Abstract
Phthalonitrile (PN) is a highly promising material in the field of high-performance thermosetting polymers due to its ability to maintain its properties even at extremely high temperatures. The goal of this study was to investigate the effects of varying curing agents on the thermal properties of cured PN resin. The curing agents were found to effectively cure the resin, as indicated by the increasing ratio of N and S contents and decreasing the C content as the proportion of curing agents increased, as observed by scanning electron microscopy and energy dispersive X-ray spectroscopy data analyses. Moreover, thermogravimetric analyses revealed that the sample with 20% curing agent showed the highest thermal decomposition rate among the 2, 5, 10, and 20% curing agent dosages. These properties can be further improved by incorporating glass fibers. Overall, these results demonstrate the successful use of curing agents to create an efficient and functional polymer with superior thermal properties that are suitable for use in harsh environments. The findings of this study are a significant step forward in advancing the use of PN as a high-performance thermosetting polymer.
1 Introduction
In recent years, there has been a growing interest in the application of high-performance thermosetting resins, which possess remarkable properties such as low water absorption, outstanding corrosion resistance, and flame retardancy. The key characteristics of high-performance thermosetting resins include high aromatic content, glass temperature (T g > 150°C), and thermal decomposition temperature (T d > 400°C) [1]. Epoxy resins are one of the most widely used high-performance thermosetting resins due to their superior mechanical and chemical properties [2,3]. Epoxy resins are synthesized through a reaction between epichlorohydrin and bisphenol-A, yielding a highly durable material that exhibits notable resistance to impact, heat, and chemicals. This trait renders epoxy resins ideal for adhesives, coatings, and composites. Similarly, phenolic resins are formulated through a reaction between phenol and formaldehyde, resulting in a material that possesses remarkable heat and chemical resistance [4,5]. Phenolic resins are commonly employed in the fabrication of high-strength composites, electrical laminates, and molded parts, owing to their exceptional mechanical properties and low flammability. Polyester resins are synthesized by the reaction of dibasic acids and glycols, which can be further augmented with a variety of additives to enhance their mechanical and chemical properties [6].
Among the emerging high-performance thermosetting resins, phthalonitrile (PN) resin has received significant attention due to its exceptional thermal stability [7,8,9,10,11]. Its heterocyclic macromolecular structure sets it apart from other resins, making it ideal for use at high service temperatures. These resins have become essential in various industries, including aerospace, microelectronics, composite materials, and more [12,13,14].
One of the most promising applications of PN is in the field of aerospace [15,16,17,18]. Due to its high thermal stability, PN can withstand extreme temperatures, making it an ideal material for use in aircraft engines and other high-temperature applications. It has been used to manufacture heat shields, exhaust nozzles, and other components in aerospace engines, where it provides outstanding resistance to thermal and mechanical stresses. Another significant application of PN is in the field of electronics [19,20,21]. The polymer is used to create printed circuit boards that have superior thermal stability and are resistant to high temperatures, making them suitable for use in harsh environments. Additionally, PN is also used as an insulating material for high-voltage power cables due to its excellent dielectric properties. PN is also being explored as a potential replacement for traditional materials in the automotive industry [22,23]. The polymer can be used to create lightweight components that are resistant to heat and chemical damage. Another exciting application of PN is in the field of 3D printing [24]. The polymer’s excellent thermal stability and chemical resistance make it an ideal material for use in 3D printing applications that require high-temperature resistance. PN-based 3D printing filaments are already available, and they have been used to create complex parts that can withstand high temperatures and harsh environments. In the field of energy, PN is being utilized to develop high-performance membranes for use in fuel cells [25]. These membranes are used to separate the fuel and oxygen in the fuel cell, so they have to be durable, stable, and possess high conductivity. PN-based membranes have demonstrated excellent performance in this application, showing high thermal stability and high proton conductivity. Although efforts have been made to improve the processability of PN resin, its high production costs remain a significant challenge [26,27,28].
The primary goal of our investigation is to meticulously examine the impact of adjusting the ratio of curing agents on the thermal properties of PN resin. Our study has shown that manipulating the proportion of curing agents can substantially improve the thermal properties and the yield of the final product. Additionally, we have also observed that the surface characteristics of the PN resin exhibit marked variations with differing ratios of curing agents. This conclusively demonstrates that the ratio of curing agents plays a crucial role in determining the thermal properties and surface characteristics of the PN resin. Our research underscores the importance of diligently controlling the ratio of curing agents to optimize the thermal properties of PN resin. Furthermore, our findings suggest that this resin holds immense potential for expanded utilization in diverse industrial applications. This article is structured as follows: Section 2 provides a comprehensive overview of the synthesis methodology for the PN resin, along with a detailed description of the relevant experimental apparatus. In Section 3, various ratios of curing agents are employed on the PN resin at reduced heating temperatures and durations compared to previous studies with the aim of enhancing energy efficiency. To achieve this, five distinct heating profiles are pre-examined to determine the optimal processing conditions. The physicochemical properties of the resulting samples are subsequently compared, and the sample exhibiting the highest temperature resistance is selected for further application in glass fiber.
2 Experimental
All of the starting materials were of reagent grade and were used without purification. PN resin was synthesized by the following route [29]. A mixture of 4-fluorobenzonitrile (200 g, 1.65 mol), benzoyl chloride (93 g, 0.66 mol), ammonium chloride (106 g, 1.98 mol), and aluminum chloride (106 g, 0.79 mol) was heated to 150°C for 24 h. Cold water (1 L) was then added to the mixture dropwise until the temperature reached 0°C. Afterward, hydrochloric acid (36.5%, 200 g) was added to the cooled mixture. The resulting mixture (Compound 1) was filtered and washed with distilled water. To obtain compound 2, 4,4′-dihydroxybiphenyl (82 g, 0.44 mol), calcium carbonate (82 g, 0.58 mol), 1-methyl-2-pyrrolidone (600 mL), and toluene (600 mL) were added to the mixture in one portion, and heated until the temperature reached 150°C. The toluene was then removed through a Dean-Stark apparatus. The mixture without toluene was then allowed to cool to room temperature. The former compound (76 g, 0.22 mol) and 1-methyl-2-pyrrolidone (500 ml) were added to the mixture at 190°C for 3 h. Then, 4-nitrophthalonitrile (89 g, 0.51 mol) and 1-methyl-2-pyrrolidone (500 mL) were added to the mixture at 50°C. The mixture was stirred overnight at 80°C and then cooled to room temperature. The resulting compound was neutralized using sodium hydroxide (5%, 6 L), and a yield of 122 g (60%) was successfully obtained. The chemical structures of the synthesized compounds were verified by scanning electron microscopy (SEM) with energy-dispersive X-ray (EDX) microanalysis using an FEI Quanta 650 FEG SEM (Thermofisher Scientific). With a high glass transition of nearly 400°C, differential scanning calorimetry results were omitted since there was no significant peak observed. Fourier-transform infrared spectrometer (FT-IR) was employed to support the curing process observed via EDX (TENSOR27, Bruker). Thermal properties were determined by time-domain thermogravimetric analysis using TGA8000 (PerkinElmer).
3 Results and discussion
The curing process involves the reaction of the PN resin with a curing agent, which leads to the formation of covalent bonds between the polymer chains. The curing temperature determines the rate and extent of this reaction, as it affects the activation energy required for the reaction to occur. One hindrance to the utilization of PN resin is its requirement for high curing temperatures [30,31]. In this work, we applied a curing stage under 400°C. The compound with varying amounts of the curing agent was heated incrementally, starting at 200°C for 2 h, then 250°C for 1 h, 300°C for 4 h, and finally 350°C for 12 h (Figure 1).

The chemical structure of the curing agent used in this work.
The ratio of the curing agent to the PN resin is also a fundamental parameter that plays a pivotal role in the preparation of these resins. The curing process is a chemical reaction between the curing agent and the PN resin, leading to the formation of crosslinks among the polymer chains. The stoichiometric ratio of the curing agent to the PN resin defines the extent of crosslinking in the final resin, thus influencing its mechanical and thermal characteristics. A curing ratio that exceeds the optimal amount leads to an over-abundance of curing agents, inducing over-crosslinking and brittleness in the final resin. Conversely, if the curing ratio is insufficient, a reduced degree of crosslinking results, leading to decreased mechanical and thermal properties of the PN resin. Moreover, the curing ratio modulates the rate of the curing reaction. Higher curing ratios generally facilitate a quicker curing process, given that more curing agent is available for the reaction. However, excessive curing ratios instigate a rapid exothermic reaction that promotes thermal degradation of the resin, and in severe cases, may even cause a fire. Therefore, the optimal curing ratio must be carefully determined. In this work, the curing ratio was in the range of 2–20%. To the best of our knowledge, investigations into the thermal properties of PN comprising a 20% concentration of curing agent have been scarcely explored in previous studies. For simplicity, the samples in this work are referred to as curing ratio-PN.
Figures 2 and 3 depict the SEM images and EDX microanalyses of the samples, respectively. Analyzing the data set, it is evident that all the samples contain significant amounts of C and O, which is a common feature of the PN resin (Table 1). Interestingly, there is a discernible increase in the N and S content in the samples as the proportion of curing agents is increased. This trend becomes more apparent when comparing the 2-PN sample to the 20-PN sample. The former, with the lowest proportion of curing agents, also has the lowest N and S content. On the other hand, the latter with a higher proportion of curing agents displays the highest N and S content. The S content is mainly due to the curing agent. The S-containing curing agent can act as co-catalysts to accelerate the curing process. This is due to their ability to react with the PN groups and generate reactive species that promote cross-linking and network formation. As a result, the curing time and temperature required for PN resin can be reduced, which improves the efficiency and cost-effectiveness of the process. S atoms can also form strong covalent bonds with the PN groups, which can prevent the molecular chains of the PN resin from unraveling at high temperatures. Therefore, it can be inferred that having a proper proportion of curing agent may improve the thermal stability of the resin. FT-IR spectroscopy was employed to further examine the curing reaction (Figure 4). All samples displayed triazine peaks at 1,520 and 1,360 cm−1. The CN group at 2,230 cm−1 weakened after the curing reaction, which is in line with relevant studies [32].

Scanning electron microscopic images of PN resins with the curing agent proportion of (a) 2%, (b) 5%, (c) 10%, and (d) 20%. EDX microanalyses (red box) are shown in Figure 3.

EDX microanalyses of PN resins with the curing agent proportion of (a) 2%, (b) 5%, (c) 10%, and (d) 20%.
Elemental characteristics of samples
| Curing agent (%) | Element | Weight (%) | Atomic (%) |
|---|---|---|---|
| 2 | C | 80.22 | 84.27 |
| N | 6.53 | 5.88 | |
| O | 11.73 | 9.25 | |
| S | 1.52 | 0.60 | |
| 5 | C | 79.06 | 83.33 |
| N | 7.45 | 6.74 | |
| O | 11.62 | 9.20 | |
| S | 1.87 | 0.74 | |
| 10 | C | 76.24 | 81.15 |
| N | 8.13 | 7.42 | |
| O | 12.98 | 10.37 | |
| S | 2.65 | 1.06 | |
| 20 | C | 74.37 | 79.57 |
| N | 9.22 | 8.41 | |
| O | 13.07 | 10.43 | |
| S | 3.34 | 1.59 |

Fourier-transform infrared spectroscopy spectra of samples (a) before and (b) after the curing process with various ratios of curing agents.
The thermal stability of a polymer is a critical factor in determining its suitability for various industrial applications. The early decomposition peaks that occur before 400°C are mainly attributed to the moisture and the breakdown of impurities present in the resin [33,34]. However, the major decomposition process commences at 500°C, indicating the presence of strong intermolecular forces after the curing process (Figure 5). Table 2 shows that the samples have high thermal stability and are capable of withstanding high temperatures due to their strong intermolecular forces. Furthermore, the thermal decomposition of 5 wt% samples was observed to occur in a specific order, with 10-PN exhibiting the highest thermal stability and 2-PN the lowest. This suggests that the utilization of a 2–5% ratio of a curing agent is insufficient for an efficacious interaction with the PN resin during the curing process. It is essential to note that the curing process is crucial in enhancing the thermal properties of the polymer, and an inefficient curing process may result in reduced thermal stability [35,36]. However, the utilization of a curing agent in excess of 10% was observed not to be as effective in enhancing the thermal properties, possibly due to surpassing the permissible curing capacity of the PN resin utilized in this study. This could lead to the formation of residual chains, subsequent to the primary decomposition of the curing agent, which may not be adequately linked to the moiety chain of the PN resin, thereby diminishing the overall efficacy of the curing process. The utilization of an excess curing agent may also result in the formation of voids and defects in the resin, which can further compromise the thermal stability of the polymer. Further research is currently underway to elucidate the intricacies of the decomposition mechanism under consideration.

Thermogravimetric analyses of samples with various ratios of curing agents in an air atmosphere.
Thermal decomposition (Td5%) of samples with various ratios of curing agents
| %Curing ratio | Td5% (°C) |
|---|---|
| 2 | 548.1 |
| 5 | 558.8 |
| 10 | 567.3 |
| 20 | 573.1 |
There are several early decomposition peaks that take place prior to 400°C, caused by the breakdown of impurities as depicted in Figure 5. The major decomposition process starts at 500°C, which suggests the presence of strong intermolecular forces after the curing process as shown in Table 2. The thermal decomposition of a 5 wt% sample was observed to occur in the following order: 10-PN > 20-PN > 5-PN > 2-PN. The present findings suggest that the utilization of a 2–5% ratio of a curing agent is insufficient for an efficacious interaction with the PN resin during the curing process. Moreover, it was observed that utilizing a curing agent in excess of 10% was not as effective in enhancing the thermal properties. This could be attributed to the fact that the use of a curing agent in excess of 10% may surpass the permissible curing capacity of the PN resin utilized in this study. It is plausible that the residual chains, subsequent to the primary decomposition of the curing agent, may not be adequately linked to the moiety chain of the PN resin, thereby diminishing the overall efficacy of the curing process. Further research is currently underway to elucidate the intricacies of the decomposition mechanism under consideration.
The incorporation of reinforcing fillers such as glass fibers has become a prevalent approach in enhancing the thermal properties of polymer composites. In recent years, it has gained considerable attention as a promising method to improve the stability and durability of polymer-based materials [37,38,39]. The highest thermal property sample (20-PN) in the current study was fabricated by affixing the 20-PN resin to the glass fiber. The process involved heating the 20-PN resin to 200°C, followed by adding the curing agent and stirring the mixture until the resin liquefied. The resulting blend was then applied to the glass fibers and dried for 24 h at 90°C in a vacuum oven, as shown in Figure 6. It is worth noting that the observed 9% increase in thermal decomposition achieved through glass fiber reinforcement, as indicated by the comparison between 20-PN alone and 20-PN-glass fiber in Figure 7, can be attributed to several underlying factors. First, glass fibers are known for their high thermal stability, which can protect the underlying resin matrix from degradation at elevated temperatures. Second, the incorporation of glass fibers can lead to the creation of a thermally stable interphase region at the interface between the fiber and resin matrix. This interphase region can improve the overall thermal stability of 20-PN by preventing degradation in the polymer matrix. Moreover, glass fibers can act as a physical barrier, blocking the diffusion of oxygen and other volatile products during the degradation process. As a result, the likelihood of thermal oxidation and mass loss is reduced. In addition to these mechanisms, the high aspect ratio of glass fibers can also play a crucial role in enhancing the thermal properties of polymer composites. The elongated shape of the fibers can help distribute stress and improve the mechanical properties of the composite, leading to a more uniform degradation behavior. This enhanced mechanical performance can also contribute to the improved thermal stability of the composite. By increasing the mechanical strength and stiffness of the composite, thermal stability can be enhanced through a reduction in the formation of microcracks and other defects in the composite.

20-PN in (a) powder form was heated to create (b) a liquified prepolymer and then introduced to (c) glass fiber.
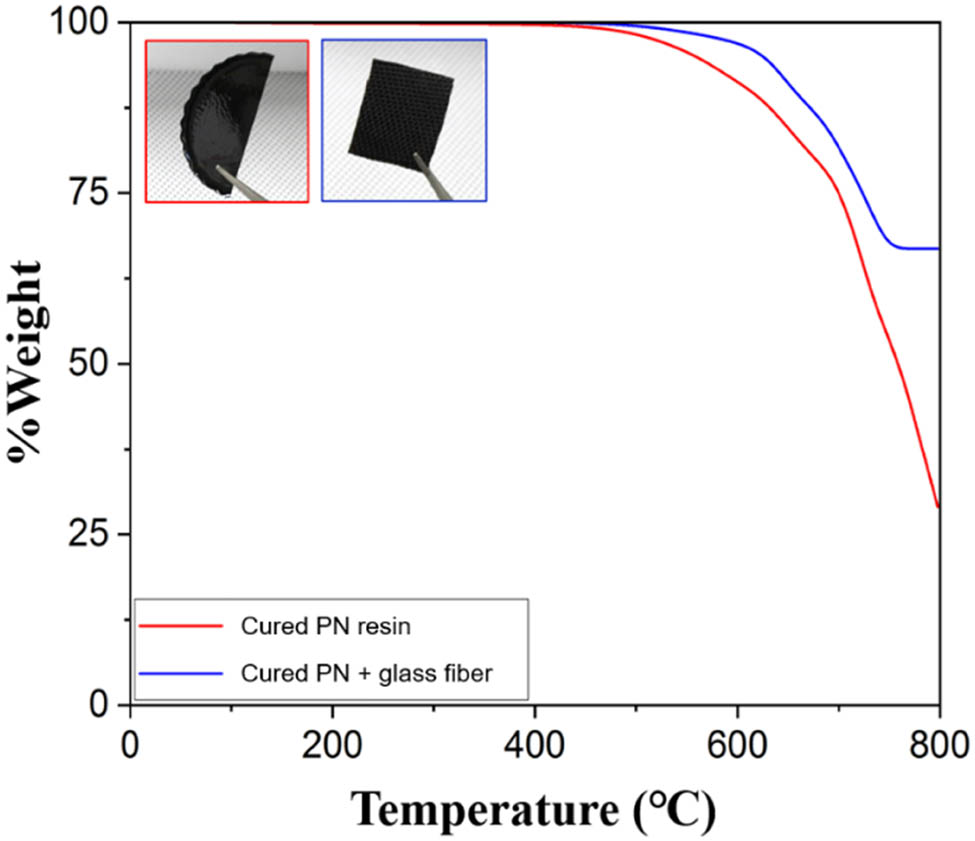
Thermogravimetric analyses of samples with various syntheses in an air atmosphere.
It is important to emphasize that the observed improvement in the thermal properties of PN through glass fiber application is a result of the combined effect of the various underlying mechanisms mentioned above. It is also worth noting that the optimal level of reinforcement required to achieve the desired thermal properties depends on the specific application requirements and the method of manufacture. In summary, the results of the current study suggest that the thermal properties of PN resin can be readily enhanced to reach temperatures as high as 600°C or beyond, contingent on the specific method of reinforcement employed. The use of glass fibers as a reinforcing filler is an effective approach to improve the thermal stability of PN composites, and this could have significant implications for various high-temperature applications.
4 Conclusions
Proper use of curing agents is essential for optimal thermal stability in high-temperature polymers like PN resin. Here, a 20% concentration showed the best physiochemical characteristics. Combining 20-PN with a glass fiber matrix further enhances thermal stability. Upcoming research should focus on refining the curing agent selection and optimizing manufacturing processes. By optimizing the curing process, as well as other manufacturing processes, the PN resin may exhibit extraordinary thermal resistance up to temperatures as high as 600°C. Such remarkable thermal stability makes PN resin an extremely promising material for applications that require withstanding high-temperature conditions.
-
Funding information: This research was funded by Agency for Defense Development, grant number 912989201.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data can be shared upon request.
References
[1] Derradji M, Wang J, Liu W. Phthalonitrile resins’ properties. Phthalonitrile Resins Compos. 2018;2:55–106.10.1016/B978-0-12-812966-1.00002-0Search in Google Scholar
[2] Capricho JC, Fox B, Hameed N. Multifunctionality in epoxy resins. Polym Rev. 2019;60(1):1–41.10.1080/15583724.2019.1650063Search in Google Scholar
[3] Liu Q, Wang D, Li Z, Li Z, Peng X, Liu C, et al. Recent developments in the flame-retardant system of epoxy resin. Materials. 2020;13(9):2145.10.3390/ma13092145Search in Google Scholar PubMed PubMed Central
[4] Xu Y, Guo L, Zhang H, Zhai H, Ren H. Research status, industrial application demand and prospects of phenolic resin. RSC Adv. 2019;9(50):28924–35.10.1039/C9RA06487GSearch in Google Scholar PubMed PubMed Central
[5] Mougel C, Garnier T, Cassagnau P, Sintes-Zydowicz N. Phenolic foams: A review of mechanical properties, fire resistance and new trends in phenol substitution. Polymer. 2019;164:86–117.10.1016/j.polymer.2018.12.050Search in Google Scholar
[6] Seraji SM, Song P, Varley RJ, Bourbigot S, Voice D, Wang H. Fire-retardant unsaturated polyester thermosets: The state-of-the-art, challenges and opportunities. Chem Eng J. 2022;430:132785.10.1016/j.cej.2021.132785Search in Google Scholar
[7] Wang G, Han Y, Guo Y, Sun J, Wang S, Zhou H, et al. Phthalonitrile terminated fluorene based copolymer with Outstanding thermal and mechanical properties. Eur Polym J. 2019;113:1–11.10.1016/j.eurpolymj.2019.01.040Search in Google Scholar
[8] Wu M, Xu J, Bai S, Chen X, Yu X, Naito K, et al. A high-performance functional phthalonitrile resin with a low melting point and a low dielectric constant. Soft Matter. 2020;16(7):1888–96.10.1039/C9SM02328CSearch in Google Scholar PubMed
[9] Kumar D, Choudhary V. Curing kinetics and thermal properties of imide containing phthalonitrile resin using aromatic amines. Thermochim Acta. 2020;693:178749.10.1016/j.tca.2020.178749Search in Google Scholar
[10] Zheng J, Zhang Y, Wang Y, Gan J, Shen L, Luo FB, et al. Synthesis and characterization of a lateral phthalonitrile functionalized main-chain Polybenzoxazine precursor. Macromol Res. 2016;24(5):409–14.10.1007/s13233-016-4061-1Search in Google Scholar
[11] Liu J, Wang S, Peng Y, Zhu J, Zhao W, Liu X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog Polym Sci. 2021;113:101353.10.1016/j.progpolymsci.2020.101353Search in Google Scholar
[12] Derradji M, Mehelli O, Liu W, Fantuzzi N. Sustainable and ecofriendly chemical design of high performance bio-based thermosets for Advanced Applications. Front Chem. 2021;9:1–8.10.3389/fchem.2021.691117Search in Google Scholar PubMed PubMed Central
[13] Liu J, Zhang L, Shun W, Dai J, Peng Y, Liu X. Recent development on Bio‐based thermosetting resins. J Polym Sci. 2021;59(14):1474–90.10.1002/pol.20210328Search in Google Scholar
[14] Chen Z, Wang L, Lin J, Du L. A theoretical insight into the curing mechanism of phthalonitrile resins promoted by aromatic amines. Phys Chem Chem Phys. 2021;23(32):17300–9.10.1039/D1CP01947CSearch in Google Scholar
[15] Bai S, Sun X, Wu M, Shi X, Chen X, Yu X, et al. Effects of pure and intercalated Halloysites on thermal properties of phthalonitrile resin nanocomposites. Polym Degrad Stab. 2020;177:109192.10.1016/j.polymdegradstab.2020.109192Search in Google Scholar
[16] Terekhov VE, Aleshkevich VV, Afanaseva ES, Nechausov SS, Babkin AV, Bulgakov BA, et al. Bis(4-cyanophenyl) phenyl phosphate as viscosity reducing comonomer for phthalonitrile resins. React Funct Polym. 2019;139:34–41.10.1016/j.reactfunctpolym.2019.03.010Search in Google Scholar
[17] Belsky KS, Sulimov AV, Bulgakov BA, Babkin AV, Kepman AV. Hydrolysis rate constants and activation parameters for phosphate- and phosphonate-bridged phthalonitrile monomers under acid, neutral and alkali conditions. Data Brief. 2017;13:10–7.10.1016/j.dib.2017.05.015Search in Google Scholar PubMed PubMed Central
[18] Ma J-Z, Cheng K, Lv J-B, Chen C, Hu J-H, Zeng K, et al. Phthalonitrile-PPO blends: Cure behavior and properties. Chin J Polym Sci. 2017;36(4):497–504.10.1007/s10118-018-2026-xSearch in Google Scholar
[19] Cheng K, Lv JB, Ma JZ, Hu JH, Chen C, Zeng K, et al. The curing behavior and properties of phthalonitrile resins using ionic liquids as a new class of curing agents. Express Polym Lett. 2017;11(11):924–34.10.3144/expresspolymlett.2017.88Search in Google Scholar
[20] Su C-T, Lin K-Y, Lee T-J, Liang M. Preparation, characterization and curing properties of epoxy-terminated poly(alkyl-phenylene oxide)s. Eur Polym J. 2010;46(7):1488–97.10.1016/j.eurpolymj.2010.04.016Search in Google Scholar
[21] Chua J, Tu Q. A molecular dynamics study of crosslinked phthalonitrile polymers: The effect of crosslink density on thermomechanical and dielectric properties. Polymers. 2018;10(1):64.10.3390/polym10010064Search in Google Scholar PubMed PubMed Central
[22] Jia Y, Bu X, Dong J, Zhou Q, Liu M, Wang F, et al. Catalytic polymerization of phthalonitrile resins by carborane with enhanced thermal oxidation resistance: Experimental and molecular simulation. Polymers. 2022;14(1):219.10.3390/polym14010219Search in Google Scholar PubMed PubMed Central
[23] Laskoski M, Dyatkin B, Osti NC, Keum JK, Mamontov E, Butler T. Understanding curing dynamics of arylacetylene and phthalonitrile thermoset blends. J Polym Sci. 2022;61(2):132–42.10.1002/pol.20220401Search in Google Scholar
[24] Nechausov S, Aleksanova A, Morozov O, Babkin A, Kepman A, Avdeev V, et al. Heat-resistant phthalonitrile-based resins for 3D printing via VAT photopolymerization. ACS Appl Polym Mater. 2022;4(10):6958–68.10.1021/acsapm.2c00874Search in Google Scholar
[25] Huang Y, Liu J, Zheng P, Feng M, Chen J, Liu X. Phthalonitrile end-capped sulfonated polyarylene ether nitriles for low-swelling proton exchange membranes. J Polym Res. 2016;23:1–6.10.1007/s10965-016-1150-ySearch in Google Scholar
[26] Aleshkevich VV, Bulgakov BA, Lipatov YV, Babkin AV, Kepman AV. High performance carbon–carbon composites obtained by a two-step process from phthalonitrile matrix composites. Mendeleev Commun. 2022;32(3):327–30.10.1016/j.mencom.2022.05.011Search in Google Scholar
[27] Guseva DV, Rudyak VY, Komarov PV, Sulimov AV, Bulgakov BA, Chertovich AV. Crosslinking mechanisms, structure and glass transition in phthalonitrile resins: Insight from computer multiscale simulations and experiments. J Polym Sci Part B Polym Phys. 2017;56(5):362–74.10.1002/polb.24548Search in Google Scholar
[28] Yang X, Lei W, Liu Q, Li Y, Li K, Wang P, et al. A tailor-made method to recycle slow-curing thermoset of phthalonitrile by constructing self-composite with the improved properties. Compos Commun. 2021;26:100791.10.1016/j.coco.2021.100791Search in Google Scholar
[29] Zong L, Liu C, Zhang S, Wang J, Jian X. Enhanced thermal properties of phthalonitrile networks by cooperating phenyl-S-triazine moieties in Backbones. Polymer. 2015;77:177–88.10.1016/j.polymer.2015.09.035Search in Google Scholar
[30] Weng Z, Hu Y, Qi Y, Zhang S, Liu C, Wang J, et al. Enhanced properties of phthalonitrile resins under lower curing temperature via complex curing agent. Polym Adv Technol. 2019;31(2):233–9.10.1002/pat.4762Search in Google Scholar
[31] He X, Liao S, Chen M, Li R, Liu Y, Liang B, et al. Study on the phthalonitrile cured via bio-tyrosine cyclic peptide: Achieving good thermal properties under low post-curing temperature. Polym Degrad Stab. 2020;181:109289.10.1016/j.polymdegradstab.2020.109289Search in Google Scholar
[32] Zhang D, Liu X, Bai X, Zhang Y, Wang G, Zhao Y, et al. Synthesis, characterization and properties of phthalonitrile-etherified Resole Resin. e-Polymers. 2020;20(1):500–9.10.1515/epoly-2020-0051Search in Google Scholar
[33] Slapnik J, Lucyshyn T, Pinter G. Relationships between the decomposition behaviour of renewable fibres and their reinforcing effect in composites processed at high temperatures. Polymers. 2021;13(24):4448.10.3390/polym13244448Search in Google Scholar PubMed PubMed Central
[34] Tsioptsias C. On the specific heat and mass loss of thermochemical transition. Chem Thermodyn Therm Anal. 2022;8:100082.10.1016/j.ctta.2022.100082Search in Google Scholar
[35] Abliz D, Duan Y, Steuernagel L, Xie L, Li D, Ziegmann G. Curing methods for advanced polymer composites - A Review. Polym Polym Compos. 2013;21(6):341–8.10.1177/096739111302100602Search in Google Scholar
[36] Pascault J-P, Williams RJ. Thermosetting polymers. Handbook of polymer synthesis, characterization, and processing. Wiley; 2013. p. 519–33.10.1002/9781118480793.ch28Search in Google Scholar
[37] Yao Y, Zeng X, Guo K, Sun R, Xu J-B. The effect of interfacial state on the thermal conductivity of functionalized al2o3 filled glass fibers reinforced polymer composites. Compos Part A Appl Sci Manuf. 2015;69:49–55.10.1016/j.compositesa.2014.10.027Search in Google Scholar
[38] Senthil Kumar MS, Mohana Sundara Raju N, Sampath PS, Chithirai Pon Selvan M. Influence of nanoclay on mechanical and thermal properties of glass fiber reinforced polymer nanocomposites. Polym Compos. 2016;39(6):1861–8.10.1002/pc.24139Search in Google Scholar
[39] Nayak RK, Ray BC. Water absorption, residual mechanical and thermal properties of hydrothermally conditioned nano-al2o3 enhanced glass fiber reinforced polymer composites. Polym Bull. 2017;74(10):4175–94.10.1007/s00289-017-1954-xSearch in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Effects of cellulose nanofibers on flexural behavior of carbon-fiber-reinforced polymer composites with delamination
- Damage mechanisms of bismaleimide matrix composites under transverse loading via quasi-static indentation
- Experimental study on hydraulic fracture behavior of concrete with wedge-splitting testing
- The assessment of color adjustment potentials for monoshade universal composites
- Metakaolin-based geopolymers filled with volcanic fly ashes: FT-IR, thermal characterization, and antibacterial property
- The effect of temperature on the tensile properties and failure mechanisms of two-dimensional braided composites
- The influence of preparation of nano-ZrO2/α-Al2O3 gradient coating on the corrosion resistance of 316L stainless steel substrate
- A numerical study on the spatial orientation of aligning fibrous particles in composites considering the wall effect
- A simulative study on the effect of friction coefficient and angle on failure behaviors of GLARE subjected to low-velocity impact
- Impact resistance capacity and degradation law of epoxy-coated steel strand under the impact load
- Analytical solutions of coupled functionally graded conical shells of revolution
- The influence of water vapor on the structural response of asphalt pavement
- A non-invasive method of glucose monitoring using FR4 material based microwave antenna sensor
- Chloride ion transport and service life prediction of aeolian sand concrete under dry–wet cycles
- Micro-damage analysis and numerical simulation of composite solid propellant based on in situ tensile test
- Experimental study on the influence of high-frequency vibratory mixing on concrete performance
- Effects of microstructure characteristics on the transverse moisture diffusivity of unidirectional composite
- Gradient-distributed ZTAp-VCp/Fe45 as new anti-wear composite material and its bonding properties during composite casting
- Experimental evaluation of velocity sensitivity for conglomerate reservoir rock in Karamay oil field
- Mechanical and tribological properties of C/C–SiC ceramic composites with different preforms
- Mechanical property improvement of oil palm empty fruit bunch composites by hybridization using ramie fibers on epoxy–CNT matrices
- Research and analysis on low-velocity impact of composite materials
- Optimizing curing agent ratios for high-performance thermosetting phthalonitrile-based glass fibers
- Method for deriving twisting process parameters of large package E-glass yarn by measuring physical properties of bobbin yarn
- A probability characteristic of crack intersecting with embedded microcapsules in capsule-based self-healing materials
- An investigation into the effect of cross-ply on energy storage and vibration characteristics of carbon fiber lattice sandwich structure bionic prosthetic foot
- Preparation and application of corona noise-suppressing anti-shedding materials for UHV transmission lines
- XRD analysis determined crystal cage occupying number n of carbon anion substituted mayenite-type cage compound C12A7: nC
- Optimizing bending strength of laminated bamboo using confined bamboo with softwoods
- Hydrogels loaded with atenolol drug metal–organic framework showing biological activity
- Creep analysis of the flax fiber-reinforced polymer composites based on the time–temperature superposition principle
- A novel 3D woven carbon fiber composite with super interlayer performance hybridized by CNT tape and copper wire simultaneously
- Effect of aggregate characteristics on properties of cemented sand and gravel
- An integrated structure of air spring for ships and its strength characteristics
- Modeling and dynamic analysis of functionally graded porous spherical shell based on Chebyshev–Ritz approach
- Failure analysis of sandwich beams under three-point bending based on theoretical and numerical models
- Study and prediction analysis on road performance of basalt fiber permeable concrete
- Prediction of the rubberized concrete behavior: A comparison of gene expression programming and response surface method
- Study on properties of recycled mixed polyester/nylon/spandex modified by hydrogenated petroleum resin
- Effect of particle size distribution on microstructure and chloride permeability of blended cement with supplementary cementitious materials
- In situ ligand synthesis affording a new Co(ii) MOF for photocatalytic application
- Fracture research of adhesive-bonded joints for GFRP laminates under mixed-mode loading condition
- Influence of temperature and humidity coupling on rutting deformation of asphalt pavement
- Review Articles
- Sustainable concrete with partial substitution of paper pulp ash: A review
- Durability and microstructure study on concrete made with sewage sludge ash: A review (Part Ⅱ)
- Mechanical performance of concrete made with sewage sludge ash: A review (Part Ⅰ)
- Durability and microstructure analysis of concrete made with volcanic ash: A review (Part II)
- Communication
- Calculation of specific surface area for tight rock characterization through high-pressure mercury intrusion
- Special Issue: MDA 2022
- Vibration response of functionally graded material sandwich plates with elliptical cutouts and geometric imperfections under the mixed boundary conditions
- Analysis of material removal process when scratching unidirectional fibers reinforced polyester composites
- Tailoring the optical and UV reflectivity of CFRP-epoxy composites: Approaches and selected results
- Fiber orientation in continuous fiber-reinforced thermoplastics/metal hybrid joining via multi-pin arrays
- Development of Mg-based metal matrix biomedical composites for acicular cruciate ligament fixation by reinforcing with rare earth oxide and hydroxyapatite – A mechanical, corrosion, and microstructural perspective
- Special Issue: CACMSE
- Preparation and application of foamed ceramic panels in interior design
Articles in the same Issue
- Regular Articles
- Effects of cellulose nanofibers on flexural behavior of carbon-fiber-reinforced polymer composites with delamination
- Damage mechanisms of bismaleimide matrix composites under transverse loading via quasi-static indentation
- Experimental study on hydraulic fracture behavior of concrete with wedge-splitting testing
- The assessment of color adjustment potentials for monoshade universal composites
- Metakaolin-based geopolymers filled with volcanic fly ashes: FT-IR, thermal characterization, and antibacterial property
- The effect of temperature on the tensile properties and failure mechanisms of two-dimensional braided composites
- The influence of preparation of nano-ZrO2/α-Al2O3 gradient coating on the corrosion resistance of 316L stainless steel substrate
- A numerical study on the spatial orientation of aligning fibrous particles in composites considering the wall effect
- A simulative study on the effect of friction coefficient and angle on failure behaviors of GLARE subjected to low-velocity impact
- Impact resistance capacity and degradation law of epoxy-coated steel strand under the impact load
- Analytical solutions of coupled functionally graded conical shells of revolution
- The influence of water vapor on the structural response of asphalt pavement
- A non-invasive method of glucose monitoring using FR4 material based microwave antenna sensor
- Chloride ion transport and service life prediction of aeolian sand concrete under dry–wet cycles
- Micro-damage analysis and numerical simulation of composite solid propellant based on in situ tensile test
- Experimental study on the influence of high-frequency vibratory mixing on concrete performance
- Effects of microstructure characteristics on the transverse moisture diffusivity of unidirectional composite
- Gradient-distributed ZTAp-VCp/Fe45 as new anti-wear composite material and its bonding properties during composite casting
- Experimental evaluation of velocity sensitivity for conglomerate reservoir rock in Karamay oil field
- Mechanical and tribological properties of C/C–SiC ceramic composites with different preforms
- Mechanical property improvement of oil palm empty fruit bunch composites by hybridization using ramie fibers on epoxy–CNT matrices
- Research and analysis on low-velocity impact of composite materials
- Optimizing curing agent ratios for high-performance thermosetting phthalonitrile-based glass fibers
- Method for deriving twisting process parameters of large package E-glass yarn by measuring physical properties of bobbin yarn
- A probability characteristic of crack intersecting with embedded microcapsules in capsule-based self-healing materials
- An investigation into the effect of cross-ply on energy storage and vibration characteristics of carbon fiber lattice sandwich structure bionic prosthetic foot
- Preparation and application of corona noise-suppressing anti-shedding materials for UHV transmission lines
- XRD analysis determined crystal cage occupying number n of carbon anion substituted mayenite-type cage compound C12A7: nC
- Optimizing bending strength of laminated bamboo using confined bamboo with softwoods
- Hydrogels loaded with atenolol drug metal–organic framework showing biological activity
- Creep analysis of the flax fiber-reinforced polymer composites based on the time–temperature superposition principle
- A novel 3D woven carbon fiber composite with super interlayer performance hybridized by CNT tape and copper wire simultaneously
- Effect of aggregate characteristics on properties of cemented sand and gravel
- An integrated structure of air spring for ships and its strength characteristics
- Modeling and dynamic analysis of functionally graded porous spherical shell based on Chebyshev–Ritz approach
- Failure analysis of sandwich beams under three-point bending based on theoretical and numerical models
- Study and prediction analysis on road performance of basalt fiber permeable concrete
- Prediction of the rubberized concrete behavior: A comparison of gene expression programming and response surface method
- Study on properties of recycled mixed polyester/nylon/spandex modified by hydrogenated petroleum resin
- Effect of particle size distribution on microstructure and chloride permeability of blended cement with supplementary cementitious materials
- In situ ligand synthesis affording a new Co(ii) MOF for photocatalytic application
- Fracture research of adhesive-bonded joints for GFRP laminates under mixed-mode loading condition
- Influence of temperature and humidity coupling on rutting deformation of asphalt pavement
- Review Articles
- Sustainable concrete with partial substitution of paper pulp ash: A review
- Durability and microstructure study on concrete made with sewage sludge ash: A review (Part Ⅱ)
- Mechanical performance of concrete made with sewage sludge ash: A review (Part Ⅰ)
- Durability and microstructure analysis of concrete made with volcanic ash: A review (Part II)
- Communication
- Calculation of specific surface area for tight rock characterization through high-pressure mercury intrusion
- Special Issue: MDA 2022
- Vibration response of functionally graded material sandwich plates with elliptical cutouts and geometric imperfections under the mixed boundary conditions
- Analysis of material removal process when scratching unidirectional fibers reinforced polyester composites
- Tailoring the optical and UV reflectivity of CFRP-epoxy composites: Approaches and selected results
- Fiber orientation in continuous fiber-reinforced thermoplastics/metal hybrid joining via multi-pin arrays
- Development of Mg-based metal matrix biomedical composites for acicular cruciate ligament fixation by reinforcing with rare earth oxide and hydroxyapatite – A mechanical, corrosion, and microstructural perspective
- Special Issue: CACMSE
- Preparation and application of foamed ceramic panels in interior design

