Abstract
Metal magnesium is mainly produced from the calcined dolomite by the silicothermic production. However, in this process, the reduction temperature is higher while the reaction speed is slow, which results in higher energy consumption and serious environmental problems. In this paper, adding aluminum into the ferrosilicon reducing agent is expected to lower the reaction temperature so as to solve the problems above. The phase transition involved in the whole reduction process including with and without aluminum addition were investigated in details by theoretical calculation and experimental research. The influence of aluminum on the magnesium oxide reduction path was analysis to clarify the internal mechanism. The results show that aluminum added into the ferrosilicon would first react with magnesium oxide to form magnesium vapor and alumina under vacuum pressure of 10 Pa when the temperature rises to 720°C. Then, calcium aluminate would be formed by the reaction of aluminum oxide and calcium oxide. Once the temperature reaches 1150°C, silicon begins to reduce the magnesium oxide to create the silicon oxide that will finally react with calcium oxide to form calcium silicate. When the temperature rises above 1150°C, both the aluminum and silicon will participate in the reduction of magnesium oxide. In the process of heating up, the mixture of aluminum, ferrosilicon and calcined dolomite forms Mg2Al4Si5O18 and Ca3Al2(OH)12 phase with the components in calcined dolomite. Mg2Al4Si5O18 and Ca3Al2(OH)12 phase finally form Ca12Al14O33 phase. The interaction between aluminum and ferrosilicon in the mixture is less; the mixture of aluminum and ferrosilicon first forms Al3FeSi2 phase, and finally has the trend of forming Al4.5FeSi phase. There is a great difference between the phase transformation of aluminum in the mixture of aluminum, ferrosilicon and calcined dolomite and that of aluminum in the mixture of aluminum and ferrosilicon.
1 Introduction
Currently, the silicothermic is the main method for the extraction of magnesium from the calcined dolomite, and the reduction process mainly uses FeSi as the reducing agent to reduce MgO under vacuum conditions at 1200°C [1]. Although the cost of ferrosilicon as reductant is lower, the reduction temperature is higher while the reaction speed is slow [2, 3, 4] , leading to high energy consumption as well as serious environment problems in reduction process [5]. With the improvement of environmental protection requirements, how to lower the energy consumption is one of the problems that the magnesium smelting industry needs to solve. There are some influence factors involved in this problem such as reduction temperature, reduction reaction rate, and the diffusion rate of magnesium vapor during the reduction process. The silicon in ferrosilicon first reacts with magnesium oxide to form SiO2 that will further combines with CaO in calcined dolomite to form calcium silicate [6,7]. The high initial reaction temperature of silicon-reduced magnesium oxide leads to high heating temperature. Ferrosilicon has a higher melting point and the solid-solid reaction process leads to slower reduction reaction rate [8,9]. The diffusion rate of magnesium vapor is mainly affected by the void ratio in the agglomerate. If the void ratio of the reduced product is large, the porosity of the agglomerate will increase.
Based on the analysis above, searching for a reducing agent with lower reduction temperature and faster reduction rate than ferrosilicon is an alternative way to solve this problem. Al and Al(46.7%)–Si(45.7%)–Fe(7.6%) alloy were considered as a promising substitute materials for FeSi [10, 11, 12, 13, 14]. The previous research results showed that these reduction agents can decrease the reduction temperature. The mechanism of magnesium oxide reduction by aluminum and Al–Si–Fe alloy is different from that of ferrosilicon. The initial reaction temperature of aluminum and magnesium oxide is lower. The reason is that aluminum and Al-Si-Fe have a low melting point and a solid-liquid reaction occurs during reduction. The phase change in the reducing process of magnesium oxide by aluminum goes through three stages. These are the formation of MgAl2O4, and Ca12Al14O33 phases, and the phase transformation from MgAl2O4 and Ca12Al7 to CaAl2O4 [10]. Although the reduction process of magnesium oxide by Al and Al–Si–Fe alloy have many advantages, the higher price of these reducing agents makes it difficult to be industrialized.
With the increasing use of aluminum and its alloy materials, more scrap aluminum and its alloy was inevitably produced. Using these scraps aluminum and its alloy as a reducing agent for magnesium oxide is expected to reduce the cost of magnesium reduction. From the current price of waste aluminum and its alloy, it is still high cost to replace ferrosilicon completely with waste aluminum and its alloy. However, it is expected to reduce the cost of reducing magnesium oxide by using aluminum as the additive of ferrosilicon and replacing part of ferrosilicon as the reducing agent. Because there are many kinds of aluminum and its alloys, it is difficult to study them one by one. Therefore, this study focuses on the behavior of aluminum and ferrosilicon in the reduction process of magnesium oxide. Therefore, in this paper, the phase transition of aluminum and ferrosilicon in the reduction process of MgO and the phase transition of the mixture of aluminum and ferrosilicon in the heating process were investigated in details.
2 Theoretical researches
The main phases of calcined dolomite are MgO and CaO. When the mixture of aluminum powder and ferrosilicon powder was used as the reductant, the reduction reaction process of calcined dolomite are as follows:
The database, as implemented in HSC chemistry software 6.0, was used to calculate the ΔG-T relationship of each reaction at a vacuum of 10 Pa. The initial calculated temperature is higher by 50°C than the melting point of aluminum. The equilibrium vapor pressure of magnesium (PMg ) is roughly equal to the residual pressure of the system. The calculation method of Gibbs free energy under vacuum conditions is shown in Eq. 12 and 13. The relationship between Gibbs free energy of each reaction and temperature under vacuum conditions is shown in Figure 1.
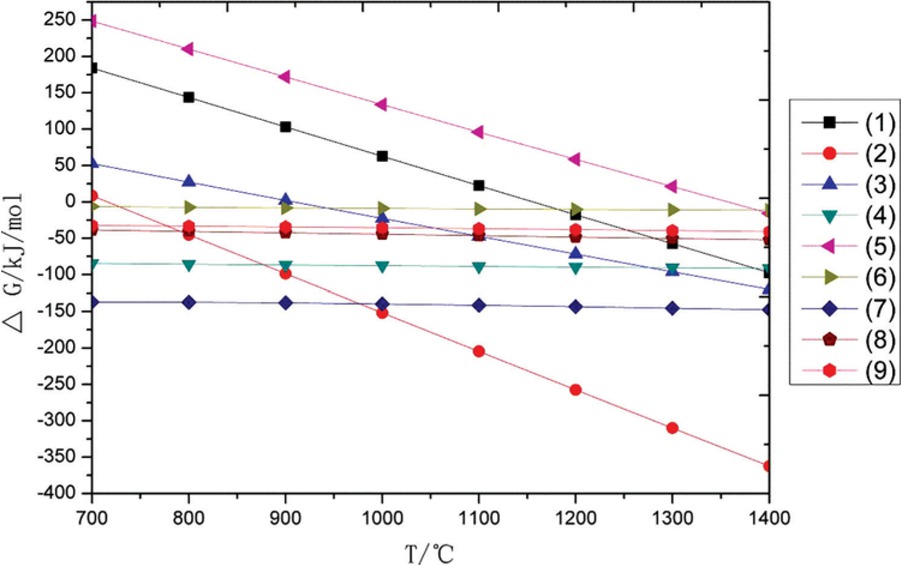
The relationship between Gibbs free energy of each reaction and temperature under vacuum conditions.
Reduction of magnesium oxide by Aluminum:
Reduction of magnesium oxide by Ferrosilicon:
where:
ΔG – the Gibbs free energy at partial pressure in the system at 10 Pa,
ΔGT – the Gibbs free energy at normal pressure,
PMg – the magnesium vapor partial pressure,
P0 – atmospheric pressure that is 101325 Pa.
In Figure 1, the Gibbs free energy of reactions 1, 2, 3 and 5 decreases sharply with the increase of temperature at a vacuum of 10 Pa. Other reactions can be performed spontaneously, such as reactions 4, 6, 7, 8 and 9. The initial reduction temperature of magnesium oxide in calcined dolomite by aluminum is about 720°C (Eq. 2) while that temperature reduced by silicon is around 1150°C (Eq. 1). The magnesium oxide in magnesium aluminate can be reduced by aluminum at 910°C (Eq. 3), but as for the silicon used as reducing agents to reduce magnesium aluminate, it need to heat to the temperature of about 1400°C (Eq. 5).
When aluminum participates alone in the reduction of magnesium oxide in calcined dolomite, the reduction reaction occurs at 720°C, and at the same time, the alumina formed by aluminum reduction of magnesium oxide reacts with the magnesia oxide to form MgO ‧ Al2O3. As the heating temperature is above 910°C, aluminum not only reduces the magnesium oxide in the calcined dolomite, but also begins to reduce the magnesium in the magnesium aluminate. However, when ferrosilicon participates alone in the reduction of magnesium oxide in calcined dolomite, the reduction reaction occurs at 1150°C, and the silica formed by ferrosilicon reduction of magnesium oxide reacts with the calcium oxide in the calcined dolomite to form 2CaO ‧ SiO2. When aluminum and ferrosilicon are both involved in the reduction of magnesium oxide, the reaction of aluminum to reduce magnesium oxide above 720°C, as the temperature is above 910°C, aluminum not only reduces the magnesium oxide in the calcined dolomite, but also begins to reduce the magnesium in the magnesium aluminate. When the heating temperature is higher than 1150°C, ferrosilicon participates in the reduction reaction, but ferrosilicon cannot reduce magnesium aluminate.
3 Experimental
3.1 Materials
Calcined dolomite is taken from Fugu County, Shaanxi Province in China. The main constituents of calcined dolomite are listed in Table 1, the main phases of calcined dolomite are CaCO3, Ca(OH)2, MgO, Mg(OH)2, etc., the X-ray diffraction results of calcined dolomite are shown in Figure 2. Aluminum powder is produced from pure aluminum, and it contains more than 99.5 wt% aluminum element. Ferrosilicon powder is taken from Fugu County, Shaanxi Province in China, and silicon content in ferrosilicon powder is greater than 75 wt%.
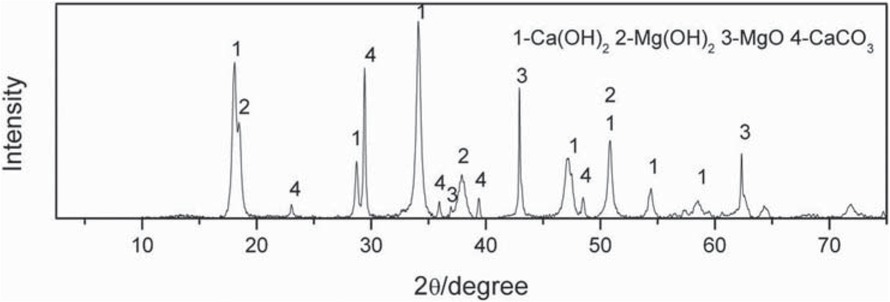
The X-ray diffraction results of calcined dolomite.
Chemical analysis of calcined dolomite.
| Phase | CaO | MgO | Fe2O3 | SiO2 | Al2O3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| Content (wt%) | 56.73 | 42.55 | 0.13 | 0.40 | 0.12 | 0.04 | 0.03 |
3.2 Experimental procedure
3.2.1 Calcined dolomite reduced by ferrosilicon with additive of aluminum
Calcined dolomite, ferrosilicon and aluminum powder were dried respectively at 105°C, and then were weighed and mixed with each other according to the prescribed ratio. Then the mixture was pressed into φ 1 cm × 1.5 cm cylinder. The cylinder was placed in a graphite crucible and put it into a vacuum furnace. The temperature is raised when the vacuum degree in the furnace is less than 10 Pa. The furnace temperature rises to the set temperature and keeps for 1 h. Stop heating when the insulation is sufficient, and cool the material to room temperature with the furnace. The material was crushed and ground, and the X-Ray detected the phase composition of the material.
3.2.2 Phase transformation of the mixture of aluminum and ferrosilicon during heating
Mix ferrosilicon and aluminum powder in proportion and press the block. Other steps are the same as in Section 3.2.1.
3.3 Experimental results and discussion
3.3.1 Calcined dolomite reduced by ferrosilicon with additive of aluminum
According to the raw material ratio of the production process of magnesium smelting enterprises using silicon thermal method, the ratio of ingredients is calcined dolomite + ferrosilicon = 84 wt% + 16 wt%. The 2 wt% ferrosilicon was replaced by aluminum powder in present study, and the ratio of the ingredients in the reduction process is calcined dolomite + ferrosilicon + aluminum powder = 84 wt% + 14 wt% + 2 wt%. The mixture is heated in a vacuum furnace at 700°C, 950°C, 1200°C, respectively. Then the slags were obtained after heating, respectively. The remaining materials are ground and examined by X-ray diffraction. The test results by X-Ray in the Figure 3.
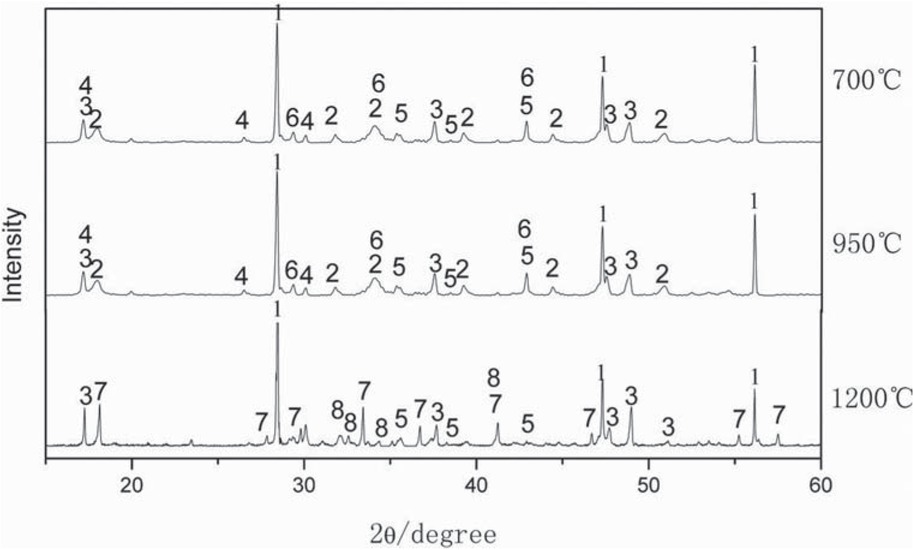
Slag phase at different temperatures.
1 – Si; 2 – Ca(OH)2; 3 – FeSi2; 4 – Ca3Al2(OH)12; 5 – MgO; 6 – Mg2Al4Si5O18; 7 – Ca12Al14O33; 8 – Ca2SiO4
It can be seen from Figure 3 that when heated to 700°C, the main phases of the slag are MgO, Ca(OH)2, Ca3Al2(OH)12, Mg2Al4Si5O18, FeSi2, and Si. When heated to 950°C. The phase composition of the material at 950°C is the same as that at 700°C. When heated further to 1200°C, the main phases of the slag are Si, FeSi2, MgO, Ca12Al14O33, and Ca2SiO4.
Compared with the main phases in calcined dolomite, when heated to 700°C, CaCO3, Mg(OH)2, Ca(OH)2 and other decomposition reactions occurred in the calcined dolomite, and new aluminum-containing compounds such as Mg2Al4Si5O18 and Ca3Al2(OH)12 were generated, indicating that aluminum has participated reactions in mixed materials. Aluminum compounds in the slag cannot be detected by X-ray diffraction due to low aluminum content. According to theoretical calculations, aluminum should participate in the reduction of magnesium oxide in the calcined dolomite at 950°C. The diffraction peak of magnesium oxide weakened in the slag after heating to 1200°C, and Ca12Al14O33 and Ca2SiO4 appeared in the slag, indicating that both aluminum and silicon participate in the reduction of magnesium oxide. Main phase in slag produced by reduction of magnesium oxide with ferrosilicon at 1200°C were Ca2SiO4, Ca12Al14O33, FeSi2, and Si. The presence of FeSi2 and Si in the slag is mainly due to over-dosing during the reduction process. It can be seen that aluminum participates in the reduction reaction and generates a new phase comparing the main phase of the three slags. At 700°C, aluminum and MgO, CaO, silicon-containing compounds, etc. in calcined dolomite form Ca3Al2(OH)12 and Mg2Al4Si5O18. Between 700°C and 950°C, the phase form of aluminum in the mixed material does not change. At 1200°C, aluminum eventually turns into Ca12Al14O33.
3.3.2 High temperature phase transition of aluminum and ferrosilicon mixture
It is difficult to detect the phase transition of aluminum in the reducing slag, because it accounts for a small proportion of the ingredients of the reduced calcined dolomite. In order to explore the phase transition of mixture with aluminum powder and ferrosilicon powder during heating. The aluminum powder is mixed with ferrosilicon and pressed a cylinder, the mixture ratio is aluminum powder + ferrosilicon = 12.5 wt% + 87.5 wt%. The cylinder is heated and kept for 1 h at 700°C, 950°C and 1200°C in vacuum furnace at 10 Pa, respectively. After heating, the heated product is detected by X-Ray detection, and the detection result is shown in Figure 4.
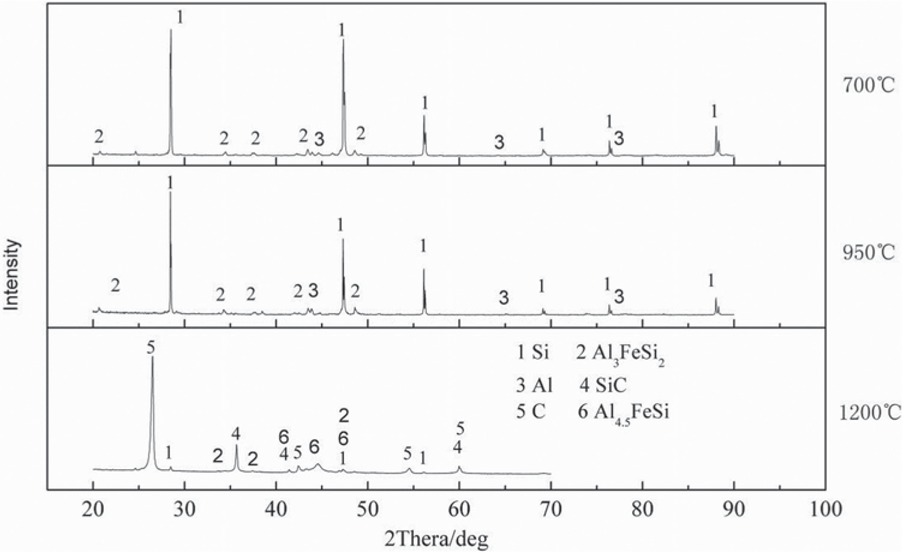
Heating phase diagram of 12.5 wt% aluminum powder and ferrosilicon mixture.
It can be seen from Figure 4 that phase transition has occurred between ferrosilicon and aluminum powder at 700°C. Extremely weak diffraction peaks of the aluminum phase, and a new phase of Al3FeSi2 was produced. The main phase in the product is Si and Al3FeSi2. The phase composition of the heated product is the same at 700°C and 950°C. When the temperature is raised to 1200°C, compared with 700°C and 950°C, the diffraction peak of Si is weakened, the appearance of diffraction characteristic peak of Al4.5FeSi phase and the main phases in the product are Si, SiC, Al4.5FeSi, Al3FeSi2, and C. C comes from graphite crucible, and SiC is produced by the reaction between Si and carbon of graphite crucible. According to the change of diffraction peak intensity, it can be inferred that at 1200°C, Al3FeSi2 phase has a trend of transition to Al4.5FeSi phases.
3.3.3 Phase transformation difference of aluminum in two mixtures
It can be seen from Figure 3 that in the process of heating up, the mixture of aluminum, calcined dolomite and ferrosilicon forms Ca3Al2(OH)12 and Mg2Al4Si5O18 with the components in calcined dolomite, and finally forms Ca12Al14O33, while the main components of ferrosilicon, silicon and FeSi, do not react with aluminum obviously in the process of heating up. In the process of heating up the mixture of aluminum and ferrosilicon, aluminum and ferrosilicon first form Al3FeSi2 phase, and finally form Al4.5FeSi phases.
4 Conclusions
The reaction temperature and reaction products of mixture of aluminum and ferrosilicon in the reduction of magnesium oxide in calcined dolomite were studied by theoretical and experimental methods. The phase transition of mixture of aluminum and ferrosilicon in heating process and in production magnesium oxide were concluded that aluminum and ferrosilicon in the temperature rising process of magnesium oxide reduction, the following conclusions can be drawn:
In the reducing process of magnesium oxide by using a mixture of aluminum and ferrosilicon as a reducing agent, under the vacuum pressure of 10 Pa as the temperature rises, aluminum first reacts with magnesium oxide to form magnesium vapor and alumina, and simultaneously aluminum oxide and calcium oxide further form calcium aluminate above 720°C. When the temperature reaches 1150°C, silicon begins to participate in the reduction reaction of magnesium oxide, while silicon oxide and calcium oxide form calcium silicate. Magnesium oxide can be reduced by both aluminum and silicon when the temperature is above 1150°C.
In the process of heating up, the mixture of aluminum, ferrosilicon and calcined dolomite forms Mg2Al4Si5O18 and Ca3Al2(OH)12 phase with the components in calcined dolomite. Mg2Al4Si5O18 and Ca3Al2(OH)12 phase finally form Ca12Al14O33 phase. The interaction between aluminum and ferrosilicon in the mixture is less; the mixture of aluminum and ferrosilicon first forms Al3FeSi2 phase, and finally has the trend of forming Al4.5FeSi phase. There is a great difference between the phase transformation of aluminum in the mixture of aluminum, ferrosilicon and calcined dolomite and that of aluminum in the mixture of aluminum and ferrosilicon.
Acknowledgements
This study was supported by the Shaanxi Provincial Department of Education special research project (18JK0480) and Shaanxi Provincial Natural Science Foundation Research Fund Project (2018JM5171) for the financial support of the project.
References
[1] Bugdayci M., Turan A., Alkan M., Yucel O., Effect of Reductant Type on the Metallothermic Magnesium Production Process. High Temp. Mater. Proc., 2018, 37(1), 1-8.10.1515/htmp-2016-0197Search in Google Scholar
[2] Yang K., Chen Q., Ren J., Coupled heat transfer and chemical reactions in magnesium production retorts. Journal of Tsinghua University Science and Technology, 2009, 49(5), 755-758.Search in Google Scholar
[3] Li R.B., Zhang S.J., Guo L.J., Wei J.J., Numerical study of magnesium (Mg) production by the Pidgeon process: Impact of heat transfer on Mg reduction process. Int. J. Heat Mass Tran., 2013, 59, 328-337.10.1016/j.ijheatmasstransfer.2012.09.027Search in Google Scholar
[4] Wang C., Zhang S., Guo L., Investigation on the effective thermal conductivity of typical Pidgeon process briquette with a combined model. Int. J. Heat Mass Tran., 2017, 115, 1348-1358.10.1016/j.ijheatmasstransfer.2017.08.064Search in Google Scholar
[5] Gao F., Nie Z.-R., Wang Z.-H., Gong X.-Z., Zuo T.-Y., Assessing environmental impact of magnesium production using Pidgeon process in China. T. Nonferr. Metal. Soc., 2008, 18(3), 749-754.10.1016/S1003-6326(08)60129-6Search in Google Scholar
[6] Li Y., Fan Y., Chen Z., Cheng F., Guo Y., Chemical, Mineralogical and Morphological Characteristics of Pidgeon Magnesium Slag. Environ. Eng. Sci., 2016, 33(4), 290-297.10.1089/ees.2015.0097Search in Google Scholar
[7] Morsi I.M., Ali H.H., Kinetics and mechanism of silicothermic reduction process of calcined dolomite in magnetherm reactor. Int. J. Miner. Process., 2014, 127, 37-43.10.1016/j.minpro.2013.11.016Search in Google Scholar
[8] Baek Y.-H., Lee B.-D., Lee K.-W., Han G.-S., Han J.-W., Study of the Thermal Reduction Behavior of Dolomite by the Pidgeon process. Korean J. Met. Mater., 2016, 54(2), 104-112.10.3365/KJMM.2016.54.2.104Search in Google Scholar
[9] Zhang C., Chu H., Gu M., Zheng S., Experimental and numerical investigation of silicothermic reduction process with detailed chemical kinetics and thermal radiation. Appl. Therm. Eng., 2018, 135, 454-462.10.1016/j.applthermaleng.2018.02.091Search in Google Scholar
[10] Fu D.-X., Feng N.-X., Wang Y.-W., Peng J.-P., Di Y.-Z., Kinetics of extracting magnesium from mixture of calcined magnesite and calcined dolomite by vacuum aluminothermic reduction. T. Nonferr. Metal. Soc., 2014, 24(3), 839-847.10.1016/S1003-6326(14)63133-2Search in Google Scholar
[11] Wang Y., You J., Peng J., Di Y., Production of Magnesium by Vacuum Aluminothermic Reduction with Magnesium Aluminate Spinel as a By-Product. JOM-J. Min. Met. Mat. S., 2016, 68(6), 1728-1736.10.1007/s11837-016-1865-6Search in Google Scholar
[12] You J., Wang Y., Deng X., Liu K., Magnesium Extraction from Calcined Dolomite by Vacuum Thermal Reduction with Solid-Waste of Al-Fe Alloy. Chin. J. Vac. Sci. Tech., 2016, 36(4), 436-441.Search in Google Scholar
[13] Liu Z.-Q., Liu J.-X., Jiang B., Qiu Q., Process for Preparing Magnesium from Dolomite by Vacuum Aluminothermic Reduction. Nonferrous Metals, 2010, 02, 56-58.Search in Google Scholar
[14] Wu X., Thermal reduction of Mg with reduction agent Al-Si-Fe ternary alloy. Nonferrous Metals, 2000, 52(2), 72-74, 97.Search in Google Scholar
© 2020 Ma et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis
Articles in the same Issue
- Obituary for Prof. Dr. Jun-ichi Yoshida
- Regular Articles
- Optimization of microwave-assisted manganese leaching from electrolyte manganese residue
- Crustacean shell bio-refining to chitin by natural deep eutectic solvents
- The kinetics of the extraction of caffeine from guarana seed under the action of ultrasonic field with simultaneous cooling
- Biocomposite scaffold preparation from hydroxyapatite extracted from waste bovine bone
- A simple room temperature-static bioreactor for effective synthesis of hexyl acetate
- Biofabrication of zinc oxide nanoparticles, characterization and cytotoxicity against pediatric leukemia cell lines
- Efficient synthesis of palladium nanoparticles using guar gum as stabilizer and their applications as catalyst in reduction reactions and degradation of azo dyes
- Isolation of biosurfactant producing bacteria from Potwar oil fields: Effect of non-fossil fuel based carbon sources
- Green synthesis, characterization and photocatalytic applications of silver nanoparticles using Diospyros lotus
- Dielectric properties and microwave heating behavior of neutral leaching residues from zinc metallurgy in the microwave field
- Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity
- Microwave-induced heating behavior of Y-TZP ceramics under multiphysics system
- Synthesis and catalytic properties of nickel salts of Keggin-type heteropolyacids embedded metal-organic framework hybrid nanocatalyst
- Preparation and properties of hydrogel based on sawdust cellulose for environmentally friendly slow release fertilizers
- Structural characterization, antioxidant and cytotoxic effects of iron nanoparticles synthesized using Asphodelus aestivus Brot. aqueous extract
- Phase transformation involved in the reduction process of magnesium oxide in calcined dolomite by ferrosilicon with additive of aluminum
- Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater
- The study on the influence of oxidation degree and temperature on the viscosity of biodiesel
- Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR
- Bacterial nanobiotic potential
- Green synthesis and characterization of carboxymethyl guar gum: Application in textile printing technology
- Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review
- Bactericidal and cytotoxic properties of green synthesized nanosilver using Rosmarinus officinalis leaves
- Synthesis of biomass-supported CuNi zero-valent nanoparticles through wetness co-impregnation method for the removal of carcinogenic dyes and nitroarene
- Synthesis of 2,2′-dibenzoylaminodiphenyl disulfide based on Aspen Plus simulation and the development of green synthesis processes
- Catalytic performance of the biosynthesized AgNps from Bistorta amplexicaule: antifungal, bactericidal, and reduction of carcinogenic 4-nitrophenol
- Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey
- Adsorption of l-α-glycerophosphocholine on ion-exchange resin: Equilibrium, kinetic, and thermodynamic studies
- Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies
- Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review
- Synthesis, characterization, and electrochemical properties of carbon nanotubes used as cathode materials for Al–air batteries from a renewable source of water hyacinth
- Optimization of medium–low-grade phosphorus rock carbothermal reduction process by response surface methodology
- The study of rod-shaped TiO2 composite material in the protection of stone cultural relics
- Eco-friendly synthesis of AuNPs for cutaneous wound-healing applications in nursing care after surgery
- Green approach in fabrication of photocatalytic, antimicrobial, and antioxidant zinc oxide nanoparticles – hydrothermal synthesis using clove hydroalcoholic extract and optimization of the process
- Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles
- Green synthesis of 3-(1-naphthyl), 4-methyl-3-(1-naphthyl) coumarins and 3-phenylcoumarins using dual-frequency ultrasonication
- Optimization for removal efficiency of fluoride using La(iii)–Al(iii)-activated carbon modified by chemical route
- In vitro biological activity of Hydroclathrus clathratus and its use as an extracellular bioreductant for silver nanoparticle formation
- Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity
- Propylene carbonate synthesis from propylene oxide and CO2 over Ga-Silicate-1 catalyst
- Environmentally benevolent synthesis and characterization of silver nanoparticles using Olea ferruginea Royle for antibacterial and antioxidant activities
- Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects
- A novel biofabrication of gold nanoparticles using Erythrina senegalensis leaf extract and their ameliorative effect on mycoplasmal pneumonia for treating lung infection in nursing care
- Phytosynthesis of selenium nanoparticles using the costus extract for bactericidal application against foodborne pathogens
- Temperature effects on electrospun chitosan nanofibers
- An electrochemical method to investigate the effects of compound composition on gold dissolution in thiosulfate solution
- Trillium govanianum Wall. Ex. Royle rhizomes extract-medicated silver nanoparticles and their antimicrobial activity
- In vitro bactericidal, antidiabetic, cytotoxic, anticoagulant, and hemolytic effect of green-synthesized silver nanoparticles using Allium sativum clove extract incubated at various temperatures
- The green synthesis of N-hydroxyethyl-substituted 1,2,3,4-tetrahydroquinolines with acidic ionic liquid as catalyst
- Effect of KMnO4 on catalytic combustion performance of semi-coke
- Removal of Congo red and malachite green from aqueous solution using heterogeneous Ag/ZnCo-ZIF catalyst in the presence of hydrogen peroxide
- Nucleotide-based green synthesis of lanthanide coordination polymers for tunable white-light emission
- Determination of life cycle GHG emission factor for paper products of Vietnam
- Parabolic trough solar collectors: A general overview of technology, industrial applications, energy market, modeling, and standards
- Structural characteristics of plant cell wall elucidated by solution-state 2D NMR spectroscopy with an optimized procedure
- Sustainable utilization of a converter slagging agent prepared by converter precipitator dust and oxide scale
- Efficacy of chitosan silver nanoparticles from shrimp-shell wastes against major mosquito vectors of public health importance
- Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization
- Characterizations and analysis of the antioxidant, antimicrobial, and dye reduction ability of green synthesized silver nanoparticles
- Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress
- Green synthesis of silver nanoparticles from Valeriana jatamansi shoots extract and its antimicrobial activity
- Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum
- Effect of calcination temperature on rare earth tailing catalysts for catalytic methane combustion
- Enhanced diuretic action of furosemide by complexation with β-cyclodextrin in the presence of sodium lauryl sulfate
- Development of chitosan/agar-silver nanoparticles-coated paper for antibacterial application
- Preparation, characterization, and catalytic performance of Pd–Ni/AC bimetallic nano-catalysts
- Acid red G dye removal from aqueous solutions by porous ceramsite produced from solid wastes: Batch and fixed-bed studies
- Review Articles
- Recent advances in the catalytic applications of GO/rGO for green organic synthesis

