Abstract
Objective
Donepezil is the most potent acetylcholinesterase (AChE) inhibitor currently available on the market for the management of Alzheimer’s disease. In this study, it was aimed to identify potent donepezil analogues.
Materials and methods
The effects of arylidene indanones (1–10) on AChE inhibition were examined using modified Ellman’s assay. Compound 4, the most potent arylidene indanone in this series, was subjected to molecular docking to anticipate its binding mode in the AChE site (PDB code: 4EY7). The pharmacokinetic profiles of all derivatives were also predicted.
Results
Compound 4 was found as the most potent AChE inhibitor with an IC50 value of 5.93 ± 0.29 μg/mL. According to molecular docking studies, compound 4 presented favorable interactions such as π–π interactions with Trp286 and Tyr337. In silico studies revealed that the compound did not violate Lipinski’s rule of five and Jorgensen’s rule of three, making it a potential orally bioavailable agent.
Conclusion
Compound 4 is a feasible candidate for further experiments related to AChE inhibition.
Öz
Amaç
Donepezil, Alzheimer hastalığının tedavisi için piyasada var olan en etkili asetilkolinesteraz (AChE) inhibitörüdür. Bu çalışmada, etkili donepezil analoglarını tanımlamak amaçlanmıştır.
Gereç ve Yöntemler
Ariliden indanonların (1–10) AChE inhibisyonu üzerine etkileri Ellman yönteminin bir modifikasyonu kullanılarak araştırılmıştır. Bu serideki en etkili ariliden indanon olan 4 no’lu bileşik AChE enzimindeki (PDB kod: 4EY7) bağlanma biçimlerini öngörmek için moleküler docking çalışmalarına tabi tutulmuştur. Bütün bileşiklerin farmakokinetik profilleri de öngörülmüştür.
Bulgular
Bileşik 4, 5.93 ± 0.29 μg/mL IC50 değeriyle en etkili AChE inhibitörü olarak bulunmuştur. Moleküler docking çalışmalarına göre, 4 no’lu bileşik Trp286 ve Tyr337 ile π–π etkileşimleri gibi uygun etkileşimler göstermiştir. In silico çalışmalar, bileşiğin Lipinski’nin beş kuralını ve Jorgensen’in üç kuralını ihlal etmediğini ve potansiyel oral biyoyararlanımı olan bir ajan olduğunu ortaya koymuştur.
Sonuç
4 No’lu bileşik AChE inhibisyonuna dayalı ileriki deneyler için olası bir adaydır.
Introduction
Alzheimer’s disease (AD) has become a prevalent and burdensome neurodegenerative disorder in elderly people across the globe [1]. The global burden of AD is increasing considerably due to the increased life expectancy. The incidence of AD is projected to more than triple by 2050 [2], and therefore the cost of long-term care for AD patients is predicted to be in the hundreds of billions of dollars [3]. Despite considerable research on AD over a century, there are only four currently available agents for the management of AD [4], [5]. Among them, donepezil, galantamine, rivastigmine decrease the breakdown of acetylcholine (ACh) in the synapse through the inhibition of cholinesterases (ChEs), whilst memantine is a N-methyl-d-aspartate (NMDA) receptor antagonist [6]. These agents only ameliorate symptoms but do not halt the progression of AD [1], [2], [3], [4].
Several enzymes, such as acetylcholinesterase (AChE), secretases, lipoxygenases, glycogen synthase kinase-3, sirtuins, caspases have been reported to participate in the pathogenesis of AD [7], [8]. Among them, AChE stands out as a promising target for anti-AD agents due to its pivotal role in the termination of signal transmission in the cholinergic system through the hydrolysis of ACh [7], [8], [9], [10]. According to cholinergic hypothesis, the decreased level of ACh results in impairment of the cholinergic neurotransmission leading to the loss of intellectual abilities [11]. As a result, the design of AChE inhibitors on the basis of the cholinergic hypothesis is still the most popular strategy to identify potent anti-AD drug candidates [4].
Chalcones have come into prominence as promising pharmacophores for the management of AD targeting a plethora of receptors and enzymes associated with the pathogenesis of AD ranging from AChE to β-secretase owing to their structural features allowing these ligands to interact with key residues of biological targets [12], [13], [14], [15], [16]. In particular, arylidene indanones identified as the rigid cousins or homologs of chalcones have emerged as potent weapons in the battle against AD due to their inhibitory potential against ChEs [17], [18], [19], [20].
Donepezil is one of the most commonly prescribed anti-AD agents used in the first-line treatment of patients with mild to severe AD due to its potent AChE inhibitory activity and favorable therapeutic profile [21], [22], [23], [24]. N-benzylpiperidine and indanone serve as crucial moieties in donepezil for the interactions with AChE [25] and therefore tremendous efforts have been devoted to the discovery of potent indanone-based donepezil analogs for the management of AD [25], [26], [27], [28], [29], [30].
In an effort to identify potent indanone-based donepezil analogs, herein arylidene indanones (1–10) [31] were evaluated as AChE inhibitors (Figure 1).
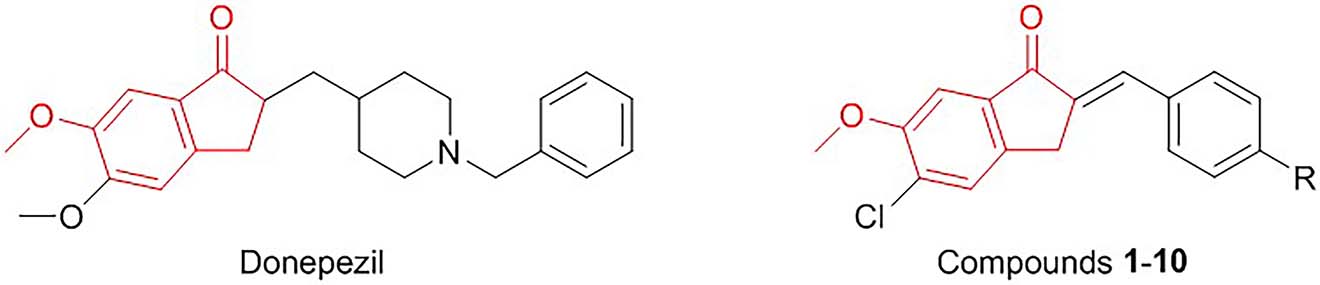
The similarity between the structures of donepezil and the arylidene indanones.
Materials and methods
Chemistry
The synthetic method and the spectral data of 5-chloro-6-methoxy-2-arylidene-2,3-dihydro-1H-inden-1-ones (1–10) were reported previously by our research team [31].
Biochemistry
The AChE inhibitory effects of the compounds were determined by Ellman’s method [32] with minor modifications [33]. The compounds were dissolved in DMSO and tested at final concentrations ranging from 5 to 80 μg/mL. 20 μL of AChE (1 U/mL), 10 μL sample were added to 2.4 mL buffer, and the mixture was incubated at 37 °C for 15 min. After 15 min incubation, 50 μL of 0.01 M 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) and 20 μL of 75 mM acetylthiocholine iodide (ATCI) were added, and the final mixture was incubated at room temperature for 30 min. Blank was prepared using 10 μL of DMSO instead of the test sample, with all other procedures similar to those used in the sample mixture. Absorbances were measured at 412 nm and 37 °C using polystyrene cuvettes with spectrophotometer (UV-1700, Shimadzu, Japan). All experiments were performed in triplicate. Donepezil was used as a positive control. Data are expressed as mean ± standard deviation (SD).
Molecular modeling
Compound 4 was docked to the active site of AChE (PDB code: 4EY7) [34] as indicated formerly [35] using Schrodinger’s Maestro molecular modeling package.
In silico absorption, distribution, excretion, metabolism (ADME) studies
The pharmacokinetic profiles of compounds 1–10 were envisaged by QikProp module of Schrödinger’s Molecular modeling package.
Results and discussion
Arylidene indanones (1–10) were investigated for their AChE inhibitory effects (Table 1). Compound 4 was identified as a notable AChE inhibitor with an IC50 value of 5.93 ± 0.29 μg/mL. This outcome revealed that p-acetamidobenzylidene moiety significantly enhanced AChE inhibitory activity.
The inhibitory effects of compounds 1-10 on AChE.
 | |||
|---|---|---|---|
| Compound | R | Inhibition% (80 µg/mL) | IC50 (µg/mL) |
| 1 | Pyrrolidinyl | 30.68 ± 0.74 | nt |
| 2 | Dimethylamino | --- | nt |
| 3 | Diethylamino | --- | nt |
| 4 | Acetamido | 87.27 ± 2.32 | 5.93 ± 0.29 |
| 5 | Piperidinyl | 23.34 ± 3.71 | nt |
| 6 | Morpholinyl | 38.87 ± 2.85 | nt |
| 7 | 4-Methyl-1-piperazinyl | 73.97 ± 1.38 | 30.67 ± 0.51 |
| 8 | 1H-Imidazol-1-yl | 59.96 ± 1.15 | 73.67 ± 1.15 |
| 9 | 1H-1,2,4-Triazol-1-yl | 95.17 ± 0.74 | 10.00 ± 0.17 |
| 10 | 2-Morpholinoethoxy | 80.77 ± 3.08 | 20.70 ± 1.13 |
| Donepezil | – | nt | 0.0065 ± 0.001 |
nt: not tested. ---: no inhibition.
Triazole-based compound 9 (IC50 = 10.00 ± 0.17 μg/mL) showed more potent AChE inhibitory activity than imidazole-based compound 8 (IC50 = 73.67 ± 1.15 μg/mL). 1,2,4-Triazole ring significantly increased AChE inhibitory activity.
The results demonstrated that compound 10 (IC50 = 20.70 ± 1.13 μg/mL) was found to be more effective on AChE than compound 6 (38.87 ± 2.85%). It can be concluded that the introduction of an ethoxy linker between the morpholine ring and the benzylidene moiety leads to a significant increase in anticholinesterase activity. On the other hand, the effects of the nitrogen heterocycles on AChE inhibitory activity were found to be in the following order: Piperazine > Morpholine > Pyrrolidine > Piperidine. Compound 7 exhibited AChE inhibitory activity with an IC50 value of 30.67 ± 0.51 μg/mL, whereas compounds 1, 5 and 6 caused less than 50% AChE inhibition at 80 μg/mL. The replacement of the nitrogen heterocycles with the dialkylamino groups resulted in the loss of AChE inhibitory activity.
Molecular docking studies were carried out to explore the possible binding mode of compound 4 in the active site of AChE (PDB code: 4EY7). Compound 4 showed good affinity to the active site of AChE (Figure 2A and 2B). Compound 4 was found to be capable of forming π–π stacking interactions with Trp286 and Tyr337 residues. The carbonyl group of the indanone scaffold formed a hydrogen bond with Phe295 residue. The docking score, glide gscore and glide emodel were found as −10.21 kcal/mol, −10.21 kcal/mol and −80.44 kcal/mol, respectively.
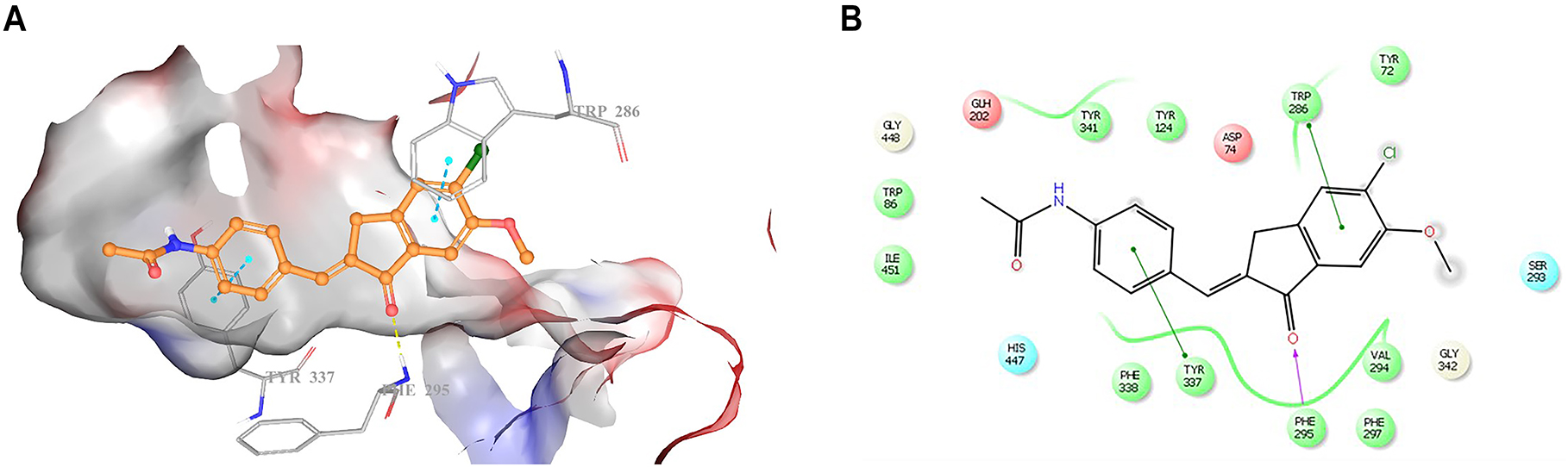
Docking pose (A) and interactions (B) of compound 4 in the active site of AChE (PDB code: 4EY7) (Hydrogen bonds are depicted as yellow dashes (A) and purple line (B), whereas π–π stacking interactions were presented as blue dashes (A) and green lines (B).
The QikProp module of Schrödinger’s Molecular modeling package was used to predict the ADME profiles of compounds 1–10 (Table 2). Compounds 3 and 5 only violated one parameter of Lipinski’s rule of five, whilst other compounds did not violate Lipinski’s rule. Based on Lipinski’s rule, these compounds can be considered as drug-like molecules. On the other hand, compounds 1, 3 and 5 only violated one parameter of Jorgensen’s rule of three, whereas other derivatives did not violate Jorgensen’s rule of three. The percentage of human oral absorption for all derivatives was also predicted to be 100.00. On the basis of these findings, all compounds are predicted to possess good oral bioavailability. Their predicted QPlogBB, SASA, PSA and QPlogKhsa values were found to be within optimum range. According to in silico ADME studies, all compounds are also able to pass through the blood-brain barrier (BBB).
In silico ADME profiles of compounds 1–10.
| Compound | QPlogBBa | SASAa | PSAa | QPlogKhsaa | Human oral absorption %a | Rule of fiveb | Rule of threec |
|---|---|---|---|---|---|---|---|
| 1 | 0.01 | 647.30 | 40.32 | 0.77 | 100.00 | 0 | 1 |
| 2 | −0.03 | 610.68 | 39.94 | 0.52 | 100.00 | 0 | 0 |
| 3 | −0.15 | 662.33 | 38.47 | 0.74 | 100.00 | 1 | 1 |
| 4 | −0.64 | 668.88 | 75.19 | 0.34 | 100.00 | 0 | 0 |
| 5 | 0.02 | 667.20 | 40.25 | 0.90 | 100.00 | 1 | 1 |
| 6 | 0.02 | 626.33 | 49.75 | 0.29 | 100.00 | 0 | 0 |
| 7 | 0.35 | 689.29 | 45.86 | 0.50 | 100.00 | 0 | 0 |
| 8 | −0.28 | 624.04 | 52.44 | 0.50 | 100.00 | 0 | 0 |
| 9 | −0.55 | 617.78 | 68.29 | 0.19 | 100.00 | 0 | 0 |
| 10 | 0.14 | 720.50 | 56.89 | 0.12 | 100.00 | 0 | 0 |
aQPlogBB: Predicted brain/blood partition coefficient (Recommended range: −3.0–1.2). SASA: Total solvent accessible surface area in square angstroms using a probe with a 1.4 Å radius (recommended range: 300.0–1000.0). PSA: Van der Waals surface area of polar nitrogen and oxygen atoms (recommended range: 7.0–200.0). QPlogKhsa: Prediction of binding to human serum albumin (Recommended range: −1.5–1.5). Human oral absorption%: Predicted human oral absorption on 0 to 100% scale. Human oral absorption higher than 80% is considered to be high, whilst human oral absorption lower than 25% is considered to be poor.
bRule of five: number of violations of Lipinski’s rule of five. The rules are: molecular weight <500, predicted octanol/water partition coefficient <5, hydrogen-bond donor atoms ≤5, hydrogen-bond acceptor atoms ≤10. Compounds that provide these rules are considered as drug-like molecules.
cRule of three: number of violations of Jorgensen’s rule of three. The three rules are: predicted aqueous solubility (QPlogS) >−5.7, predicted apparent Caco-2 cell permeability (QPPCaco in nm/s) >22 nm/s, # Primary metabolites <7. Compounds with fewer (and preferably no) violations of these rules are more likely to be orally available agents (Schrödinger Release 2016-2: Schrödinger, LLC, New York, NY, USA).
Conclusion
In this paper, we focused on the assessment of a series of arylidene indanones as AChE inhibitors. The most active AChE inhibitor in this series was found as compound 4. According to docking studies, compound 4 was capable of forming proper interactions with Trp286, Tyr337 and Phe295 in the active site of AChE. Based on ADME studies, compound 4 is expected to possess a favorable ADME profile. The results highlight that compound 4 is a hit compound for the generation of AChE inhibitors with enhanced efficacy and selectivity through the molecular modification of compound 4.
Research funding: None declared.
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: The authors state no conflict of interest.
References
1. Chen, Y, Fu, AKY, Ip, NY . Synaptic dysfunction in Alzheimer’s disease: mechanisms and therapeutic strategies. Pharmacol Ther 2019;195:186–98. https://doi.org/10.1016/j.pharmthera.2018.11.006.Suche in Google Scholar PubMed
2. Godyń, J, Jończyk, J, Panek, D, Malawska, B . Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep 2016;68:127–38. https://doi.org/10.1016/j.pharep.2015.07.006.Suche in Google Scholar PubMed
3. Tayeb, HO, Yang, HD, Price, BH, Tarazi, FI . Pharmacotherapies for Alzheimer’s disease: beyond cholinesterase inhibitors. Pharmacol Ther 2012;134:8–25. https://doi.org/10.1016/j.pharmthera.2011.12.002.Suche in Google Scholar PubMed
4. Li, Q, He, S, Chen, Y, Feng, F, Qu, W, Sun, H . Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer’s disease. Eur J Med Chem 2018;158:463–77. https://doi.org/10.1016/j.ejmech.2018.09.031.Suche in Google Scholar PubMed
5. Anand, R, Gill, KD, Mahdi, AA . Therapeutics of Alzheimer’s disease: past, present and future. Neuropharmacology 2014;76:27–50. https://doi.org/10.1016/j.neuropharm.2013.07.004.Suche in Google Scholar PubMed
6. Noetzli, M, Eap, CB . Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin Pharmacokinet 2013;52:225–41. https://doi.org/10.1007/s40262-013-0038-9.Suche in Google Scholar PubMed
7. Islam, BU, Tabrez, S . Management of Alzheimer’s disease—an insight of the enzymatic and other novel potential targets. Int J Biol Macromol 2017;97:700–9. https://doi.org/10.1016/j.ijbiomac.2017.01.076.Suche in Google Scholar PubMed
8. Silva, T, Reis, J, Teixeira, J, Borges, F . Alzheimer’s disease, enzyme targets and drug discovery struggles: from natural products to drug prototypes. Ageing Res Rev 2014;15:116–45. https://doi.org/10.1016/j.arr.2014.03.008.Suche in Google Scholar PubMed
9. Lazarević-Pašti, T, Leskovac, A, Momić, T, Petrović, S, Vasić, V . Modulators of acetylcholinesterase activity: from Alzheimer’s disease to anti-cancer drugs. Curr Med Chem 2017;24:3283–309. https://doi.org/10.2174/0929867324666170705123509.Suche in Google Scholar PubMed
10. Guzior, N, Więckowska, A, Panek, D, Malawska, B . Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer’s disease. Curr Med Chem 2015;22:373–404. https://doi.org/10.2174/0929867321666141106122628.Suche in Google Scholar PubMed PubMed Central
11. Singh, M, Kaur, M, Kukreja, H, Chugh, R, Silakari, O, Singh, D . Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur J Med Chem 2013;70:165–88. https://doi.org/10.1016/j.ejmech.2013.09.050.Suche in Google Scholar PubMed
12. Zhuang, C, Zhang, W, Sheng, C, Zhang, W, Xing, C, Miao, Z . Chalcone: a privileged structure in medicinal chemistry. Chem Rev 2017;117:7762–810. https://doi.org/10.1021/acs.chemrev.7b00020.Suche in Google Scholar PubMed PubMed Central
13. Gomes, MN, Muratov, EN, Pereira, M, Peixoto, JC, Rosseto, LP, Cravo, PV, et al. Chalcone derivatives: promising starting points for drug design. Molecules 2017;22:1210. https://doi.org/10.3390/molecules22081210.Suche in Google Scholar PubMed PubMed Central
14. Rampa, A, Bartolini, M, Pruccoli, L, Naldi, M, Iriepa, I, Moraleda, I, et al. Exploiting the chalcone scaffold to develop multifunctional agents for Alzheimer’s disease. Molecules 2018;23:1902. https://doi.org/10.3390/molecules23081902.Suche in Google Scholar PubMed PubMed Central
15. Zhang, X, Rakesh, KP, Bukhari, SNA, Balakrishna, M, Manukumar, HM, Qin, HL . Multi-targetable chalcone analogs to treat deadly Alzheimer’s disease: current view and upcoming advice. Bioorg Chem 2018;80:86–93. https://doi.org/10.1016/j.bioorg.2018.06.009.Suche in Google Scholar PubMed
16. Liu, HR, Liu, XJ, Fan, HQ, Tang, JJ, Gao, XH, Liu, WK . Design, synthesis and pharmacological evaluation of chalcone derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem 2014;22:6124–33. https://doi.org/10.1016/j.bmc.2014.08.033.Suche in Google Scholar PubMed
17. Menezes, JCJMDS . Arylidene indanone scaffold: medicinal chemistry and structure–activity relationship view. RSC Adv 2017;7:9357–72. https://doi.org/10.1039/C6RA28613E.Suche in Google Scholar
18. Patil, SA, Patil, R, Patil, SA . Recent developments in biological activities of indanones. Eur J Med Chem 2017;138:182–98. https://doi.org/10.1016/j.ejmech.2017.06.032.Suche in Google Scholar PubMed
19. Huang, L, Miao, H, Sun, Y, Meng, F, Li, X . Discovery of indanone derivatives as multi-target-directed ligands against Alzheimer’s disease. Eur J Med Chem 2014;87:429–39. https://doi.org/10.1016/j.ejmech.2014.09.081.Suche in Google Scholar PubMed
20. Rizzo, S, Bartolini, M, Ceccarini, L, Piazzi, L, Gobbi, S, Cavalli, A, et al. Targeting Alzheimer’s disease: novel indanone hybrids bearing a pharmacophoric fragment of AP2238. Bioorg Med Chem 2010;18:1749–60. https://doi.org/10.1016/j.bmc.2010.01.071.Suche in Google Scholar PubMed
21. Prvulovic, D, Schneider, B . Pharmacokinetic and pharmacodynamic evaluation of donepezil for the treatment of Alzheimer’s disease. Expert Opin Drug Metab Toxicol 2014;10:1039–50. https://doi.org/10.1517/17425255.2014.915028.Suche in Google Scholar PubMed
22. Adlimoghaddam, A, Neuendorff, M, Roy, B, Albensi, BC . A review of clinical treatment considerations of donepezil in severe Alzheimer’s disease. CNS Neurosci Ther 2018;24:876–88. https://doi.org/10.1111/cns.13035.Suche in Google Scholar PubMed PubMed Central
23. Brewster, JTII, Dell’Acqua, S, Thach, DQ, Sessler, JL . Classics in chemical neuroscience: donepezil. ACS Chem Neurosci 2019;10:155–67. https://doi.org/10.1021/acschemneuro.8b00517.Suche in Google Scholar PubMed
24. Cheewakriengkrai, L, Gauthier, S . A 10-year perspective on donepezil. Expert Opin Pharmacother 2013;14:331–8. https://doi.org/10.1517/14656566.2013.760543.Suche in Google Scholar PubMed
25. Rodrigues Simões, MC, Dias Viegas, FP, Moreira, MS, de Freitas Silva, M, Riquiel, MM, Mattos da Rosa, P, et al. Donepezil: an important prototype to the design of new drug candidates for Alzheimer’s disease. Mini Rev Med Chem 2014;14:2–19. https://doi.org/10.2174/1389557513666131119201353.Suche in Google Scholar PubMed
26. Yerdelen, KO, Koca, M, Anil, B, Sevindik, H, Kasap, Z, Halici, Z, et al. Synthesis of donepezil-based multifunctional agents for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 2015;25:5576–82. https://doi.org/10.1016/j.bmcl.2015.10.051.Suche in Google Scholar PubMed
27. Gabr, MT, Abdel-Raziq, MS . Structure-based design, synthesis, and evaluation of structurally rigid donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg Med Chem Lett 2018;28:2910–13. https://doi.org/10.1016/j.bmcl.2018.07.019.Suche in Google Scholar PubMed
28. van Greunen, DG, Cordier, W, Nell, M, van der Westhuyzen, C, Steenkamp, V, Panayides, JL, et al. Targeting Alzheimer’s disease by investigating previously unexplored chemical space surrounding the cholinesterase inhibitor donepezil. Eur J Med Chem 2017;127:671–90. https://doi.org/10.1016/j.ejmech.2016.10.036.Suche in Google Scholar PubMed
29. Costanzo, P, Cariati, L, Desiderio, D, Sgammato, R, Lamberti, A, Arcone, R, et al. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med Chem Lett 2016;7:470–5. https://doi.org/10.1021/acsmedchemlett.5b00483.Suche in Google Scholar PubMed PubMed Central
30. Green, KD, Fosso, MY, Garneau-Tsodikova, S . Multifunctional donepezil analogues as cholinesterase and BACE1 inhibitors. Molecules 2018;23:3252. https://doi.org/10.3390/molecules23123252.Suche in Google Scholar PubMed PubMed Central
31. Özdemir, A, Gökbulut, S, Sever, B, Akalın Çiftçi, G, Altıntop, MD . Synthesis and evaluation of a new series of arylidene indanones as potential anticancer agents. Anti-Cancer Agents Med Chem 2018;18:1394–404. https://doi.org/10.2174/1871520618666171206111923.Suche in Google Scholar PubMed
32. Ellman, GL, Courtney, KD, Anders, V, Featherstone, RM . A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9.Suche in Google Scholar PubMed
33. Altintop, MD, Özdemir, A, Kaplancikli, ZA, Turan-Zitouni, G, Temel, HE, Akalın Çiftçi, G . Synthesis and biological evaluation of some pyrazoline derivatives bearing a dithiocarbamate moiety as new cholinesterase inhibitors. Arch Pharm Chem Life Sci 2013;346:189–99. https://doi.org/10.1002/ardp.201200384.Suche in Google Scholar PubMed
34. Cheung, J, Rudolph, M, Burshteyn, F, Cassidy, MS, Gary, EN, Love, J, et al. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 2012;55:10282–86. https://doi.org/10.1021/jm300871x.Suche in Google Scholar PubMed
35. Altıntop, MD, Temel, HE, Sever, B, Akalın Çiftçi, G, Kaplancıklı, ZA . Synthesis and evaluation of new benzodioxole-based thiosemicarbazone derivatives as potential antitumor agents. Molecules 2016;21:1598. https://doi.org/10.3390/molecules21111598.Suche in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note
Artikel in diesem Heft
- Frontmatter
- Research Articles
- Development of new total RNA isolation method for tissues with rich phenolic compounds
- Myofibrillar degeneration with diphtheria toxin
- In vitro and in silico studies on AChE inhibitory effects of a series of donepezil-like arylidene indanones
- In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi
- Purification and characterization of glucose-6-phosphate dehydrogenase from Eisenia fetida and effects of some pesticides and metal ions
- Nephroprotective effects of eriocitrin via alleviation of oxidative stress and DNA damage against cisplatin-induced renal toxicity
- The impact of orally administered gadolinium orthovanadate GdVO4:Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes
- An anxiolytic drug buspirone ameliorates hyperglycemia and endothelial dysfunction in type 2 diabetic rat model
- Effects of mesenchymal stem cell and amnion membrane transfer on prevention of pericardial adhesions
- How potential endocrine disruptor deltamethrin effects antioxidant enzyme levels and total antioxidant status on model organisms
- Antiproliferative effect of rosehip tea phenolics in prostate cancer cell lines
- Investigation of MMP-9 rs3918242 and TIMP-2 rs8179090 polymorphisms in renal cell carcinoma tissues
- Investigation of SR-BI gene rs4238001 and rs5888 polymorphisms prevalence and effects on Turkish patients with metabolic syndrome
- Assessment of the frequency and biochemical parameters of conjunctivitis in COVID-19 and other viral and bacterial conditions
- Short Communication
- Lack of hotspot mutations other than TP53 R249S in aflatoxin B1 associated hepatocellular carcinoma
- Letter to the Editors
- Cornuside, identified in Corni fructus, suppresses melanin biosynthesis in B16/F10 melanoma cells through tyrosinase inhibition
- The extract of male bee and beehive from Bombus terrestris has biological efficacies for promoting skin health
- COVID-19 laboratory biosafety guide
- Retraction note

