The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
-
Anton Tkachenko
, Anatolii Onishchenko
Abstract
Objectives
To assess the state of phospholipid bilayer of red blood cells (RBCs) in rats orally exposed to gadolinium orthovanadate GdVO4:Eu3+ nanoparticles (VNPs) during two weeks using fluorescent probes − ortho-hydroxy derivatives of 2,5-diaryl-1,3-oxazole.
Methods
Steady-state fluorescence spectroscopy: a study by the environment-sensitive fluorescent probes − 2-(2′-OH-phenyl)-5-phenyl-1,3-oxazole (probe O1O) and 2-(2′-OH-phenyl)-phenanthro[9,10]-1,3-oxazole (probe PH7).
Results
No significant changes are detected in the spectra of the fluorescent probes bound to the RBCs from the rats orally exposed to nanoparticles in comparison with the corresponding spectra of the probes bound to the cells from the control group of animals. This indicates that, in case of the rats orally exposed to nanoparticles, no noticeable changes in physico-chemical properties (i.e., in the polarity and the proton-donor ability) are observed in the lipid membranes of RBCs in the region, where the probes locate.
Conclusions
No changes in the physical and chemical properties of the erythrocyte membranes are detected in the region from glycerol backbones of phospholipids to the center of the phospholipid bilayer in the rats orally exposed to VNPs during 2 weeks.
ÖZ
Amaç
Bu çalışmanın amacı iki hafta boyunca 2,5 diaril-1,3-oksazol orto-hidroksi türevleri floresan probları kullanılarak Gadolinyum ortovanada GdVO4’e oral yolla maruz kalan sıçanlarda RBC’lerin fosfolipid çift tabakasının durumunu değerlendirmektir.
Yöntemler
Floresans spektroskopisi: çevreye duyarlı floresan probları−2-(2′-OH-fenil)-5-fenil-1,3-oksazol (prob O10) ve 2-(2′-OH-fenil)-fenantro [9,10]-1,3-oksazol (PH7 probu).
Sonuçlar
Kontrol grubundaki hayvanların hücrelerine bağlı probların karşılık gelen spektrumlarına kıyasla, nanoparçacıklara oral olarak maruz kalan sıçanların RBC’lerine bağlı floresan probların spektrumlarında önemli bir değişiklik tespit edilmemiştir. Bu, nanopartiküllere oral yolla maruz kalan sıçanlarda, probların bulunduğu bölgedeki RBC’lerin lipit membranlarında fiziko-kimyasal özelliklerde (yani polarite ve proton-verici kabiliyetinde) belirgin bir değişiklik gözlenmediğini göstermektedir.
Tartışma
İki hafta boyunca oral olarak VNP’lere maruz kalan sıçanlarda eritrosit membranlarının fiziksel ve kimyasal özelliklerinde, fosfolipitlerin gliserol omurgalarından fosfolipid çift tabakasının merkezine kadar hiçbir değişiklik tespit edilmemiştir.
Introduction
Nanotechnology has revolutionized the field of medicine during the last decade. Nanoparticles (NPs) have found numerous applications in various biomedical fields. It has been reported that NPs can be used as targeted drug delivery systems, biosensors, for gene delivery, bioimaging and magnetic hyperthermia [1], [2], [3], [4].
Nanoparticles that contain rare Earth elements form one of the types of nanostructured materials. The future of their biomedical application seems to be promising due to their stability and low toxicity [5]. In particular, it has been reported that scintillating gadolinium orthovanadate NPs doped with europium ions GdVO4:Eu3+ (VNPs) can be involved in free radical scavenging in vitro [6]. There is some evidence that VNPs have beneficial health-related effects in experimental models. Administration of VNPs normalized sexual behavior and restored fertility in rats with neonatal reproductive dysfunction [7]. However, their practical implementation for therapeutic purposes raises questions on their safety, and the entire emerging field of nanomedicine requires developing safety assessment tests that can be used to predict the toxicity of nanomaterials. One of the mechanisms by which nanoparticles can exert toxicity at the cellular level is disruption of cell and organelle membranes, especially the lysosomal ones [8]. Cell membrane damage affects its permeability and decreases the viability of cells [9]. The loss of lysosomal membrane integrity results in the leakage of the intralysosomal content rich in proteolytic and other hydrolytic enzymes with the significant damage to cells [10]. Furthermore, the toxic effects of NPs can be observed due to their ability to generate reactive oxygen species (ROS) [8], [11]. Reactive oxygen species are involved in free radical oxidation of polyunsaturated fatty acids (PUFAs) abundant in phospholipid bilayer of cell membranes causing lipid peroxidation. Lipid peroxidation, in its turn, increases the membrane permeability making the cells more vulnerable [12]. Thus, the evaluation of cell membrane integrity can be used to assess the toxicity of VNPs. Erythrocytes can be used as target cells for evaluating the toxicity of VNPs in vivo, since they are directly exposed to VNPs released into the bloodstream after their absorption in the intestine. Lipid peroxidation increases the membrane viscosity and, therefore, decreases the membrane fluidity in cells, including red blood cells (RBCs) [13], [14], which can be detected using fluorescent probes.
In this study, the state of phospholipid bilayer of RBCs was estimated by using fluorescent probes O1O (2-(2′-ОН-phenyl)-5-phenyl-1,3-oxazole) and PH7 (2-(2′-OH-phenyl)-phenanthro[9,10]-1,3-oxazole). The mentioned fluorescent probes were chosen because their fluorescent characteristics depend on the physico-chemical properties of their microenvironment: the proton-donor ability, the polarity and viscosity of the microenvironment [15], [16], [17], [18], [19].
It is well known that the excited state proton transfer (ESIPT) reaction takes place when the ortho-hydroxy 2,5-diaryl-1,3-oxazole is in the excited state [15], [16], [17], [18], [19]: hydroxyl group in the ortho-position of the lateral benzene ring acts as proton donor, whereas the nitrogen atom of oxazole ring acts as proton acceptor (Figure 1). Phototautomer form (T*) is formed in result of the ESIPT-reaction. The phototautomer is fluorescent in significantly longer wavelengths compared with the initial (or so-called “normal”) form (N*) [15], [16], [17], [18], [19].
![Figure 1: Scheme of excited state intramolecular proton transfer (ESIPT) in 2-(2′-hydroxyphenyl)-5-phenyl-1,3-oxazole (probe O1O) [24]. The upwards arrow represents the electronic excitation and the downwards arrow shows the emission of light (fluorescence). Corresponding maximum of absorption and the ranges of emission are shown in nanometers.](/document/doi/10.1515/tjb-2019-0427/asset/graphic/j_tjb-2019-0427_fig_001.jpg)
Scheme of excited state intramolecular proton transfer (ESIPT) in 2-(2′-hydroxyphenyl)-5-phenyl-1,3-oxazole (probe O1O) [24]. The upwards arrow represents the electronic excitation and the downwards arrow shows the emission of light (fluorescence). Corresponding maximum of absorption and the ranges of emission are shown in nanometers.
The presence of two-band fluorescence enables us to perform ratiometric measurement, i.e., to use the ratio of the phototautomer form and the initial form fluorescence intensities (IT*/IN*) as a parameter for assessment of the physical and chemical properties of the microenvironment.
Usage of ratiometric fluorescent probes allows eliminating not only the measurement error caused by the deviation of the fluorescent probe concentration (e.g., uneven content of fluorescent probe in various membranes), but also the measurement errors due to deviation in configuration and adjustment of equipment for measurements of fluorescence (e.g., deviation in the intensity of the source of excitation light, changes in the sensitivity of the photodetector, changes in focusing, etc.) [20], [21], [22], [23].
Location and orientation of probes O1O (2-(2′-OH-phenyl)-5-phenyl-1,3-oxazole) and PH7 (2-(2′-OH-phenyl)-phenanthro[9,10]-1,3-oxazole) in the cell membrane is shown in Figure 2. Probe O1O is located in the area of glycerol backbones of phospholipids (closer to the center of the lipid bilayer), in the area of carbonyl groups of phospholipids and in the area of hydrocarbon chains of phospholipids (near the carbonyl groups of phospholipids). Probe PH7 is located in the area of hydrocarbon chains of phospholipids (closer to the center of the bilayer).
![Figure 2: Location and orientation of fluorescent probes O1O (2-(2′-ОН-phenyl)-5-phenyl-1,3-oxazole) and PH7 (2-(2′-OH-phenyl)-phenanthro[9, 10]-1,3-oxazole) in phospholipid membranes [15], [24]. Two molecules of phosphatidylcholine from the outer leaflet are shown to denote the location of the probe.](/document/doi/10.1515/tjb-2019-0427/asset/graphic/j_tjb-2019-0427_fig_002.jpg)
The location and orientation of probes O1O and PH7 in the cell membrane are proposed on the basis of their fluorescent properties in lipid membranes [15], [24], calculations of their location using a method of molecular dynamics [24] and their structural similarity to the fluorescent probes with a known location and orientation in lipid membranes [25].
The aim of our research was to assess the state of cell membranes in RBCs of rats consuming VNP solution and obtaining a dose of 20 μg/kg of weight during 2 weeks by means of the fluorescent probes O1O (2-(2′-OH-phenyl)-5-phenyl-1,3-oxazole) and PH7 (2-(2′-OH-phenyl)-phenanthro[9,10]-1,3-oxazole).
Materials and methods
Study design and animals
A total of 20 female adult Wistar Albino Glaxo (WAG) rats whose weight ranged from 160 to 190 g were provided by the vivarium of Kharkiv National Medical University. The rats were divided into two equal groups in a random order. Group A included the animals orally administered a 1% VNP water solution on a daily basis except on Sundays (n=10). A dose of 20 μg/kg of weight was given. Group B consisted of intact animals served as controls (n=10). The rats were housed in cages in standard laboratory conditions of room temperature (24 ± 2 °C) and relative humidity of 50–60%. Access to water and food was provided ad libitum. Cervical dislocation technique was used to sacrifice the animals used in this study. Blood was collected using sterile sodium citrate vacutainer tubes. The blood samples were used to prepare erythrocyte suspensions.
Experimental procedures carried out in this study were performed in accordance with the EU Directive 2010/63/EU on the protection of animals used for scientific purposes, based on the Council of Europe Convection for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS123).
Characteristics of nanoparticles
Water colloidal solution of GdVO4:Eu3+ nanoparticles were obtained using the method reported earlier [26]. Eight milliliter of sodium ethylenediaminetetraacetate (EDTA 2 Na) solution (0.01 mol/L) was added to water solution of rare-earth chlorides (10 mL, 0.01 mol/L). It was followed by the addition of Na3VO4 (8 mL, 0.01 mol/L) drop by drop (рН=13). The mixture was intensively stirred by a magnetic stirrer until the formation of yellowish transparent solution was observed. The solution was colorless and transparent and scattered light under the side illumination (Tindal cone). To remove excessive ions, it was cooled and dialyzed against water for 24 h. We used a dialysis membrane with a molecular weight cutoff of 12 kDa whose pore size reached 2.5 nm.
Fluorescence measurements
For all the fluorescence measurements, the cells were fluorescently labeled via the same procedure. Because the solubility of fluorescent probes O1O and PH7 is limited in water, we dissolved the probes in acetonitrile to the initial concentration of ∼2.10−4 mol/L (stock solution). An aliquot of the probe stock solution was added to the RBC suspensions to achieve a final probe concentration of ∼5.10−6 mol/L (such a concentration of the probe corresponds to a lipid-to-probe molar ratio of ∼200:1). Then, the cell suspensions were incubated with the probes at room temperature for 1 h before fluorescence measurements took place. The fluorescence spectra were measured on a fluorescence spectrometer “Lumina” (Thermo Fisher Scientific) at room temperature. The measurements were performed in a 10 × 10 mm cuvette. The fluorescence spectra of the probes were determined in the range of 340–630 nm, with an increment of 0.1 nm. Data were collected with 0.02 s interval. The slits on the excitation and emission monochromators were 5 nm. The excitation wavelength was 330 nm. The accuracy of the fluorescence intensity measurements of the samples was 8.3 and 21.5% for probes PH7 and O1O, respectively.
Wash protocol for RBCs
To prepare RBC suspension for incubation with the fluorescent probes, RBCs were washed. Briefly, when the rats were sacrificed, their whole blood was collected into sterile sodium citrate vacutainer blood collection tubes. Then 100 μL of blood was added to 12 × 75 mm capped polysterene test tubes containing 1 mL of normal saline solution (0.9% NaCl, Yuria-Pharm, Ukraine). This was followed by centrifugation at 1000 rpm during 5 min. The supernatant was discarded. Erythrocytes were resuspended in 1% of normal saline solution and centrifuged again in the same conditions. This step was repeated to have a total of three washes. One millilitrt of 0.9% NaCl and 10 μL of RBC mass were mixed. The suspension obtained as a result was at once used for the incubation with the fluorescent probes O1O (2-(2′-OH-phenyl)-5-phenyl-1,3-oxazole) and PH7 (2-(2′-OH-phenyl)-phenanthro[9,10]-1,3-oxazole.
Statistical analysis
Non-parametric Mann–Whitney U test was selected to compare two independent groups of variables. Its choice was substantiated by the outcome of the Shapiro–Wilk normality test showing the non-Gaussian distribution. The data obtained as a result of the statistical analysis were presented in the form of medians and interquartile ranges. Differences were considered statistically significant at p<0.05. Statistically analyses were performed by GraphPad Prism 5.0 (GraphPad software, USA).
Results
For this study, the composition of spindle-like Gd(0,9) Eu(0,1)VO4 nanoparticles was synthesized. Their average size was 8 × 25 nm. A transmission electron microscopy (TEM) image of nanoparticles used in this research is available in Figure 3.
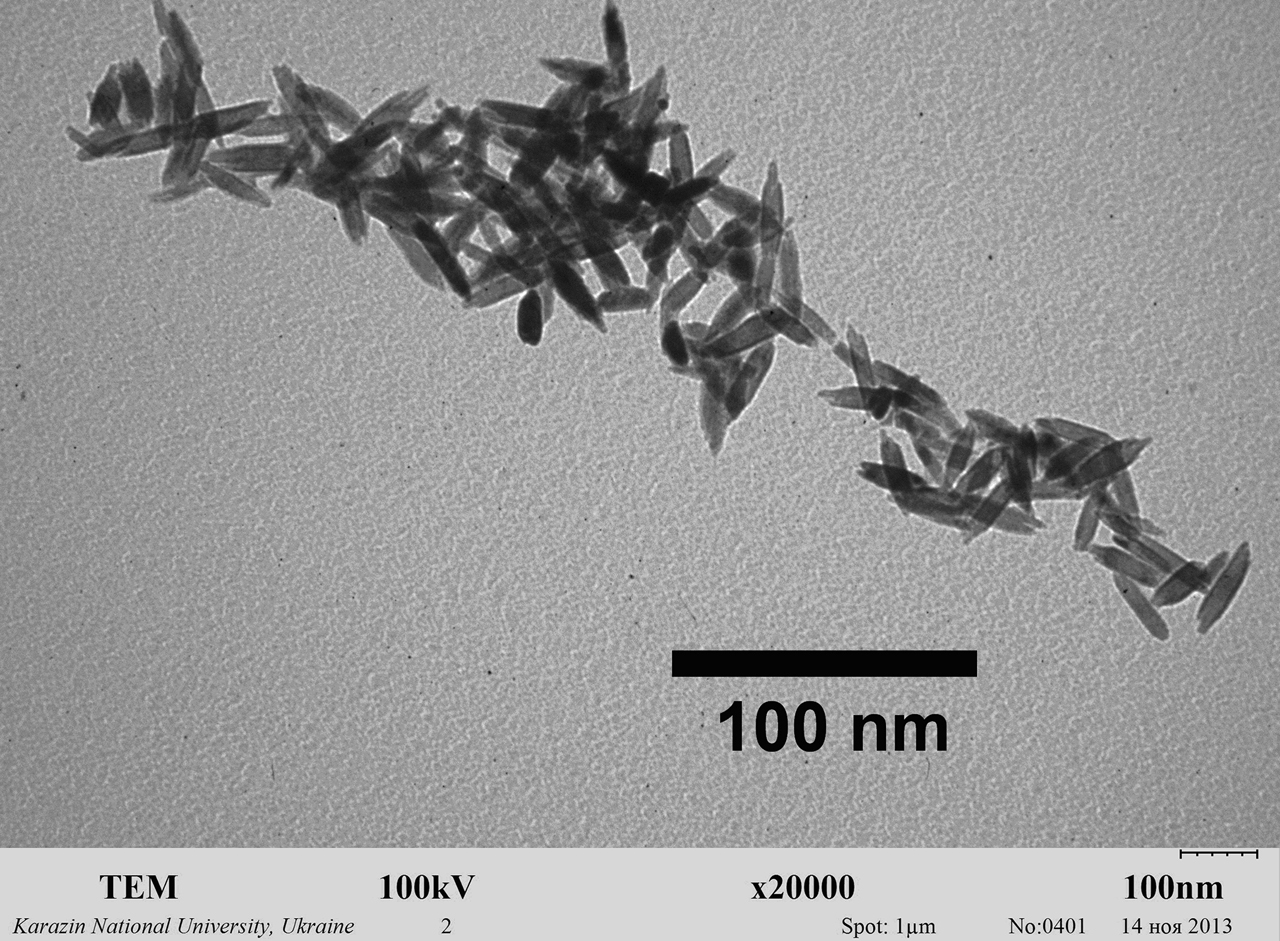
Representative transmission electron microscopy (TEM) image of orthovanadate GdVO4:Eu3+ nanoparticles obtained for this study in colloidal solutions. Their average size is 8 × 25 nm.
The results of the fluorescence measurements are presented in Figure 4 and Table 1.
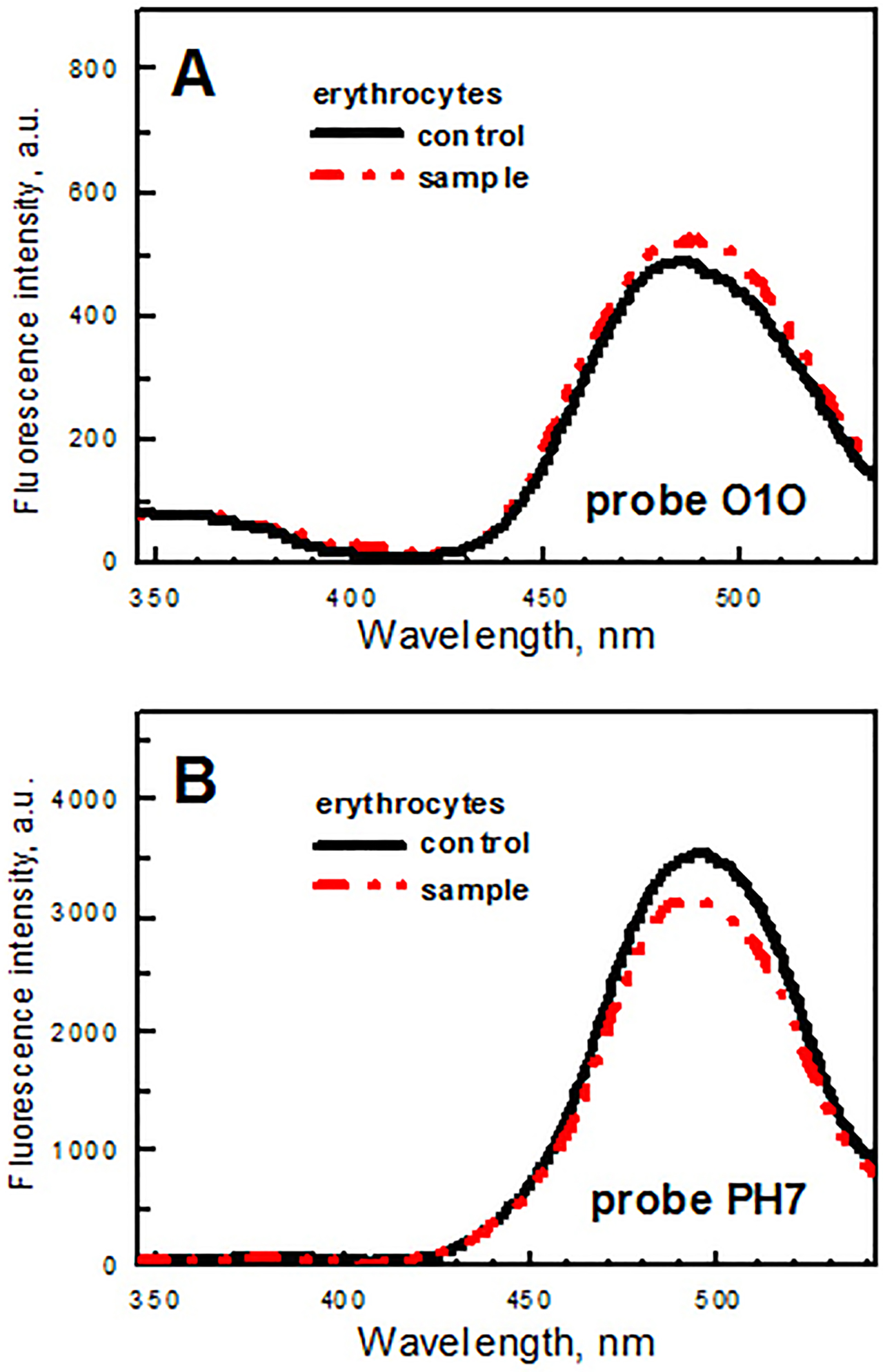
Fluorescence spectra of probes O1O (panel A) and PH7 (panel B) in erythrocyte suspensions: (a) the control group of rats (black solid line), (b) the animals orally exposed to gadolinium orthovanadate GdVO4:Eu3+ nanoparticles (VNPs) during two weeks (red dash-dot-dot line).
The ratio of the fluorescence intensities of the phototautomer and normal forms (IT*/IN*) of probes O1O and PH7 in RBC membranes of rats (Me [IQR]).
| Groups of animals | Probe O1O (I485/I372) | Probe PH7 (I492/I382) |
|---|---|---|
| Control | 6.8 [4.6; 9.2] | 35.0 [31.4; 38.9] |
| Rats orally exposed to nanoparticles | 6.2 [4.0; 8.3] p>0.05 | 33.0 [29.9;36.3] p>0.05 |
Differences were considered statistically significant at p<0.05.
No noticeable statistically significant (p>0.05) changes are observed in the spectrum of fluorescent probe O1O bound to the RBCs from the rats orally exposed to VNPs during two weeks in comparison with the corresponding spectrum of the probe bound to the cells from the control group of animals (Figure 4). The ratios of the fluorescence intensities of the phototautomer and normal forms (IT*/IN*) of probe O1O calculated for the rats orally exposed to nanoparticles and for the control group coincided within the accuracy of analysis (Table 1). This suggests that, in case of the rats orally exposed to nanoparticles, no noticeable changes in physico-chemical properties (i.e., in the polarity and the proton-donor ability) are observed in the lipid membranes of RBCs in the regions, where probe O1O locates: in the area of glycerol backbones of phospholipids (closer to the center of the lipid bilayer), in the area of carbonyl groups of phospholipids and in the area of hydrocarbon chains of phospholipids (near the carbonyl groups of phospholipids).
In case of probe PH7, slight difference between the spectrum of the probe bound to the RBCs from the rats orally exposed to VNPs during two weeks and the corresponding spectrum of the probe bound to the cells from the control group of animals is observed (Figure 4). However, it was statistically insignificant (p>0.05). Taking into account the accuracy of the fluorescence intensity measurements of the samples (8.3%), the observed difference between the spectra is negligible. The ratio of the fluorescence intensities of the phototautomer and normal forms (IT*/IN*) of probe PH7 calculated for the rats orally exposed to nanoparticles coincided within the accuracy of analysis with the corresponding ratio calculated for the control group (Table 1). This indicates that, in comparison to the control group (group B), no significant changes in physico-chemical properties (i.e. in the polarity and the proton-donor ability) of the lipid membranes of RBCs are observed for experimental group (group A) in the membrane area, where probe PH7 locates: i.e., in the area of hydrocarbon chains of phospholipids closer to the center of the bilayer.
In general, one can make a conclusion that no changes in the physical and chemical properties of the erythrocyte membranes are detected in the region from glycerol backbones of phospholipids to the center of the phospholipid bilayer in the rats orally exposed to VNPs during 2 weeks.
Discussion
It is important to note that human RBCs are present in blood vessels for 120 days and directly contact with NPs after their absorption into the bloodstream from intestine. Furthermore, they contain oxygen-transporting protein hemoglobin and, thus, constantly experience high oxygen tension [27]. In addition, oxidative stress-induced damage to RBC membranes may affect oxygen delivery to tissues leading to hypoxia [28]. All these facts make RBC membrane a sensitive and valuable marker for evaluating VNP toxicity.
Despite several beneficial effects of VNPs reported earlier, some experiments demonstrate that at high doses (0.5 mL, concentration—0.2 g/L) in case of intramuscular injections to rats, VNPs can transiently activate lipid peroxidation, evidenced by elevation of circulating malondialdehyde and conjugated dienes [29]. However, at lower therapeutic doses (20 μg/kg of weight) used in this study at oral exposure we did not observe changes in the RBC membrane, which are characteristic of lipid peroxidation (i.e., an increase in viscosity and a decrease in fluidity). A more ordered, rigid and viscous membrane is observed in conditions of lipid peroxidation activation due to the free radical oxidation of polyunsaturated fatty acids (PUFAs) found in phospholipid molecules. They are characterized by higher flexibility and, thus, the ability to pack more loosely compared with saturated fatty acids. Thus, when PUFAs are oxidized by ROS, the relative amount of saturated fatty acids in phospholipids increases, providing the increase in viscosity and the decrease in fluidity [13], [30], [31]. Thus, the lack of statistically significant changes in the fluorescence of two probes in RBC suspensions observed in this study is indicative of the absence of lipid peroxidation activation under the influence of VNPs. Our findings allow us to assume that fatty acid composition of phospholipids in RBC membranes is not affected and PUFAs remain unoxidized by ROS.
We believe that evaluation of the state of RBC membrane by the fluorescent probe technique can be used as a relatively simple, cost-effective and informative method to assess the toxicity of nanoparticles.
Our research has some limitations. Firstly, we did not assess the features of fatty acid composition of erythrocyte membrane phospholipids to judge directly whether PUFAs are oxidized or not. Secondly, levels of oxidative stress markers such as TBA (thiobarbituric acid)-reactive substances, conjugated dienes, reduced glutathione, membrane SH groups, and others were not evaluated, since the use of two independent fluorescent probes is sufficient to demonstrate oxidative stress-associated changes in RBCs.
Conclusion
No changes in the physical and chemical properties of the erythrocyte membranes are detected in the region from glycerol backbones of phospholipids to the center of the phospholipid bilayer in rats orally exposed to gadolinium orthovanadate GdVO4:Eu3+ nanoparticles during 2 weeks, i.e., VNPs at the concentration studied don’t promote ROS-mediated cell membrane damage in RBCs.
Research funding: The study was not funded in any way.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
References
1. Jeevanandam, J, Barhoum, A, Chan, YS, Dufresne, A, Danquah, MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol 2018;9:1050–74. https://doi.org/10.3762/bjnano.9.98.Search in Google Scholar PubMed PubMed Central
2. Khan, HA, Sakharkar, MK, Nayak, A, Kishore, U, Khan, A. Nanoparticles for biomedical applications: an overview. In: Narayan, R, editor Nanobiomaterials: nanostructured materials for biomedical applications. Cambridge Cb1 6ah, Cambs, England: Woodhead Publ Ltd, Abington Hall Abington; 2018.10.1016/B978-0-08-100716-7.00014-3Search in Google Scholar
3. Ramos, AP, Cruz, MAE, Tovani, CB, Ciancaglini, P. Biomedical applications of nanotechnology. Biophys Rev 2017;9:79–89. https://doi.org/10.1007/s12551-016-0246-2.Search in Google Scholar PubMed PubMed Central
4. McNamara, К, Tofail, SAM. Nanoparticles in biomedical applications. Adv Phys 2017;2:54–88. https://doi.org/10.1080/23746149.2016.1254570.Search in Google Scholar
5. Escudero, A, Becerro, A, Carrillo-Carrión, C, Núñez, NO, Zyuzin, MV, Laguna, M, et al. Rare earth based nanostructured materials: synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017;6:881–921. https://doi.org/10.1515/nanoph-2017-0007.Search in Google Scholar
6. Hubenko, K, Yefimova, S, Tkacheva, T, Maksimchuk, P, Borovoy, I, Klochkov, V, et al. Reactive oxygen species generation in aqueous solutions containing GdVO4:Eu3+ nanoparticles and their complexes with methylene blue. Nanoscale Res Lett 2018;13:100. https://doi.org/10.1186/s11671-018-2514-5.Search in Google Scholar PubMed PubMed Central
7. Belkina, IO, Smolenko, NP, Klochkov, VK, Malukin, YV, Chistyakova, EE, Karpenko, NA, et al. The effect of gadolinium orthovanadate nanoparticles by neonatal induced reproductive disease in male rats. Fiziol Zh 2016;62:76–82. https://doi.org/10.15407/fz62.05.076.Search in Google Scholar
8. Wolfram, J, Zhu, M, Yang, Y, Shen, J, Gentile, E, Paolino, D, et al. Safety of nanoparticles in medicine. Curr Drug Targets 2015;16:1671–81. https://doi.org/10.2174/1389450115666140804124808.Search in Google Scholar PubMed PubMed Central
9. Li, X, Xiao, Y, Cui, Y, Tan, T, Narasimhulu, CA, Hao, H, et al. Cell membrane damage is involved in the impaired survival of bone marrow stem cells by oxidized low-density lipoprotein. J Cell Mol Med 2014;18:2445–53. https://doi.org/10.1111/jcmm.12424.Search in Google Scholar PubMed PubMed Central
10. Papadopoulos, C, Meyer, H. Detection and clearance of damaged lysosomes by the endo-lysosomal damage response and lysophagy. Curr Biol 2017;27:1330–41. https://doi.org/10.1016/j.cub.2017.11.012.Search in Google Scholar PubMed
11. Manke, A, Wang, L, Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013;2013:942916. https://doi.org/10.1155/2013/942916.Search in Google Scholar
12. Van der Paal, J, Neyts, EC, Verlackt, CCW, Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem Sci 2016;7:489–98. https://doi.org/10.1039/c5sc02311d.Search in Google Scholar
13. Reiter, RJ, Tan, D-X, Galano, A. Melatonin reduces lipid peroxidation and membrane viscosity. Front Physiol 2014;5:377. https://doi.org/10.3389/fphys.2014.00377.Search in Google Scholar
14. Tsuda, K. Oxidative stress and membrane fluidity of red blood cells in hypertensive and normotensive men: an electron spin resonance investigation. Int Heart J 2010;51:121–4. https://doi.org/10.1536/ihj.51.121.Search in Google Scholar
15. Posokhov, YO, Kyrychenko, A, Korniyenko, Y. Derivatives of 2,5-diaryl-1,3-oxazole and 2,5-diaryl-1,3,4-oxadiazole as environment-sensitive fluorescent probes for studies of biological membranes. In: Geddes, CD, editor Reviews in Fluorescence 2017. Switzerland: Springer Nature Switzerland AG; 2018. Chapter 9.10.1007/978-3-030-01569-5_9Search in Google Scholar
16. Doroshenko, AO, Posokhov, EA, Verezubova, AA, Ptyagina, LM, Skripkina, VT, et al. Radiationless deactivation of excited phototautomer form and molecular structure of ESIPT-compounds. Photochem Photobiol Sci 2002;1:92–9. https://doi.org/10.1039/b107255m.10.1039/b107255mSearch in Google Scholar
17. Doroshenko, AO, Posokhov, EA, Verezubova, AA, Ptyagina, LM. Excited state intramolecular proton transfer reaction and luminescent properties of the ortho-hydroxy derivatives of 2,5-diphenyl-1,3,4-oxadiazole. J Phys Org Chem 2000;13:253–65. https://doi.org/10.1002/1099-1395(200005)13:5<253::aid-poc238=3.0.co;2-d.10.1002/1099-1395(200005)13:5<253::AID-POC238>3.0.CO;2-DSearch in Google Scholar
18. Doroshenko, AO, Posokhov, EA. Proton phototransfer in a series of ortho-hydroxy derivatives of 2,5-diphenyl-1,3-оxazole and 2,5-diphenyl-1,3,4-оxadiazole in polystyrene films. Theor Exp Chem 1999;35:334–7. https://doi.org/10.1007/bf02522792.Search in Google Scholar
19. Doroshenko, AO, Posokhov, EA, Shershukov, VM, Mitina, VG, Ponomarev, OA. Intramolecular proton-transfer reaction in an excited state in a series of ortho-hydroxy derivatives of 2,5-diaryloxazole. High Energy Chem 1997;31:388–94.Search in Google Scholar
20. O’Connor, N, Silver, RB. Ratio imaging: practical considerations for measuring intracellular Ca2+ and pH in living cells. Methods Cell Biol 2013;114:387–406. https://doi.org/10.1016/b978-0-12-407761-4.00016-6.Search in Google Scholar
21. O’Connor, N, Silver, RB. Ratio imaging: practical considerations for measuring intracellular Ca2+ and pH in living cells. Methods Cell Biol 2007;81:415–33. https://doi.org/10.1016/s0091-679x(06)81019-8.Search in Google Scholar
22. Demchenko, AP. The problem of self-calibration of fluorescence signal in microscale sensor systems. Lab Chip 2005;5:1210–23. https://doi.org/10.1039/b507447a.Search in Google Scholar
23. Silver, RB. Ratio imaging: practical considerations for measuring intracellular calcium and pH in living tissue. Methods Cell Biol 1998;56:237–51. https://doi.org/10.1016/s0091-679x(08)60429-x.Search in Google Scholar
24. Posokhov, Y, Kyrychenko, A. Location of fluorescent probes (2-hydroxy derivatives of 2,5-diaryl-1,3-oxazole) in lipid membrane studied by fluorescence spectroscopy and molecular dynamics simulation. Biophys Chem 2018;235:9–18. https://doi.org/10.1016/j.bpc.2018.01.005.Search in Google Scholar PubMed
25. Dobretsov, GE. Fluorescence probes in cell, membrane and lipoprotein investigations. Moscow: Nauka; 1989.Search in Google Scholar
26. Klochkov, VK, Malyshenko, AI, Sedyh, OO, Malyukin, YuV. Wet-chemical synthesis and characterization of luminescent colloidal nanoparticles: ReVO4:Eu3+ (Re=La, Gd, Y) with rod-like and spindle-like shape. Funct Mat 2011;1:111–5.Search in Google Scholar
27. Maurya, PK, Kumar, P, Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J Methodol 2015;5:216–22. https://doi.org/10.5662/wjm.v5.i4.216.Search in Google Scholar PubMed PubMed Central
28. Mohanty, JG, Nagababu, E, Rifkind, JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 2014;5:84. https://doi.org/10.3389/fphys.2014.00084.Search in Google Scholar PubMed PubMed Central
29. Klochkov, VK, Kaliman, VP, Karpenko, NA, Kavok, NS, Malyukina, MY, Yefimova, SL, et al. In vivo effects of rare-earth based nanoparticles on oxidative balance in rats. Biotechnol Acta. 2016;6:72–81. https://doi.org/10.15407/biotech9.06.072.Search in Google Scholar
30. Catalá, Á. Lipid peroxidation modifies the assembly of biological membranes the lipid whisker model. Front Physiol 2015;5:520. https://doi.org/10.3389/fphys.2014.00520.Search in Google Scholar PubMed PubMed Central
31. Ayala, A, Muñoz, MF, Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014;2014:360438. https://doi.org/10.1155/2014/360438.Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note
Articles in the same Issue
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note

