Abstract
Objectives
Cardiac damage in patient with diphtheritic myocarditis is reported as the leading cause of mortality. Diphtheria toxin (DTx) is a well-known bacterial toxin inducing various cytotoxic effects. Mainly, catalytic fragment inhibits protein synthesis, induces cytotoxicity, and depolymerizes actin filaments. In this study, we aimed to demonstrate the extent of myofibrillar damage under DTx treatment to porcine cardiac tissue samples.
Methods
Tissue samples were incubated with DTx for 1–3 h in culture conditions. To analyze whole toxin (both fragments) distribution, conjugation of DTx with FITC was performed. Measurements were carried out with fluorescence spectrophotometer before and after dialysis. Immunofluorescence microscopy was used to show localization of DTx-FITC (15 nM) on cardiac tissue incubated for 2 h. Ultrastructural characterization of cardiac tissue samples treated with DTx (15 or 150 nM) was performed with transmission electron microscopy.
Results
DTx exerts myofibrillar disorganization. Myofilament degeneration, mitochondrial damage, vacuolization, and abundant lipid droplets were determined with 150 nM of DTx treatment.
Conclusions
This finding is an addition to depolymerization of actin filaments as a result of the DTx-actin interactions in in vitro conditions, indicating that myofilament damage can occur with DTx directly besides protein synthesis inhibition. Ultrastructural results support the importance of filamentous actin degeneration at diphtheritic myocarditis.
Öz
Amaç
Kardiyak hasarın, difteri miyokardit hastalarında önde gelen mortalite nedeni olduğu bildirilmektedir. Difteri toksini (DT), çeşitli sitotoksik etkileri indükleyen ve iyi bilinen bir bakteriyel toksindir. Temel olarak, toksinin katalitik fragmenti protein sentezini inhibe eder, sitotoksisiteyi tetikler ve aktin filamentlerini depolimerize eder. Bu çalışmada, DT uygulanan domuz kardiyak doku örneklerindeki miyofibril hasarının derecesini göstermeyi amaçladık.
Yöntemler
Dokular kültür koşullarında DT ile 1–3 saat bekletildi. Tüm toksinin (her iki fragmentin) dağılımını incelemek üzere DT, FITC ile konjüge edildi. Ölçümler diyaliz öncesi ve diyaliz sonrası floresans spektrofotometresinde yapıldı. Lokalizasyon belirlemek üzere DT-FITC (15 nM) kardiyak dokuya iki saat uygulandı ve immünfloresan mikroskopi kullanıldı. DT (15 nM, 150 nM) uygulanan doku örneklerinin ince yapı analizi transmisyon elektron mikroskopisi ile yapıldı.
Bulgular
DT miyofibriler organizasyonu bozmaktadır. DT’nin 150 nM uygulanması ile miyofilament dejenerasyonu, mitokondriyal hasar, vaküolizasyon ve lipid damlalarında artış tespit edildi.
Sonuç
Bu bulgu, DT-aktin etkileşimi sonucunda in vitro koşullarda aktin filamentlerinde görünen depolimerizasyon bulgusuna ek olarak, protein sentez inhibisyonunun yanı sıra DT ile miyofilament hasarının doğrudan meydana gelebileceğini gösterir. Ultrastrüktürel sonuçlar, difteri miyokarditinde filamentöz aktin dejenerasyonunun önemini desteklemektedir.
Introduction
Diphtheria emerges as a significant health problem for even developed countries in consequence of migration from countries with poor routine vaccination policies [1]. Diphtheria outbreak was reported in resource-limited countries and global incidence is increased from 8,819 patients to 16,648 cases in 1 year [2]. The heart is one of the affected organs once bacterial exotoxin is produced by toxigenic strains of Corynebacterium diphtheria then secreted and circulated through the blood stream. Younger children and older adults are prone to get disease and cases associated with cardiac complications lead to mortality [3]. Myocarditis, an important predictor of death, may occur in patients with respiratory diphtheria. The case-fatality rate was reported as 10% in the United States [4], and 14.3% in Indonesia, which has limited access to healthcare and vaccination [5]. Postmortem studies from diphtheritic patients with acute cardiac failure exhibit distorted myocardium with granular degeneration and loss of cross striations [6]. Myofibrillar degeneration, induced with DTx on cardiac muscle from the viewpoint of F-actin stability damage is important.
DTx is an A-B two-peptide protein of 58 kDa [7]. Fragment A (FA) of DTx corresponding to C-domain has catalytic activity and fragment B (FB) consisting of R- and T-domains is responsible for receptor-mediated endocytosis of DTx and translocation of FA into cytosol [8], [9]. Delivery of C-domain from endosomal compartment to cytoplasm is a sequential process. Acidification of endosomal lumen triggers conformational change of DTx to refold and to initiate translocation of FA across the endosomal membrane [10]. The transfer of FA into cytosol requires both disulfide bond reduction and translocation enabling factors that leads FA to catalyze the transfer of ADP-ribosyl group of nicotine amide dinucleotide [11]. ADP-ribosylated diphthamide residue of eukaryotic elongation factor 2 (eEF2) leads protein synthesis inhibition [12]. Interactions between FA and cytosolic proteins such as actin [13] and Hsp90 [14] have been proposed to have important role in endosomal trafficking and cytotoxicity of FA [15], [16]. DTx-induced cytotoxicity is not limited to inhibiting of protein synthesis machinery. Once FA is internalized, ADP-ribosylation of eEF2 is pursued by internucleosomal DNA cleavage [17] and depolymerization of actin filaments [18].
Experimental models of cardiomyocyte degeneration under DTx treatment were excluded rodents [19], [20], [21] due to necessity of precursor of heparin-binding EGF-like growth factor (pro-HB-EGF) known as DTx receptor [22]. Based on previous reports of F-actin depolymerization in cell culture systems, tissue samples were used in this study. Here to address the extent of thin filament breakdown, we used porcine cardiac tissue samples and analyzed them with microscopy following their incubation in the presence of DTx.
Material and methods
Tissue preparation
Cardiac tissue samples (2 g) were dissected from ventricles of pig heart in collaboration with clinicians and surgeons [23]. The experiments on animals were conducted in accordance with the local Ethical Committee laws and regulations as regards care and use of experimental animals. This study was approved by Local Ethics Committee of Experimental Animals of Cerrahpasa University, Faculty of Medicine. Tissue samples were immediately incubated in 6-well plates using DMEM F-12 medium supplemented with 10% fetal calf serum (FCS, Gibco) at 37 °C in 5% CO2. Samples were incubated either in the absence of toxin as control or in the presence of DTx-FITC conjugate (15 nM) for 2 h for immunofluorescence imaging to show the distribution of the diphtheria toxin (DTx) on cardiac muscle. For ultrastructural analysis, tissue samples were incubated with DTx (15 nM) or without toxin for 1–3 h. Tissue samples were incubated with 150 nM of DTx for 2 h to determine if there are any differences in myofibril disarray when the DTx concentration is 200 times higher than minimum lethal dose indicated for human [24].
Conjugation of DTx with FITC
For conjugation, 2 mg of fluorescein isothiocyanate (FITC) (Sigma, St. Louis, MO, USA) was first dissolved in 1 mL anhydrous dimethyl sulfoxide (DMSO) using a protocol as described [25]. FITC (5 µM) was used for 20 µg of DTx (Calbiochem; St. Louis, MO, USA, Refik Saydam; Turkey) and mixed immediately in 450 μL Reaction Buffer (500 mM Carbonate, pH 9.2). The reaction tube was wrapped in foil then incubated and rotated at room temperature (RT) for 1 h. The DTx–FITC conjugate was exchanged into Storage Buffer (10 mM Tris, 150 mM NaCl, 0.1% NaN3, and pH 8.2) by dialysis with membrane tube (Mini GeBAflex-tube, 25,000 MWCO) during overnight and unbound FITC was removed. Fluorescence measurements were carried out using a fluorescence spectrophotometer (PerkinElmer; Waltham, MA, USA, LS 45 Luminescence Spectrometer) equipped with FLWINLAB software. The excitation wavelength of the fluorescence was set at a range around 490–520 nm. The emitted fluorescence was measured from 450 to 650 nm for free and DTx-conjugated FITC (DTx-FITC) before and after dialysis.
Immunofluorescence microscopy and imaging
Cardiac muscle tissues were embedded in a frozen section compound (FSC22 Surgipath, Richmond, IL, USA) and were snap frozen in isopentane (2-methylbutane, Sigma–Aldrich Co.) cooled in liquid nitrogen. Cryostat sections (4 µm) were taken with microtome (Leica SM 2000, Germany) on poly-l-lysine-coated slides. Cryosections were fixed for 5 min with acetone and were washed three times for 5 min with Phosphate Buffered Saline (PBS). The glass microscope slides coverslipped with ProLong Gold antibleaching reagent (Invitrogen Molecular Probes, OR, USA). The tissue samples incubated in the absence of fluorescently tagged toxin (DTx–FITC) were used for controls. DTx–FITC-treated tissue samples were examined with U-MWB2 filter cube. Images were captured using Olympus BX51 Research Microscope equipped with a DP72 camera.
Transmission electron microscopy
For transmission electron microscopy each cardiac muscle tissue sample (1 mm3 of thickness) was washed with PBS, fixed with 2.5% glutaraldehyde at RT for 1 h then washed again with PBS then post-fixed with 0.1% osmium tetroxide solution. After routine dehydration processing in a graded acetone series and in two stages of graded mixtures of acetone–resin (1:1, 60 min; 1:3, 60 min and pure resin, 60 min) specimens were embedded in capsules filled with epoxy resin, Epon 812 (Fluka). Capsules were incubated at 60 °C for 18 h for polymerization. Ultrathin sections of 60 nm thickness were made with an ultramicrotome (C:Reichert OM U3) and were placed on grids with 100 mesh. For contrast enhancement, the sections were stained with 2% uranyl acetate for 30 min and with lead nitrate for 10 min. Sections were examined using a Jeol Jem 1011 transmission electron microscope (JEOL, Tokyo, Japan) at 80 kV accelerating voltage. The images were taken by Megaview III digital camera equipped with Soft Imaging System Analysis program.
Results
FITC conjugation of DTx
The fluorometric measurements of DTx–FITC conjugate were carried out before and after dialysis. The excitation wavelength of the fluorescence was set at 493 nm. The fluorescence emission wavelength for DTx–FITC and free FITC were detected as two peaks at 500 and 518 nm, respectively (Figure 1A). After dialysis maximum emitted fluorescence wavelength was detected for DTx–FITC conjugate at 500 nm (Figure 1B). Insets in Figure 1A, B show the fluorescence intensity measured over time at emission wavelength of 518 nm. A decrease of nine fold was detected after dialysis, confirming the disappearance of unbound FITC.
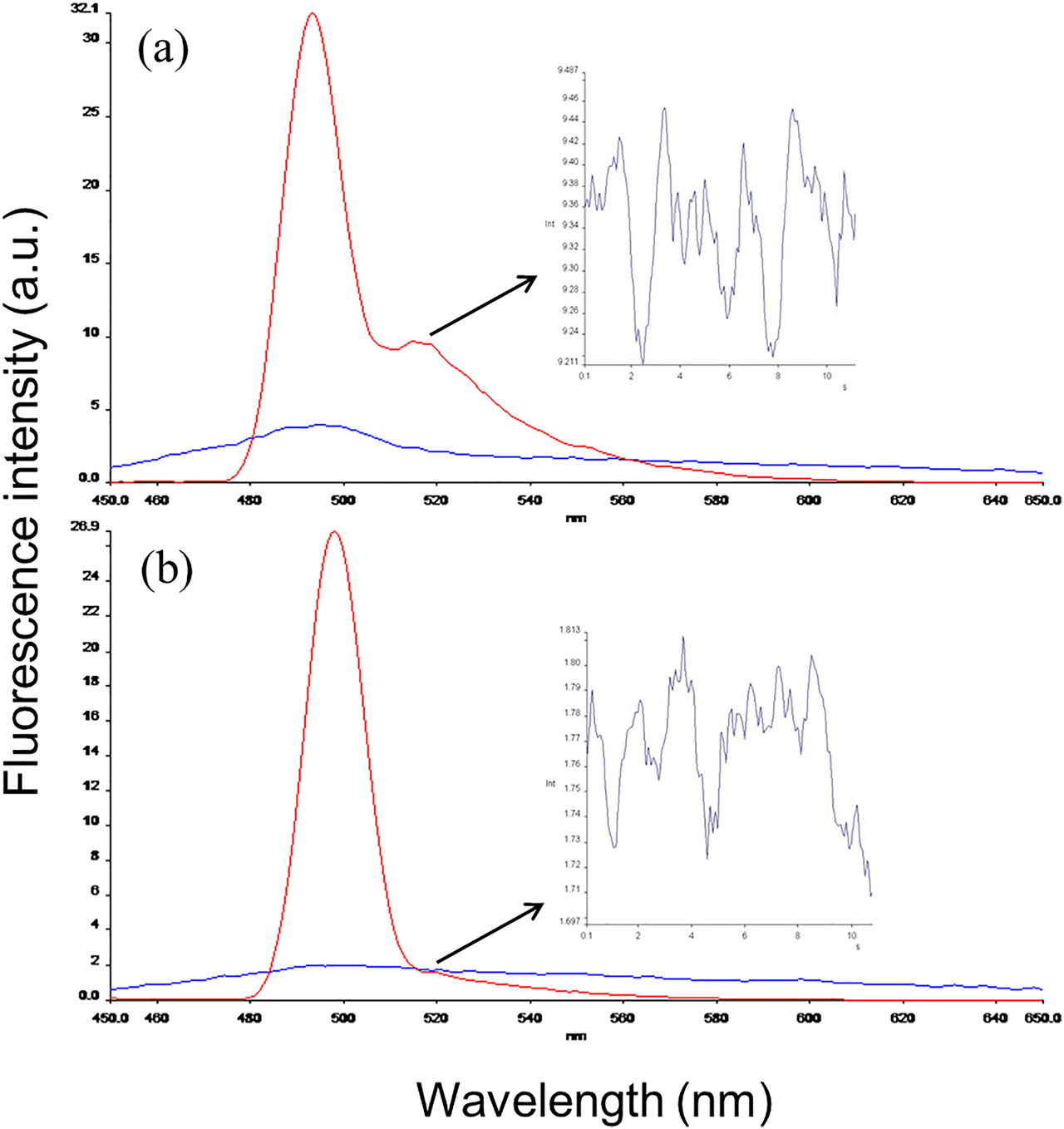
Excitation and emission spectra of DTx–FITC conjugate before and after dialysis. (a) Emission spectra of free and conjugated FITC before dialysis (line with two peaks). (b) The emitted fluorescence for DTx–FITC conjugate was detected with a high peak at 500 nm as measured previously in (a). Decrease of fluorescence intensity measurements (arrows) at 518 nm was shown with insets indicating removed free FITC.
Localization of DTx–FITC conjugate in cardiac muscle tissue
Immunofluorescence studies were conducted to determine the localization of whole toxin, DTx–FITC, in cardiac tissue samples (Figure 2). Cryosections were imaged at 100× of magnifications. Tissue samples incubated in the absence of toxin were used as control, imaged without any staining, as shown in Figure 2A. Tissue samples, incubated in the presence of DTx-FITC (15 nM, for 2 h), were shown in Figure 2B. Localizations of DTx-conjugated FITC suggests widespread distribution of toxin on myofibers when cardiac tissue samples were incubated with DTx–FITC in culture conditions.
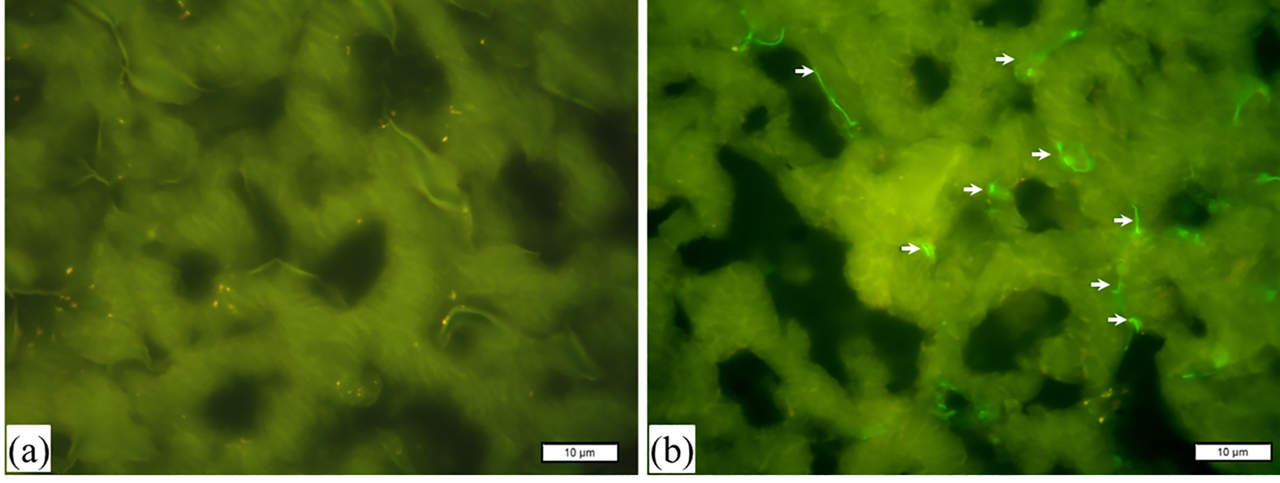
Fluorescence microphotographs of cryosections. Control samples of porcine cardiac tissue are shown in (a) without staining. Cardiac muscle tissue samples, incubated in the presence of DTx–FITC (15 nM) for 2 h, are shown in (b). Localizations of whole toxin (green) are indicated with arrows. Magnification, ×100.
Ultrastructural characterization of DTx-treated cardiac tissue samples
Cardiac tissue samples were analyzed at the ultrastructural level following 2 h of incubation in culture conditions. Analysis by TEM revealed control cardiac tissue samples displayed an intact myofibrillar and mitochondrial structure following 1 h of incubation (data not shown). Tissue dilatation and few lipid droplets within myofibers were seen in control sections after 2 h of incubation in culture condition (Figure 3A, D). Comparing with control sections, DTx (15 nM)-treated samples exhibited myofibrillar disorganization and Z-line alterations within 2 h of incubation (Figure 3B, E). Furthermore, the accumulation of mitochondria around dilated myofibrils was observed. Abnormalities in myofibrillar architecture were more prominent when DTx concentrations were increased from 15 to 150 nM (Figure 3C, F). Dilation, higher degree of disorganization in intercalated discs, widely distributed abundant lipid droplets and vacuoles were detected within the damaged myofibers (Figure 3C).That point out loss of cross striations and the granular degeneration. Z-disc displayed misalignment, and mitochondria showed striking loss of cristae in addition to swelling (Figure 3F). These qualitative changes are the classical ultrastructural hallmarks of myofibrillar degeneration similar to that seen in diphtheritic myocarditis.
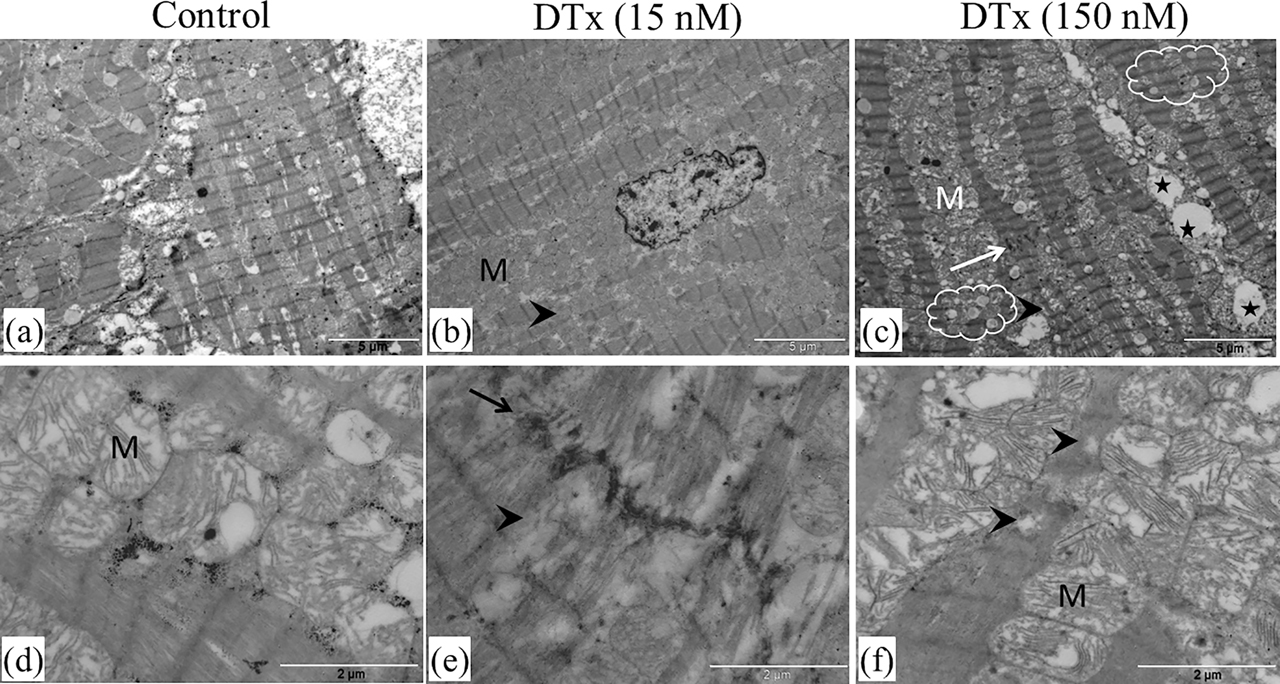
Ultrastructure of porcine cardiac tissue samples. Electron micrographs showing control tissue samples incubated in medium for 2 h (a, d). Tissue sections incubated in the presence of DTx (15 nM) display myofibrillar disruption (arrowhead) and intercalated discs disorganization (arrow), respectively, in (b) and (e). Accumulation of mitochondria around dilated myofibrils is detected (b). Tissue sections incubated in the presence of DTx (150 nM) have a higher degree of disorganization (c, f). Vacuolization (stars), myofibrillar disarray (arrow) and numerous lipid droplets (clouds) are prominent (c). The aggravated mitochondrial degeneration is also found (f). M, mitochondria. Scale bar: 5 μm (a, b, and c); 2 μm (d, e, and f).
Discussion
In this study, we combined two approaches to determine actin filament severing effect of DTx on cardiac tissue sample. Due to possible T-domain–actin interaction, it is crucial to have whole protein toxin before the completion of sequestration of catalytic domain, therefore, first we conjugated FITC with DTx. We then incubated cardiac tissue samples in classic culture conditions with DTx–FITC conjugate and visualized cryosections by immunofluorescent microscopy. Next, we have taken advantage of capabilities of transmission electron microscopy to analyze ultrastructure of myofibrils following in vitro DTx treatment.
Our results reveal DTx colocalization on thin filaments of myofibrils in line with results obtained previously for the DTx localization on actin filament in HUVECs [18]. Catalytic domain of DTx has been shown to bind to F-actin in a stoichiometric manner [13], [18]. The binding of FA to F-actin has been determined to take place at the positive end of the filament, consequently, further polymerization of G-actin is blocked and results with a time-dependent breakdown of the F-actin [18]. Depolymerizing of actin filaments by cytochalasin D usage resulted a block of FA release from DTx-loaded early endosomes into cytosol thus actin filaments are thought to have regulatory capacity and to play an important role in FA delivery processes [15], [16]. One of the components of protein synthesis mechanisms eEF2, the target of catalytic domain of DTx, has been also found to be effective in delivery of FA besides actin cytoskeleton and cytosolic translocation factors [15]. The high efficiency of FA delivery is hence determined as Km = 2.2 nM [18]. Molecular dynamics simulation of FA and F-actin revealed the most possible interaction occurs between Tyr204 of DTx and Gly48 of G-actin [26]. We have also showed the interaction between mutant DTx, CRM197, and G-actin by experimental methods and molecular docking simulations. We determined that Lys42 on FA of CRM197 interacts with Gly197 of G-actin, moreover Cys218, Cys233 residues residing on T-domain of CRM197 interact with Arg62 and Ser60 of G-actin, respectively [27]. A recent and compatible Nuclear Magnetic Resonance (NMR) study indicates that lysine residues on FA of CRM197, partially folded polypeptide, are more accessible for conjugations [28]. The rearrangement of the T-domain of DTx (residues, 194–386) during sequential events of C-domain delivery was determined and transmembrane alpha helix TH1 was reported to be the first completely unfolded segment of T-domain [29]. Those findings and DTx colocalization on thin filaments of myofibrils suggest that FA delivery into cytosol might well be supported by toxin–actin interaction thus abundant actin molecules in thin filaments become target for DTx.
Ultrastructural analysis of cardiac tissue samples incubated with DTx confirmed myofibrilolysis, dilatation, and mitochondrial damage. Our findings are in line with the previous studies conveyed on diphtheria patients [30], [31], [32], [33]. Structural deformities were shown to aggravate depending on the toxin concentration. DTx-associated breakdown of F-actin in human umbilical vein endothelial cells (HUVECs) has been reported to display shrinkage of cell size moreover a decrease of 60% in the amount of F-actin has been determined in postmicrosomal pellets of DTx-treated cells [18]. Within this line, we have also shown diminishing areas of cell-to-cell contact in the presence of mutant DTx, CRM197 [27]. Therefore, abnormalities in sarcomeric structure observed here reflect disruption of F-actin stability under DTx treatment especially its effect was observed aggravated in dose-dependent manner. Breakdown of actin filaments is likely the cause of high troponin levels found in patients with diphtheritic myocarditis [34]. Our results may have important implications for cardiac symptoms. Host cell actin filament degradation and disruption of myofibrils in the course of diphtheria may play a role in the cardiac symptoms of the disease. Furthermore, striking ultrastructural changes in mitochondrial architecture in our results are in accordance with previous findings [30]. Swollen mitochondria accompanied with disorganized cristae and lipid droplets may indicate triggered apoptotic process which was previously determined in DTx-sensitive cell lines. Cytolysis in human myeloid leukemia U937 cells [35], [36], tumor cell lysis in renal cell carcinoma cell lines [37], induction of pro–apoptotic caspase-3 plus reduction of anti–apoptotic Mcl-1 and Bcl-2 in HUVECs [38] and cell rounding in HeLa cells [14] point to DTx is associated with programmed cell death besides the onset of the inhibition of protein synthesis. DTx has been used for developing of recombinant immunotoxins, and Denileukin diftitox (ONTAK, DAB389IL-2) has been approved by the US Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphoma [39]. Along this treatment, vascular leak syndrome with affected endothelial barrier function has been reported [40] as one of the side effects in which depolymerization of F-actin may play significant part due to interactions between catalytic domain of DTx and actin cytoskeleton. Actin filament stabilizers may help to prevent side effects of the treatment.
Naturally the classical cell culture system used in this study for cardiac tissue samples has some limitations. To diminish the inconvenience of the culture conditions, DTx were treated in high concentrations, and the incubation time was restricted with 2 h. As the disruption of myofibrils was more prominent in higher concentration of DTx our findings provide robust evidence for degenerative effects of DTx on thin filaments of cardiac tissue. This indicates that DTx-treated porcine myocardial fibers may be used as a model for dysrhythmia and conduction disturbances. In case to discard the inhibition of protein synthesis effect of DTx, the mutant form, CRM197 can be used since CRM197 also interacts with actin and may induce morphologic changes in myocardial fibers.
In conclusion, we defined myofibrillar degeneration induced with DTx on cardiac muscle tissue specimen from the viewpoint of F-actin stability damage. The degradation of F-actin may appear as the underlying pathophysiology of diphtheria infection in addition to inhibition of protein synthesis.
Award Identifier / Grant number: T-53/15122006
Acknowledgments
The authors are thankful to L. Ebru Akyön and technician Ali Öztürk for their assistance.
Research funding: This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University Project no. T-53/15122006.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
References
1. Hoskisson, PA. Microbe profile: Corynebacterium diphtheriae – an old foe always ready to seize opportunity. Microbiology 2018;164:865–7. https://doi.org/10.1099/mic.0.000627.Search in Google Scholar
2. World Health Organization. Diphtheria reported cases. Availble from: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html [Accessed Sep 2019].Search in Google Scholar
3. Jain, A, Samdani, S, Meena, V, Sharma, MP. Diphtheria: it is still prevalent!!!. Int J Pediatr Otorhinolaryngol 2016;86:68–71. https://doi.org/10.1016/j.ijporl.2016.04.024.Search in Google Scholar
4. Faulkner, A, Bozio, CH, Acosta, A,Tiwari, TSP. Centers for disease control and prevention. Manual for the surveillance of vaccine-preventable diseases (Chapter 1): diphtheria. 2018, https://www.cdc.gov/vaccines/pubs/surv-manual/chpt01-dip.html.Search in Google Scholar
5. Nawing, HD, Pelupessy, NM, Alimadong, H, Albar, H. Clinical spectrum and outcomes of pediatric diphtheria. Paediatr Indones 2019;59: 38–43. https://doi.org/10.14238/pi59.1.2019.38-43.Search in Google Scholar
6. Hadfield, TL, McEvoy, P, Polotsky, Y, Tzinserling, VA, Yakovlev, AA. The pathology of diphtheria. J Infect Dis 2000;181:S116. https://doi.org/10.1086/315551.Search in Google Scholar
7. PappenheimerJrAM. Diphtheria toxin. Annu Rev Biochem 1977;46:69–94. https://doi.org/10.1146/annurev.bi.46.070177.000441.Search in Google Scholar
8. Choe, S, Bennett, MJ, Fujii, G, Curmi, PM, Kantardjieff, KA, Collier, RJ, et al. The crystal structure of diphtheria toxin. Nature 1992;357:216–22. https://doi.org/10.1038/357216a0.Search in Google Scholar
9. Bennett, MJ, Choe, S, Eisenberg, D. Refined structure of dimeric diphtheria toxin at 2.0 A resolution. Protein Sci 1994;3:1444–63. https://doi.org/10.1002/pro.5560030911.Search in Google Scholar
10. Ladokhin, AS, Legmann, R, Collier, RJ, White, SH. Reversible refolding of the diphtheria toxin T-domain on lipid membranes. Biochemistry 2004;43:7451–8. https://doi.org/10.1021/bi036157w.Search in Google Scholar
11. Van Ness, BG, Howard, JB, Bodley, JW. ADP-ribosylation of elongation factor 2 by diphtheria toxin, NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem 1980;255:10710–6.10.1016/S0021-9258(19)70365-2Search in Google Scholar
12. Collier, RJ. Effect of diphtheria toxin on protein synthesis: inactivation of one of the transfer factors. J Mol Biol 1967;25:83–98. https://doi.org/10.1016/0022-2836(67)90280-x.Search in Google Scholar
13. Bektaş, M, Varol, B, Nurten, R, Bermek, E. Interaction of diphtheria toxin (fragment A) with actin. Cell Biochem Funct 2009;27:430–9.10.1002/cbf.1590Search in Google Scholar
14. Schuster, M, Schnell, L, Feigl, P, Birkhofer, C, Mohr, K, Roeder, M, et al. The Hsp90 machinery facilitates the transport of diphtheria toxin into human cells. Sci Rep 2017;7:613. https://doi.org/10.1038/s41598-017-00780-x.Search in Google Scholar
15. Bektaş, M, Hacıosmanoğlu, E, Özerman, B, Varol, B, Nurten, R, Bermek, E. On diphtheria toxin fragment A release into the cytosol–cytochalasin D effect and involvement of actin filaments and eukaryotic elongation factor 2. Int J Biochem Cell Biol 2011;43:1365–72.10.1016/j.biocel.2011.05.017Search in Google Scholar
16. Özerman Edis, B, Hacıosmanoğlu, E, Varol, B, Bektaş, M. Intracellular trafficking of diphtheria toxin and its mutated form, CRM197, in the endocytic pathway. North Clin Istanb 2018;5:89–95.10.14744/nci.2017.55798Search in Google Scholar
17. Bruce, C, Baldvin, RL, Lessnik, SL, Wisnieski, BJ. Diphtheria toxin and its ADP-ribosyltransferase-defective homologue CRM197 possess deoxyribonuclease activity. Proc Natl Acad Sci USA 1990;87:2995–98. https://doi.org/10.1073/pnas.87.8.2995.Search in Google Scholar
18. Varol, B, Bektaş, M, Nurten, R, Bermek, E. The cytotoxic effect of diphtheria toxin on the actin cytoskeleton. Cell Mol Biol Lett 2012;17:49–61. https://doi.org/10.2478/s11658-011-0036-6.Search in Google Scholar
19. Zabejinski, MM, Ivanova, VV, Zaborov, AM, Nasirov, RA. New animal model of diphtheritic myocarditis. Exp Toxicol Pathol 2000;52:67–70. https://doi.org/10.1016/s0940-2993(00)80020-2.Search in Google Scholar
20. Akazawa, H, Komazaki, S, Shimomura, H, Terasaki, F, Zou, Y, Takano, H, et al. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem 2004;279:41095–103. https://doi.org/10.1074/jbc.m313084200.Search in Google Scholar
21. Padilla-Carlin, DJ, McMurray, DN, Hickey, AJ. The Guinea pig as a model of infectious diseases. Comp Med 2008;58:324–40.Search in Google Scholar
22. Naglich, JG, Metherall, JE, Russell, DW, Eidels, L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 1992;69:1051–61. https://doi.org/10.1016/0092-8674(92)90623-k.Search in Google Scholar
23. Okyar, A, Yildiz, A, Aksu, B, Çinar, C, Ozsoy, Y, Baktir, G. Effect of terpenes as penetration enhancers on percutaneous penetration of tiaprofenic acid through pig skin. Acta Pharm Sci 2008;50:247–56.Search in Google Scholar
24. Gill, DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev 1982;46:86–94. https://doi.org/10.1128/mmbr.46.1.86-94.1982.Search in Google Scholar
25. Roederer, M. Conjugation of monoclonal antibodies. Available from: https://www.drmr.com/abcon/ [Aug 2004].Search in Google Scholar
26. Ünlü, A, Bektaş, M, Şener, S, Nurten, R. The interaction between actin and FA fragment of diphtheria toxin. Mol Biol Rep 2013;40:3135–45. https://doi.org/10.1007/s11033-012-2387-0.Search in Google Scholar
27. Özerman Edis, B, Varol, B, Hacıosmanoğlu, E, Ünlü, A, Bektaş, M. Cross-reacting material 197 (CRM197) affects actin cytoskeleton of endothelial cells. Gen Physiol Biophys 2017;36:383–9. https://doi.org/10.4149/gpb_2017006.Search in Google Scholar
28. Sauvé, S, Gingras, G, Aubin, Y. NMR study of mutations of glycine-52 of the catalytic domain of diphtheria toxin. J Pharm Biomed Anal 2018;150:72–9. https://doi.org/10.1016/j.jpba.2017.11.056.Search in Google Scholar
29. Kurnikov, IV, Kyrychenko, A, Flores-Canales, JC, Rodnin, MV, Simakov, N, Vargas-Uribe, M, et al. pH-triggered conformational switching of the diphtheria toxin T-domain: the roles of N-terminal histidines. J Mol Biol 2013;425:2752–64. https://doi.org/10.1016/j.jmb.2013.04.030.Search in Google Scholar
30. Burch, GE, Sun, SC, Sohal, RS, Chu, KC, Colcolough, HL. Diphtheritic myocarditis. A histochemical and electron microscopic study. Am J Cardiol 1968;21:261–68. https://doi.org/10.1016/0002-9149(68)90328-7.Search in Google Scholar
31. Celik, T, Selimov, N, Vekilova, A, Kursaklioglu, H, Iyisoy, A, Kilic, S, et al. Prognostic significance of electrocardiographic abnormalities in diphtheritic myocarditis after hospital discharge: a long-term follow-up study. Ann Noninvasive Electrocardiol 2006;11:28–33. https://doi.org/10.1111/j.1542-474x.2006.00062.x.Search in Google Scholar
32. Ceyhan, M, Ozsurekci, Y, Aydin, MM, Akcali, KC, Talim, B, Celik, M, et al. Determination of the presence of diphtheria toxin in the myocardial tissue of rabbits and a female subject by using an immunofluorescent antibody method. J Clin Med Res 2015;7:472–78. https://doi.org/10.14740/jocmr2142w.Search in Google Scholar PubMed PubMed Central
33. Van Damme, K, Peeters, N, Jorens, PG, Boiy, T, Deplancke, M, Audiens, H, et al. Fatal diphtheria myocarditis in a 3-year-old girl-related to late availability and administration of antitoxin? Paediatr Int Child Health 2017;29:1–5.10.1080/20469047.2017.1378796Search in Google Scholar PubMed
34. Samdani, S, Jain, A, Meena, V, Meena, CB. Cardiac complications in diphtheria and predictors of outcomes. Int J Pediatr Otorhinolaryngol 2018;104:76–8. https://doi.org/10.1016/j.ijporl.2017.10.032.Search in Google Scholar PubMed
35. Kochi, SK, Collier, RJ. DNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesis. Exp Cell Res 1993;208:296–302. https://doi.org/10.1006/excr.1993.1249.Search in Google Scholar PubMed
36. Komatsu, N, Oda, T, Muramatsu, T. Involvement of both caspase-like proteases and serine proteases in apoptotic cell death induced by ricin, modeccin, diphtheria toxin, and pseudomonas toxin. J Biochem 1998;124:1038–44. https://doi.org/10.1093/oxfordjournals.jbchem.a022197.Search in Google Scholar PubMed
37. Mizutani, Y, Bonavida, B, Yoshida, O. Cytotoxic effect of diphtheria toxin used alone or in combination with other agents on human renal cell carcinoma cell lines. Urol Res 1994;22:261–66. https://doi.org/10.1007/bf00541904.Search in Google Scholar
38. Varol, B, Özerman, B, Hacosmanoğlu, E, Bektaş, M, Nurten, R. Evaluation of the cytotoxic effect of diphtheria toxin on human umbilical vein endothelial cells. Joint meeting of the Federation of European Physiological Societies (FEPS) and the Hungarian Physiological Society. Budapest, Hungary, Wiley, Acta Physiol, 2014;211:168 p (P12.6).Search in Google Scholar
39. Li, M, Liu, ZS, Liu, XL, Hui, Q, Lu, SY, Qu, LL, et al. Clinical targeting recombinant immunotoxins for cancer therapy. Onco Targets Ther 2017;10:3645–65. https://doi.org/10.2147/ott.s134584.Search in Google Scholar PubMed PubMed Central
40. Park-Windhol, C, D’Amore, PA. Disorders of vascular permeability. Annu Rev Pathol 2016;11:251–81. https://doi.org/10.1146/annurev-pathol-012615-044506.Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note
Articles in the same Issue
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note

