The basis of the method is the reaction of the enzyme with methanol, in the presence of an optimal concentration of H2O2 [21]. Crayfish tissues were homogenized with 0.05 M phosphate buffer at pH 7.0. CAT activity was expressed as units representing the calculated rate constant (K)/mg protein.
Glutathione Peroxidase Activity (GPx)
GPx activity was measured according to the protocol of the Glutathione Peroxidase Assay Kit (Cayman Chemical Company). Tissue samples were homogenized in Tris-HCl containing EDTA (1/10; w/v). Tissue Glutathione Peroxidase Activity results were stated as nanomoles of NADPH oxidized per minute per mg protein, using an extinction coefficient for NADPH at 340 nm.
Two replicates were performed for all tests. Besides protein concentrations for all tissues were performed by the method of Lowry et al. [22].
Statistical analyses
The results are means ± SD. Analysis of data concerning the differences between groups was made using non-parametric Mann Whitney-U Test and Kruskal Wallis with GRAPHPAD InStat 3 Statistic Programme.
Results
Haemolymph analysis
Following exposure to 0.05 μg/L of deltamethrin for 48 h and 7 days, the THCs values were decreased significantly when compared to control groups (p < 0.05). Means (THC/mL) of the control values were 80,989.58 ± 13,390 and 79,427.08 ± 9,909 as control and control (solvent), respectively. The decrease was around 27% after 48 h (50,885.42 ± 3,971.8) and 50% after 7 days (34,895.83 ± 3,125.8) when compared to control groups. There is no significant difference between duration times of exposure groups. The results are shown in Figure 1.
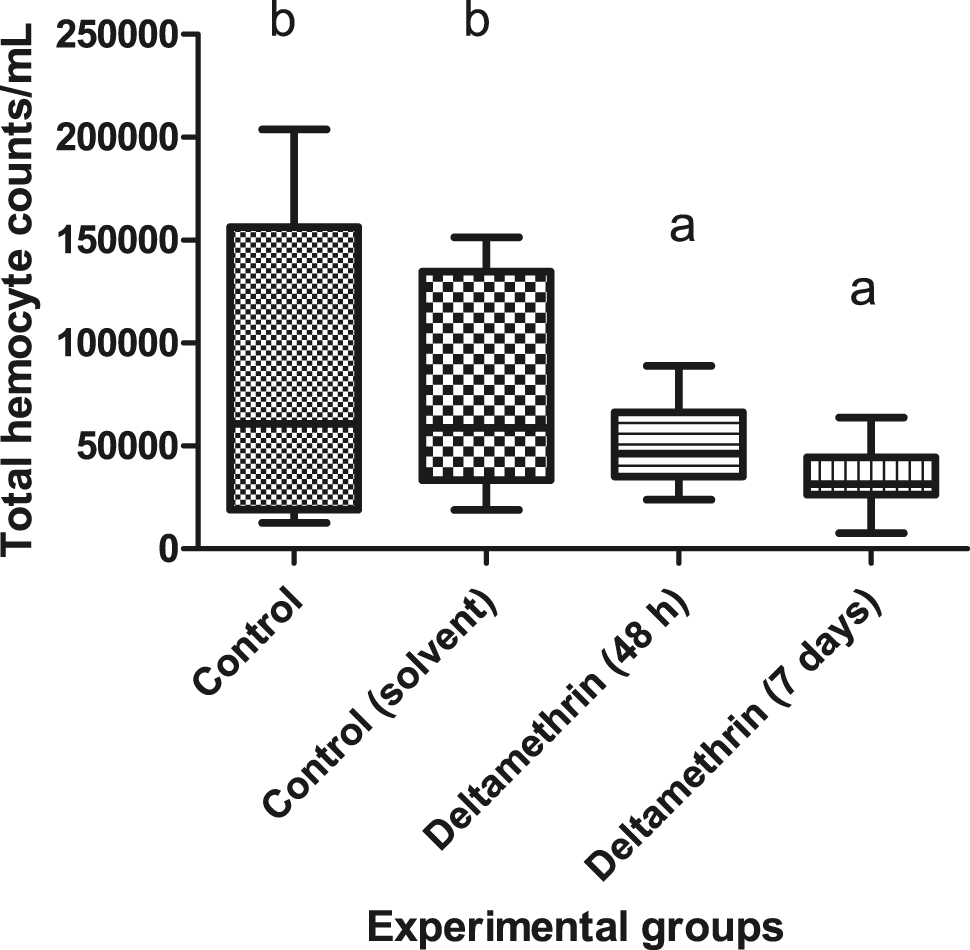
Total haemocyte counts of crayfish after exposure to 0.005 μg/L of deltamethrin for 48 h and 7 days. Means in the figure with different small letters are significantly different (p < 0.05).
Haemolymph TAS levels of deltamethrin exposed crayfish were slightly increased when compared to control groups but not statistically significantly different. The haemolymph TAS results are shown in Figure 2. Means of the haemolymph TAS values for control and control (solvent), 48 h exposed group and 7 days exposed group were 1.50 ± 0.02, 1.52 ± 0.05, 1.60 ± 0.06 and 1.57 ± 0.04 mmol/L, respectively. There was no significant correlation between THCs and TAS (r2 = 0.045).

Haemolymph total antioxidant status of crayfish after exposed to 0.005 μg/L of deltamethrin for 48 h and 7 days.
Antioxidant enzyme activities of tissue samples
SOD enzyme activities of gill tissues were significantly increased when compared to control groups (p < 0.05). Means of gill tissue SOD enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 4.33 ± 0.42, 4.54 ± 0.89, 5.26 ± 1.04 and 5.13 ± 0.45 U/mg protein, respectively. Gill tissue SOD activities were increased about 17%. SOD activities of hepatopancreas tissues were not altered statistically. Means of the hepatopancreas tissue SOD enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 1.50 ± 0.59, 1.48 ± 0.46, 1.74 ± 0.34 and 1.55 ± 0.23 U/mg protein, respectively. SOD activities of muscle tissues were significantly rised (p < 0.05) after exposed to deltamethrin. Means of muscle tissue SOD enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 3.69 ± 0.20, 3.91 ± 0.14, 5.40 ± 0.33 and 4.64 ± 0.27 U/mg protein, respectively. The SOD activity results of gill, hepatopancreas and muscle tissues are depicted in Figure 3.
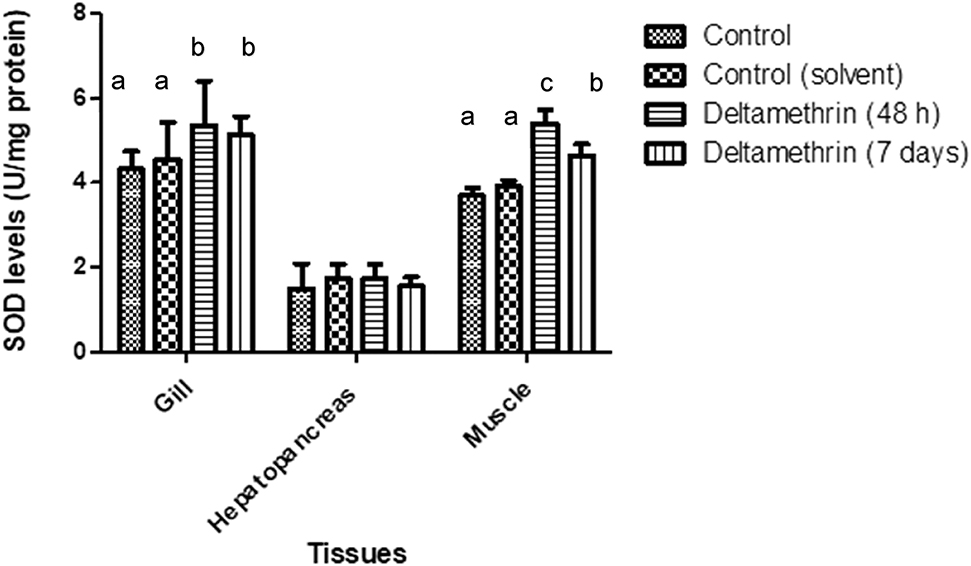
SOD levels of gill, hepatopancreas and muscle tissues of crayfish after exposed to 0.005 μg/L of deltamethrin for 48 h and 7 days. Means in the figure for each tissue with different small letters are significantly different (p < 0.05).
CAT enzyme activities of gill tissues were significantly increased when compared to control groups (p < 0.05). Means of gill tissue CAT enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 0.059 ± 0.024, 0.048 ± 0.001, 0.09 ± 0.003 and 0.08 ± 0.001 mmol/min/mg protein, respectively. CAT activities were increased about 31% after 48 h and 20% after 7 days. Hepatopancreas CAT levels were increased significantly after 48 h (p < 0.05), but returned back to control levels after 7 days. Means of hepatopancreas tissue CAT enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 0.077 ± 0.010, 0.081 ± 0.003, 0.176 ± 0.089 and 0.070 ± 0.001 mmol/min/mg protein, respectively. The CAT activities of muscle tissues were not affected by deltamethrin exposure. Means of muscle tissue CAT enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 0.021 ± 0.05, 0.022 ± 0.01, 0.020 ± 0.004 and 0.019 ± 0.040 mmol/min/mg protein, respectively. The CAT activity results of gill, hepatopancreas and muscle tissues are shown in Figure 4.
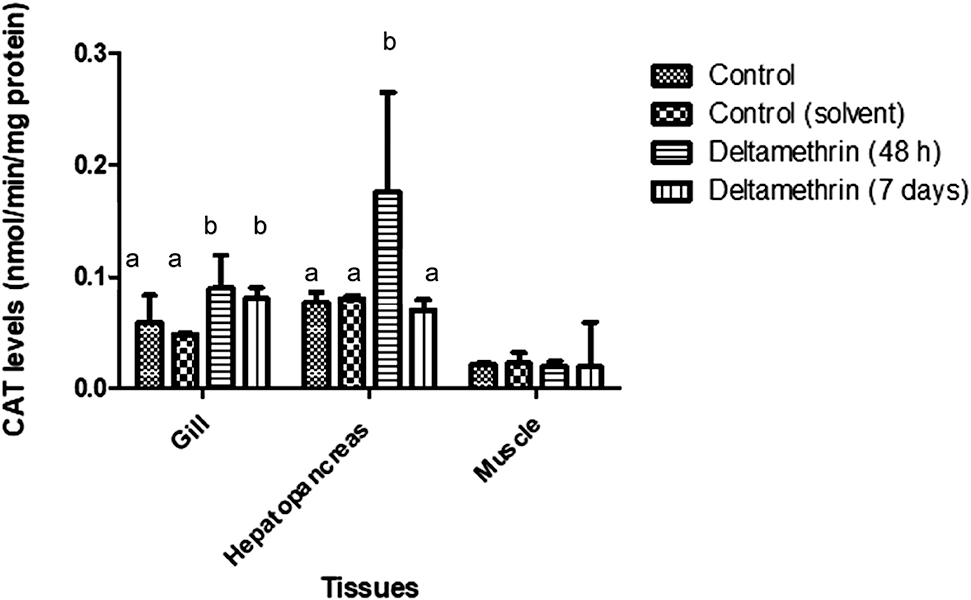
CAT levels of gill, hepatopancreas and muscle tissues of crayfish after exposed to 0.005 μg/L of deltamethrin for 48 h and 7 days. Means in the figure for each tissue with different small letters are significantly different (p < 0.05).
GPx enzyme activities of gill tissues were significantly increased when compared to control groups (p < 0.05). Means of gill tissue GPx enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 1.136 ± 0.12, 1.120 ± 0.02, 1.48 ± 0.158 and 1.831 ± 0.03 mmol/min/mg protein, respectively. GPx activities were increased about 25% after 48 h and 38% after 7 days. Means of hepatopancreas tissue GPx enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 0.382 ± 0.05, 0.401 ± 0.01, 0.530 ± 0.193 and 0.501 ± 0.039 mmol/min/mg protein, respectively. Hepatopancreas GPx activities were increased significantly after the exposure to deltamethrin during the both exposure period (p < 0.05). Means of muscle tissue GPx enzyme activities for control and control (solvent), 48 h exposed group and 7 days exposed group were 0.416 ± 0.05, 0.402 ± 0.20, 0.424 ± 0.052 and 0.432 ± 0.055 mmol/min/mg protein, respectively. Muscle GPx activities were not altered after deltamethrin exposure. The GPx activity results of gill, hepatopancreas and muscle tissues are shown in Figure 5.
Gill tissues SOD, CAT and GPx activities, hepatopancreas CAT and GPx activities and muscle SOD activities increased significantly compared to control groups (p < 0.05), while hepatopancreas SOD activities and muscle CAT and GPx activities were not altered significantly.
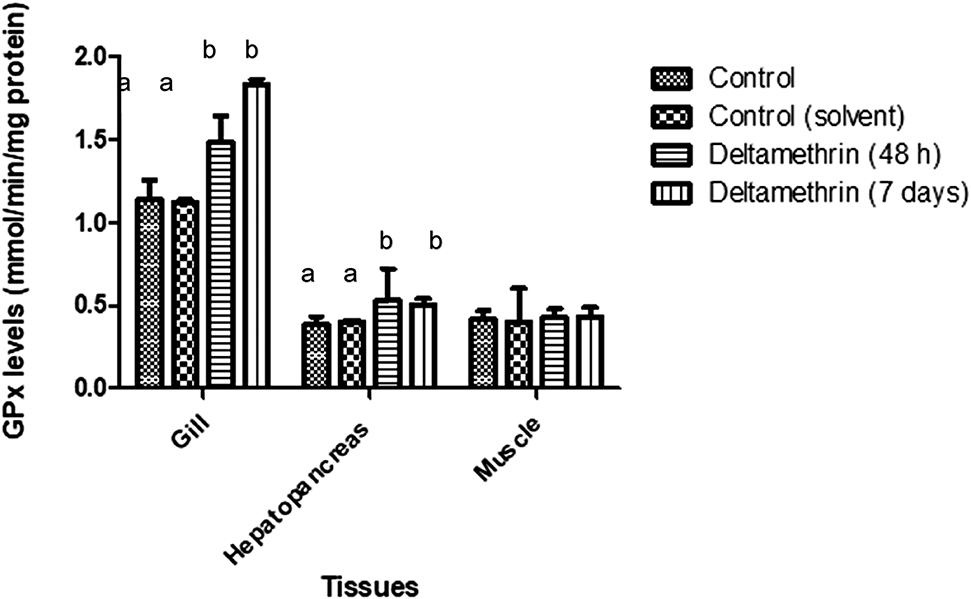
GPx levels of gill, hepatopancreas and muscle tissues of crayfish after exposed to 0.005 μg/L of deltamethrin for 48 h and 7 days. Means in the figure for each tissue with different small letters are significantly different (p < 0.05).
Discussion
The determination of enzyme activities in aquatic animals are extensively evaluated for monitoring the existence of the contaminants in aquatic ecosystems and sensitive biochemical bioindicators before hazardous effects occur on the food web through humans. The SOD, CAT and GPx activities provide a primary line of defence under stress [23], [24]. In recent study, haemocyte numbers were evaluated as stress marker for crayfish and showed that the total number of haemocytes in crayfish, A. leptodactylus significantly decreased following the exposure to 0.05 μg/L of deltamethrin for 48 h and 7 days.
Decreases in THCs are normally related to several environmental contaminants and stress factors for different crustaceans: Jussila et al. [25], Yildiz and Benli [26] and Smith and Johnston [27], reported significant decline in THCs in shrimp, Crangon crangon following treatment to Polychlorinated biphenyl. Qin et al. [28]; determined decrease at the THCs of freshwater crab Sinopotamon henanense following exposure to 58 and 116 mg/L cadmium for 96 h.
TAS is an overall indicator of antioxidant status by representing the enzyme and nonenzyme activities of original antioxidants in the body [29]. As TAS values increase, the antioxidant defence against free radical reaction and reactive O2 intermediates rises [30]. Detection of TAS in aquatic invertebrates have not fully reported in the open literature. So, the results also important and original as the first report about this bioindicator in invertebrate model organisms although we had increased levels for TAS evaluation but not statistically important. In addition, plasma TAS levels have been evaluated as an indicator of stress factors and immune responses at aquatic vertebrates [31], [32], [33].
Detection of SOD activity has been widely used as a bioindicator in fish related to health enhancement, monitoring of pollution stress like pesticides, disease indication, and thermal or osmotic stress. However, studies of SOD with crustaceans are limited [34]. In our study, gill and muscle tissues for SOD enzyme activities were significantly increased, while hepatopancreas SOD activities did not alter. SOD detoxifies the superoxide anion (O2–) forming hydrogen peroxide (H2O2) and the latter, as a ROS, is decomposed by CAT and GPx [35]. Deltamethrin induce toxic effect in aquatic organisms through their high rate of gill absorption, lipophilicity and deficiency in the fish enzyme system to hydrolyse pyrethroids [36]. Borkovic et al. [37] determined lower total SOD activities in the hepatopancreas compared to the gills SOD activities of muscle Spiny cheek crayfish (Orconectes limosus) at the River Danube. Similar to our results, the elevated activity of SOD in the gill tissue was observed in crayfish after exposed to carbaryl for 48 h and 7 days [38].
The CAT and GPX activity in the present study was significantly increased in the gill tissue in both exposure times while no changes were observed for muscle tissue. Similar to our results, CAT activities were changed in crayfish after exposed to 0.144 and 1.44 mg/L prometryne at 11 days [39]. CAT activities were increased in the hepatopancreas tissue than in the gill tissues of crayfish from River Danube [37]. Fernandez et al. [40] and Benli et al. [38] determined high CAT activity after exposure to 48 h carbamate pesticide of carbaryl. In different studies, the increased GPx activity in the hepatopancreas tissue of two different crayfish species after exposed to carbaryl were also observed [38], [40].
As a result, for most ecotoxicologic bioindicators biochemical biomarkers are popular findings, here in our study crayfish as an invertebrate model might be useful candidates for comparative researches with mammals related with biochemical markers. Some of the special features of crustaceans might be important for the interpretation of data from bioindicator studies for developing of ecotoxicological endpoints.
Funding source: Gazi University Scientific Project Unit
Award Identifier / Grant number: 18/2010-01
Acknowledgements
This study was supported by Gazi University Scientific Project Unit with Project no: 18/2010-01.
Conflict of interest: Authors declare no conflict of interest.
Çıkar çatışması: Yazarlar çıkar çatışması bildirmemişlerdir.
References
1. Sumpter JP. Protecting aquatic organisms from chemicals: the harsh realities. Philos Trans R Soc A 2009;367:3877–89. https://doi.org/10.1098/rsta.2009.0106.Suche in Google Scholar
2. Parvez S, Raisuddin S. Copper modulates non-enzymatic antioxidants in the freshwater fish Channa punctata (Bloch) exposed to deltamethrin. Chemosphere 2006;62:1324–32. https://doi.org/10.1016/j.chemosphere.2005.07.025.Suche in Google Scholar
3. Smith TM, Stratton. GW. Effects of synthetic pyrethroid insecticides on nontarget organisms. Res Rev 1986;97:93–119. https://doi.org/10.1007/978-1-4612-4934-4_4.Suche in Google Scholar
4. Rehman H, Ali M, Atif F, Kaur M, Bhatia K, Raisuddin K. The modulatory effect of deltamethrin on antioxidants in mice. Clin Chem Acta 2006;369:61–65. https://doi.org/10.1016/j.cca.2006.01.010.Suche in Google Scholar
5. Yalın S, Çömelekoğlu Ü, Mazmancı B, Ballı E, Eroğlu P, Sögüt F, et al. Effect of Funalia trogii in heart tissue of rats exposed to deltamethrin. Turk J of Biochem 2012;37:239–44. https://doi.org/10.5505/tjb.2012.52724.Suche in Google Scholar
6. Oliveira C, Almeida J, Guilhermino L, Soares AMVM, Gravato C. Acute effects of deltamethrin on swimming velocity and biomarkers of the common prawn Palaemon serratus. Aquat Toxicol 2012;124–125:209–16. https://doi.org/10.1016/j.aquatox.2012.08.010.Suche in Google Scholar
7. Velisek J, Dobsikova R, Svobodova Z, Modra H, Luskova V. Effect of deltamethrin on the biochemical profile of common carp (Cyprinus carpio L.). Bull Environ Contam Toxicol 2006;76:992–8. https://doi.org/10.1007/s00128-006-1016-9.Suche in Google Scholar
8. Sayeed I, Parvez S, Pandey S, Hafeez B, Haque R, Raisuddin S, et al. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish, Channa punctatus Bloch. Ecotox Environ Saf 2003;56:295–301. https://doi.org/10.1016/s0147-6513(03)00009-5.Suche in Google Scholar
9. Banerjee BD, Seth V, Ahmed RS. Pesticide-induced oxidative stress: perspectives and trends. Rev Environ Health 2001;16:1–40. https://doi.org/10.1515/reveh.2001.16.1.1.Suche in Google Scholar PubMed
10. Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaiee A. Pesticides and oxidative stress: a review. Med Sci Monit 2004;10:141–7.Suche in Google Scholar
11. Fiorino E, Sehonova P, lhalova L, Blahova J, Svobodova Z, Faggio C. Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pol Res 2018;25:8542–9. https://doi.org/10.1007/s11356-017-1141-5.Suche in Google Scholar PubMed
12. Burgos-Aceves MA, Cohen A, Smith Y, Faggio C. MicroRNAs and their role on fish oxidative stress during xenobiotic environmental exposures. Ecotox Environ Saf 2018;148:995–1000. https://doi.org/10.1016/j.ecoenv.2017.12.001.Suche in Google Scholar
13. Sehonova P, Svobodova Z, Dolezelova P, Vosmerova P, Faggio C. Effects of waterborne antidepressants on non-target animals living in the aquatic environment: a review. Sci of the Total Environ 2018;631–632:789–94. https://doi.org/10.1016/j.scitotenv.2018.03.076.Suche in Google Scholar
14. Kumar A, Sasmal D, Sharma N. Mechanism of deltamethrin induced thymic and splenic toxicity in mice and its protection by piperine and curcumin: In vivo study. Drug Chem Toxicol 2018;41:33–41. https://doi.org/10.1080/01480545.2017.1286352.Suche in Google Scholar
15. Sepici-Dinçel A, Alparslan N, Benli ACK, Selvi M, Sarıkaya R, Özkul İA, et al. Haemolymph biochemical parameters reference intervals and total haemocyte counts of narrow clawed crayfish Astacus leptodactylus (Eschscholtz, 1823), Ecol Indicat 2013;24:305–9. https://doi.org/10.1016/j.ecolind.2012.07.002.Suche in Google Scholar
16. Köksal G. Astacus leptodactylus in Europe. In: Holdich DM, Lowery R, editors. Freshwater crayfish biology, management and exploitation. London, UK: Croom Helm; 1988:365–400 pp.Suche in Google Scholar
17. Meng L, Meyer PNR, Leary ML, Mohammed YF, Ferber SD, Lin JW, et al. Effects of Deltamethrin on crayfish motor axon activity and neuromuscular transmission. Neurosci Lett 2016;617:32–8. https://doi.org/10.1016/j.neulet.2016.01.061.Suche in Google Scholar
18. Benli ACK. The influence of etofenprox on narrow clawed crayfish (Astacus leptodactylus Eschscholtz, 1823): Acute toxicity and sublethal effects on histology, haemolymph parameters, and total haemocyte counts. Environ Toxicol 2015;30:887–94. https://doi.org/10.1002/tox.21963.Suche in Google Scholar
19. Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 1993;84:407–12. https://doi.org/10.1042/cs0840407.Suche in Google Scholar
20. Sun Y, Oberley LW, Li YA. Simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34:497–500.10.1093/clinchem/34.3.497Suche in Google Scholar
21. Aebi H. Catalase in vitro. Methods Enzymol 1984;105:121–6.10.1016/S0076-6879(84)05016-3Suche in Google Scholar
22. Lowry OH, Rosebrough HJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75.10.1016/S0021-9258(19)52451-6Suche in Google Scholar
23. Schvezov N, Gustavo A, Lovrich M, Romero C. Oxidative stress during re-immersion of the king crab Lithodes santolla (Molina, 1782) (Decapoda: Anomura: Lithodidae) after air exposure. J Crustac Biol 2017;37:195–203. https://doi.org/10.1093/jcbiol/rux004.Suche in Google Scholar
24. Stara A, Kouba A, Velisek J. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii). J Biomed Biotec 2014;1:680131. https://doi.org/10.1155/2014/680131.Suche in Google Scholar
25. Jussila J, Jago J, Tsvetnenko E, Dunstan B, Evans LH. Total and differential haemocyte counts in western rock lobsters (Panulirus cygnus George) under postharvest stress. Mar Freshwater Res 1997;48:863–7.10.1071/MF97216Suche in Google Scholar
26. Yildiz H, Benli ACK. Nitrite toxicity to crayfish, Astacus leptodactylus, the effects of sublethal nitrite exposure on hemolymph nitrite, total hemocyte counts, and hemolymph glucose. Ecotox Environ Saf 2004;59:370–5. https://doi.org/10.1016/j.ecoenv.2003.07.007.Suche in Google Scholar
27. Smith VJ, Johnston PA. Differential haemotoxic effect of PCB congeners in the common shrimp, Crangon crangon. Comp Biochem Physiol C 1992;101:641–9. https://doi.org/10.1016/0742-8413(92)90099-s.Suche in Google Scholar
28. Qin Q, Qin S, Wang L, Lei W. Immune responses and ultrastructural changes of haemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat Toxicol 2012;106–107:140–6. https://doi.org/10.1016/j.aquatox.2011.08.013.Suche in Google Scholar
29. Xiao N, Wang XC, Diao YF, Liu R, Tian KL, Effect of initial fluid resuscitation on subsequent treatment in uncontrolled hemorrhagic shock in rats. Shock 2004;21:276–80. https://doi.org/10.1097/01.shk.0000110622.42625.cb.Suche in Google Scholar
30. Chien YH., Pan CH, Hunter B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture 2003;216:177–91. https://doi.org/10.1016/S0044-8486(02)00056-X.Suche in Google Scholar
31. Selvi M, Cavaş T, Benli ACK, Koçak Memmi B, Cinkılıç N. Sublethal toxicity of esbiothrin relationship with total antioxidant status and in vivo genotoxicity assessment in fish (Cyprinus carpio L., 1758) using the micronucleus test and comet assay. Environ Toxicol 2013;28:644–51. https://doi.org/10.1002/tox.20760.Suche in Google Scholar PubMed
32. Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014;224:164–75. https://doi.org/10.1016/j.cbi.2014.10.016.Suche in Google Scholar PubMed
33. Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf 2018;162:147–59. https://doi.org/10.1016/j.ecoenv.2018.06.070.Suche in Google Scholar
34. Holmblad T, Söderhäll K. Cell adhesion molecule and antioxidation enzymes in a crustacean, possible role in immunity. Aquaculture 1999;172:111–23. https://doi.org/10.1016/S0044-8486(98)00446-3.Suche in Google Scholar
35. Bell KL, Smith VJ. In vitro superoxide production by hyaline cells of the shore crab Carcinus maenas (L.). Dev Comp Immunol 1993;17:211–19. https://doi.org/10.1016/0145-305X(93)90040-W.Suche in Google Scholar
36. Viran R, Erkoç FÜ, Polat H, Koçak O. Investigation of acute toxicity of deltamethrin on guppies Poecilia reticulate. Ecotoxicol Environ Saf 2003;55:82–5. https://doi.org/10.1016/s0147-6513(02)00096-9.Suche in Google Scholar
37. Borkovic SS, Pavlovic SZ, Kovacevic TB, Stajn AS, Petrovic VM, et al. Antioxidant defence enzyme activities in hepatopancreas, gills and muscle of Spiny cheek crayfish (Orconectes limosus) from the River Danube. Comp Biochem Phys C 2008;147:122–8. https://doi.org/10.1016/j.cbpc.2007.08.006.Suche in Google Scholar PubMed
38. Benli ACK, Şahin D, Koçak B, Sepici Dinçel A, Karbarile maruz kalan tatlı su istakozlarında (Astacus leptodactylus Eschscholtz, 1823) antioksidan enzim düzeyleri. Turk J Bioch 2012;37:162–6. https://doi.org/10.5505/tjb.2012.24633.Suche in Google Scholar
39. El-Atti AM, Mahmoud MA, Desouky MA, Mohamadien A, Radwa MS. Effects of titanium dioxide nanoparticles on red swamp crayfish, Procambarus clarkii: Bioaccumulation, oxidative stress and histopathological biomarkers. Egypt J Aquat Res 2019;45:11–18. https://doi.org/10.1016/j.ejar.2019.01.001.Suche in Google Scholar
40. Fernández AV, de Almeida EA, López-Barea J. Biochemical and proteomic effects in Procambarus clarkii after chlorpyrifos or carbaryl exposure under sublethal conditions. Biomarkers 2009;14:299–310. https://doi.org/10.1080/13547500902913211.Suche in Google Scholar PubMed
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note

