Abstract
Two new mononuclear complexes, [Zn(3-Hcpa)2(H2O)4] (1) and [Zn(3,5,6-tcpa)2(H2O)4] [Zn(3,5,6-tcpa)2(H2O)2] (2) (3-H2cpa = 3-carboxy-phenoxyacetic acid, 3,5,6-Htcpa = 3,5,6-trichloro pyridine-2-oxyacetic acid), were synthesized and structurally characterized. The single-crystal X-ray diffraction analysis showed that in 1 the ZnII ion lies on an inversion center of an octahedron formed by four aqua ligands and two carboxy oxygen atoms of two unidentate 3-Hcpa− anions in trans-arrangement. Complex 2 is a co-crystal consisting of two discrete and stereochemically different complexes: one with an octahedrally, and the other a tetrahedrally coordinated zinc center. The six-coordination about the first ZnII ion comprises four oxygen atoms from water (H2O) molecules and two from the carboxy groups of monodentate trans-related 3,5,6-tcpa− ligands. The four-coordination about the second ZnII ion is comprised of two H2O ligands and two unidentate carboxy oxygen atoms from 3,5,6-tcpa− ligands. O–HLO hydrogen bond and/or ClLCl halogen bond interactions play an important part in construction of the three-dimensional (3D) networks for 1 and 2. The photoluminescence spectra reveal that both 1 and 2 display luminescent properties in the violet region.
1 Introduction
The design, syntheses and applications of metal complexes based on flexible ligands has become an increasingly important research field in crystal engineering over the past decades, because of the unusual structural diversities and potential applications for gas adsorption, heterogeneous catalysis, proton conduction and other fields in contrast with those based on rigid ligands [1]. In principle, flexible ligands usually contain at least one sp3-hybrid atom (usually C, N, O or S) in their backbones and parts of the molecules can rotate around a single bond. Moreover, the flexible sections can participate in various metal-ligand coordination modes. In our previous research, the four flexible ligands ethylenedithiodiacetic acid (H2edtda) [2], 3-carboxy-phenylacetic acid (3-H2cmb) [3], [4], and 2,2′-oxydiacetic acid (2,2′-H2oda) [5], [6], [7], [8] together with 2-carboxy-phenoxyacetic acid (2-H2cpa) [9], [10] have been introduced to construct novel d- or f-block metal complexes. The results showed that the oxygen acetic acid type ligands which contain sp3-oxygen atoms, such as 2,2′-H2oda and 2-H2cpa, are more effective and prolific in offering flexibility than H2edtda and 3-H2cmb. Thus, our attention has turned to the oxygen acetic acid-type ligands, and we recently focused on two types of them, namely, 3-carboxy-phenoxyacetic acid (3-H2cpa) and 3,5,6-trichloropyridine-2-oxyacetic acid (3,5,6-Htcpa). On the one hand, 3-H2cpa has been confirmed to be an excellent semi-rigid and semi-flexible ligand to build intriguing structures [11], [12], [13], [14], [15], [16], while ZnII compounds are rare [17], [18], [19]. 3,5,6-Htcpa is a herbicide widely used in agriculture and gardening. One binuclear CdII cluster [20], one-dimensional (1D) NiII [21] and CoII [22] polymers containing this ligand have been published by us lately. 3,5,6-Htcpa is the analogue of 2,4,5-trichloro-phenoxyacetic acid (2,4,5-Htcpa) except for the N atom in the 1-position. Several complexes based on 2,4,5-Htcpa have been reported, including mono-, bi-, tri- or tetranuclear compounds of MnII [23], CuII [24], [25] and FeIII [26], [27]. 3,5,6-Htcpa should thus be a good choice for constructing related systems. In addition, Zn-carboxylates are known to display excellent luminescence properties [28], [29], [30], [31]. Based on the above considerations, this paper describes the synthesis and crystal structures of two new mononuclear complexes [Zn(3-Hcpa)2(H2O)4] (1) and [Zn(3,5,6-tcpa)2(H2O)4][Zn(3,5,6-tcpa)2(H2O)2] (2) formed by 3-H2cpa and 3,5,6-Htcpa (Scheme 1). The solid state luminescence properties of them also have been discussed carefully.

The formulas for complexes 1 and 2.
2 Experimental
The 3-carboxy phenoxyacetic acid (3-H2cpa) was prepared by the reaction of chloroacetic acid (Tianjin Damao Chemical Reagent Factory, Tianjin, P. R. China) with 3-hydroxybenzoic acid (Tianjin Bodi Chemical Company Limited, Tianjin, P. R. China) [32], and all other chemicals were of analytical reagent grade and used without further purification. Elemental analyses for C, H and N were carried out on an Elementar Vario EL elemental analyzer (Elementar, Hesse, Germany). Diffraction studies on single crystals were conducted using a Rigaku Oxford Diffraction SuperNova area-detector diffractometer (Rigaku Corporation, Wilmington, MA, USA) using mirror optics-monochromatized MoKα radiation (λ=0.71073 Å) [33]. The fluorescence spectra were recorded on an F-7000 FL spectrophotometer (Hitachi High-Technologies Corporation, Tokyo, Japan) in the solid state at room temperature. The wavelength was scanned at 20 nm s−1. Solid samples of 1 was locked between two glass slides and then measured.
2.1 Preparation of tetraaqua-bis(3-carboxy phenoxyacetate-κO)-zinc (II), [Zn(3-Hcpa)2(H2O)4] (1)
Zn(CH3COO)2·2H2O (Tianjin Yongda Chemical Reagent Company Limited, Tianjin, P. R. China) (0.5 mmol, 0.110 g), 3-carboxy phenoxy acetic acid (3-H2cpa, 0.5 mmol, 0.099 g) were dissolved in 20 mL distilled water and the mixture was neutralized with NaOH (Tianjin Damao Chemical Reagent Factory, Tianjin, P. R. China) (0.1 mol L−1) to pH=4.0. The solution was sealed in a 25 mL Teflon reactor (Gongyi zhanjie Credit Instrument Marketing Department, Gongyi, Henan Province, P. R. China, http://81138565701.shop.fengj.com) and kept under autogeneous pressure at T=393 K for 3 days. After cooling to room temperature at a rate of 279 K·h−1, and filtering, colorless block-shaped crystals had grown from the filtrate by slow evaporation 3 weeks later. Yield: 14 mg (11% based on 3-H2cpa). –C18H22O14Zn (%): calcd. C 40.93, H 4.17; found C 40.99, H 4.11.
2.2 Preparation of tetraaqua-bis(3,5,6- trichloropyridine-2-oxyacetato-κO)- zinc(II) diaqua-bis(3,5,6-trichloropyridine-2-oxyacetato-κO)-zinc(II), [Zn(3,5,6-tcpa)2(H2O)4][Zn(3,5,6-tcpa)2 (H2O)2] (2)
Complex 2 was obtained by a procedure similar to that used for the preparation of 1 except for using Zn(CH3COO)2·2H2O (0.3 mmol, 0.066 g) and 3,5,6-trichloropyridine-2-oxyacetic acid (Hubei Yebang Technology Company Limited, Xiaogan, Hubei Province, P. R. China) (3, 5, 6-Htcpa, 0.3 mmol, 0.077 g) as the starting reactants, and the mixture was adjusted to pH=7.0 with 0.3 mol L−1 KOH (Tianjin Damao Chemical Reagent Factory, Tianjin, P. R. China). After cooling to room temperature, colorless block shaped crystals were obtained and collected. Yield: 33 mg (35%, based on 3,5,6-Htcpa). –C28H24Cl12N4O18Zn2 (%): calcd. C 17.13, H 1.90, N 4.44; found C 17.20, H 1.96, N 4.38.
2.3 Single-crystal X-ray structure determinations
Colorless crystals with dimensions of 0.25×0.22×0.20 mm3 (1) and 0.25×0.23×0.22 mm3 (2) were selected for measurement. A total of 3580 (1) and 30935 (2) reflections were collected in the ranges of 6.74≤2θ≤56.69° (1) and 6.51≤2θ≤56.87° (2) using ω-2θ scans, of which 2143 (1) and 5062 (2) were unique with Rint=0.0350 (1) and 0.0681 (2). Using Olex2 (a complete structure solution, refinement and analysis program, http://www.olex2.org) [34], the structures of 1 and 2 were solved with the Shelxt (crystal structure refinement software, http://shelx.uni-ac.gwdg.de/SHELX/) [35] structure solution program using intrinsic phasing and refined with the Shelxl (an ongoing developmental software for Integrated space-group and crystal structure determination) [36] refinement package using least-squares minimization. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were generated geometrically and treated by a mixture of independent and constrained refinement. The crystal data and collection parameters for 1 and 2 are summarized in Table 1. Selected bond lengths and bond angles are given in Table 2. Hydrogen bonds and ClLCl halogen bond parameters are listed in Table 3.
Crystal structure data for 1 and 2.
| Formula | C18H22O14Zn | C28H24 Cl12N4O18Zn2 |
| Mr | 527.72 | 1260.65 |
| Temperature, K | 293.3(2) | 289.2(3) |
| Crystal size, mm3 | 0.25×0.22×0.20 | 0.25×0.23×0.22 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P1̅ (no. 2) | I2/a (no. 15) |
| a, Å | 4.9608(4) | 19.3069(4) |
| b, Å | 5.8456(5) | 6.0908(10) |
| c, Å | 18.3803(16) | 37.8119(8) |
| α, ° | 97.059(7) | 90 |
| β, ° | 94.867(7) | 98.090(2) |
| γ, ° | 98.080(7) | 90 |
| V, Å3 | 520.88(8) | 4402.22(15) |
| Z | 1 | 4 |
| Dcalcd, g cm−3 | 1.68 | 1.90 |
| μ(MoKα), mm−1 | 1.3 | 1.9 |
| F(000), e | 272 | 2512 |
| hkl range | −6≤h≤6 −6≤k≤+7 −24≤l≤+23 | −24≤h≤25 −7≤k≤+7 −48≤l≤+46 |
| ((sinθ)/λ)max, Å−1 | 0.668 | 0.670 |
| Refl. measured | 3580 | 30935 |
| Refl. unique | 2143 | 5062 |
| Rint | 0.0350 | 0.0681 |
| Param. refined | 154 | 294 |
| R(F)/wR(F2)a,b (all refl.) | 0.0656/0.0964 | 0.0708/0.0843 |
| GoF (F2)c | 1.038 | 1.032 |
| Weighting factors A/Bb | 0.0304/0 | 0.0263/0 |
| Δρfin (max/min), e Å−3 | 0.31/−0.46 | 0.55/−0.47 |
aR(F)=Σ||Fo|–|Fc||/Σ|Fo|; bwR(F2)=[Σw(Fo2–Fc2)2/Σw(Fo2)2]1/2; w=[σ2(Fo2)+(AP)2+BP]−1, where P=(Max(Fo2, 0)+2Fc2)/3; cGoF=S=[Σw(Fo2–Fc2)2/(nobs–nparam)]1/2.
Selected bond lengths (Å) and bond angles (°) for 1 and 2.
| Complex 1 | ||||||
| Zn1–O1 | 2.097(2) | Zn1–O6 | 2.148(2) | |||
| Zn1–O7 | 2.048(2) | O6a–Zn1–O6 | 180 | |||
| O1a–Zn1–O1 | 180 | O7a–Zn1–O7 | 180 | |||
| O7–Zn1–O6 | 90.64(9) | O7–Zn1–O1 | 91.90(8) | |||
| O7a–Zn1–O1 | 88.10(9) | O1–Zn1–O6 | 92.26(8) | |||
| O1a–Zn1–O6 | 87.74(8) | O7a–Zn1–O6 | 89.36(9) | |||
| Complex 2 | ||||||
| Zn1–O1 | 2.129(2) | Zn2–O6 | 1.952 (2) | |||
| Zn1–O4 | 2.095(2) | Zn2–O9 | 1.976(2) | |||
| Zn1–O5 | 2.075(2) | O6–Zn2–O6c | 127.70(1) | |||
| O1b–Zn1–O1 | 180 | O6c–Zn2–O9 | 110.72(8) | |||
| O5b–Zn1–O5 | 180 | O6–Zn2–O9 | 102.51(8) | |||
| O5b–Zn1–O1 | 92.15(7) | O9c–Zn2–O9 | 99.34(11) | |||
| O4b–Zn1–O4 | 180 | O4–Zn1–O1 | 89.25(7) | |||
| O5b–Zn1–O4 | 90.49(7) | O5–Zn1–O4 | 89.51(7) | |||
| O4b–Zn1–O1 | 90.75(7) | O5–Zn1–O1 | 87.85(7) | |||
Symmetry operations: a−x, 1−y, 1−z; b1−x, 1−y, 1−z; c1/2−x, y, 1−z.
Hydrogen bond geometries (Å, °) and/or Cl⋯Cl bond geometries (Å) for 1 and 2.
| Donor-HLAcceptor | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) | Contact type [37] |
|---|---|---|---|---|---|
| Complex 1 | |||||
| O6–H6ALO1a | 0.86 | 2.21 | 2.856(3) | 132.2 | inter |
| O6–H6ALO3a | 0.86 | 2.24 | 3.024(2) | 151.6 | inter |
| O6–H6BLO2b | 0.85 | 1.89 | 2.712(3) | 160.7 | inter |
| O7–H7ALO6c | 0.86 | 2.07 | 2.865(3) | 154.0 | inter |
| O7–H7BLO2 | 0.84 | 1.95 | 2.675(3) | 143.4 | intra |
| O5–H5ALO4d | 0.82 | 1.82 | 2.631(3) | 170.8 | inter |
| Complex 2 | |||||
| O5–H5ALO6 | 0.85 | 2.06 | 2.842(2) | 151.5 | intra(o–t) |
| O9–H9ALO1 | 0.85 | 1.98 | 2.722(2) | 145.4 | intra(t–o) |
| O9–H9BLO7e | 0.85 | 2.09 | 2.708(3) | 128.5 | inter(t–t′) |
| O4–H4ALO7f | 0.86 | 2.16 | 2.836(2) | 134.9 | inter(o–t′) |
| O4–H4BLO2 | 0.86 | 1.88 | 2.646(3) | 146.9 | intra(o–o) |
| ClLCl bonds | ∠C4–ClLCl6g | ∠Cl2LCl6g–C12g | d(Cl2LCl6g) | o–t′ | |
| C4–Cl2L Cl6g–C12g | 157.89 | 150.91 | 3.246 | ||
Symmetry operations: a−1+x, y, z; bx, 1+y, z; c−1−x, 1−y, 1−z; d2−x, −y, 2−z; e1/2−x, 1+y, 1−z; f1/2+x, 1−y, z; gx, 3/2−y, 1/2+z. intra=intra-molecular, inter=inter-molecular, o=octahedral, t=tetrahedral. Primed letters for the co-crystal 2 stand for interactions with neighboring units related by the symmetry operations listed in the first column; see also ref. [37].
CCDC 1821888 (1) and 1936363 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viawww.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Description of the structure of [Zn(3-Hcpa)2(H2O)4] (1)
The molecular structure determination revealed that compound 1 is a mononuclear species and the asymmetric unit comprises one ZnII ion, two monodeprotonated 3-carboxy-phenoxyacetate ligands (3-Hcpa−) and four coordinated water molecules.
The ZnII ion lies on a center of inversion and is six-coordinate with four water molecules [Zn1–O6, 2.148(2) Å; Zn1–O7, 2.048(2) Å; mean 2.098(2) Å] and two trans-related unidentate carboxy oxygen atoms of 3-Hcpa− [Zn1–O1, 2.097(2) Å] in an octahedral geometry (Fig. 1). The mean Zn–Owater bond distance almost equals to those of the Zn–Ocarboxy bonds (Table 2). The trans-O–Zn–O bond angles are 180° (by symmetry) and the cis ones range from 87.74(8)° to 92.26(8)°. These parameters support a nearly idealized octahedron for 1.

The molecular structure of 1, showing the atom numbering scheme. Displacement ellipsoids are shown at the 30% probability level. The dashed lines show the intra-molecular O–HLO hydrogen bonds.
The packing of the complex 1 is constructed by O–HLO hydrogen bonding interactions (Fig. 2 and Table 2). The complexed H2O as donor (O7) is involved in intra-molecular hydrogen bonds with uncomplexed carboxy oxygen atoms as acceptors (O2; O7–H7BLO2). The donors and acceptors for inter-molecular hydrogen bonds are as follows, respectively: the protonated carboxy oxygen atom of the 3-Hcpa− group as donor (O5) and the neighboring equivalent as acceptor (O4d; O5–H5ALO4d); coordinated water molecule as donors (O6) and the uncoordinated carboxy oxygen atom (O2b; O6–H6BLO2b) as acceptor. (See Table 3 for a full list of the symmetry operations.) Through the above hydrogen bondings, 1 is connected into the layer structure in the bc plane, with the ZnLZn separation of 21.959 Å (Fig. 2a). Along the a axis, the coordinated water molecules act as donors (O6, O7), and the coordinated carboxy oxygen (O1a; O6–H6ALO1a), ether oxygen (O3a; O6–H6ALO3a) as well as coordinated H2O molecules (O6c; O7–H7ALO6c) are acceptors. These hydrogen bonds link the layers of 1 into a 3D network finally (Fig. 2c), with the Zn⋯Zn separation of 4.961 Å between adjacent layers (Fig. 2b).

(a) The O–HLO hydrogen bonds projected on the bc plane in 1; (b) another O–HLO hydrogen bonds along the a axis in 1; (c) 3D network formed by hydrogen bondings in 1.
Comparing 1 with its homologues [Ni(3-Hcpa)2(H2O)4] (1-Ni) [11] and [Co(3-Hcpa)2(H2O)4] (1-Co) [12], some important similarities and differences can be found as follows: (i) Among all structures, the average M–Owater distance is almost the same with the M–Ocarboxy distance, but the two M–Owater distances differ significantly. This may be attributed to the formation of three inter-molecular hydrogen bonds for one Owater atom but two for the other Owater atom. (ii) The trans O–M–O bond angles are 180° (by symmetry) and the cis ones are close to 90°, suggesting idealized octahedral geometries for all of them. (iii) O–HLO bonds stabilize the 3D networks. (iv) The ZnLZn separation (21.96 Å) in the layer of 1 is a little longer than the NiLNi (21.89 Å) distance in 1-Ni and the CoLCo (18.27 Å) distance in 1-Co, but the ZnLZn separation (4.96 Å) between adjacent layers is shorter than those of NiLNi (5.79 Å) and CoLCo (5.87 Å).
Compound 1 is the first ZnII complex in which the partly deprotonated 3-Hcpa− anion acts as a monodentate ligand. In the reported ZnII coordination polymers {[Zn(4,4′-bipy)(H2O)4](3-cpa)·H2O}n [17], {[Zn(3-cpa)(imidazole)2]·3H2O}n [18] and [Zn(3-cpa)(1H-benzimidazole)2]n [19], the fully deprotonated 3-cpa2− anion is a free (un-coordinated) counterion in the first one, and acts as bis-monodentate ligands in the latter two.
3.2 Description of the structure of [Zn(3,5,6-tcpa)2(H2O)4][Zn(3,5,6-tcpa)2(H2O)2] (2)
Compound 2 is an interesting co-crystal of two mononuclear units, featuring two discrete and stereochemically different complexes: one octahedral [Zn(3,5,6-tcpa)2(H2O)4], the other tetrahedral [Zn(3,5,6-tcpa)2(H2O)2].
In the first one, the six-coordinated ZnII ion lies on a crystallographical inversion center and the coordination sphere consists of four oxygens from water molecules [Zn1–O4, 2.095(2) Å; Zn1–O5, 2.075(2) Å, mean 2.085(2) Å] and two from carboxy groups of unidentate trans-related 3,5,6-tcpa− anions [Zn–O1, 2.129(2) Å] (Fig. 3 and Table 2). The mean Zn–Owater distance [2.085(2) Å] is much shorter than those of the Zn–Ocarboxy bond. The trans O–Zn–O bond angles equals to 180° (by symmetry) and the cis ones are in the range of 90.49(7) to 92.15(7)°. These parameters indicate a slightly elongated octahedron.

The molecular structure of 2, showing the atom numbering scheme. Displacement ellipsoids are shown at the 30% probability level. The dashed lines show the intra-molecular O–HLO hydrogen bonds.
In turn, the four-coordinated unit contains two water molecules [Zn–O9, 1.976(2) Å] and two unidentate 3,5,6-tcpa− ligands [Zn–O6, 1.952(2) Å] in a distorted tetrahedral geometry, and shows approximate two-fold rotational symmetry (Fig. 3). The O–Zn–O bond angles fall in the range of 99.34(11) to 127.70(1)°.
The 3D supramolecular architecture of the complex 2 arises from two weak non-covalent interactions, i.e. O–H⋯O hydrogen bonding and weak ClLCl halogen bonding (Fig. 4 and Table 2).

(a) The O–HLO hydrogen bonds along the a axis in 2; (b) The O9–H9BLO7e hydrogen bond and Cl2LCl6g halogen bond interactions projected on the bc plane in 2 (for symmetry operations see Table 3); (c) 3D framework connected by hydrogen bonds and halogen bond interactions of 2.
The intra-molecular hydrogen bonding is established between the complexed water molecules as donors (O4, O5, O9) with uncomplexed (O2; O4–H4BLO2) and complexed carboxy oxygen atoms (O6, O1; O5–H5ALO6, O9–H9ALO1) as acceptors (Fig. 3). For the inter-molecular ones, the water molecules serve as donors (O4, O9) for uncomplexed carboxy oxygen atoms as acceptors (O7e, O7f; O9–H9B⋯O7e, O4–H4A⋯O7f) (Fig. 4). In other words, all aqua ligands are involved in hydrogen bondings with either adjacent octahedral (two) or tetrahedral units (three) (Table 2).
The halogen⋯halogen interactions are found between C4–Cl2 and Cl6g–C12g of neighboring octahedral and tetrahedral units with a Cl2⋯Cl6g separation of 3.246 Å (Fig. 4b). In general, inter-molecular C–X1⋯X2–C interactions (X=F, Cl, Br, I) are classified into two types according to the values of the angles θ1=∠C–X1⋯X2 and θ2=∠X1⋯X2–C. The interactions with similar angles (θ1=θ2) are called Type I. The condition of θ1=180° and θ2=90° is defined as the interactions of Type II. In 2, the geometry of halogen⋯halogen interactions is characterized by ∠C4–Cl2⋯Cl6g (θ1=157.89°) and ∠Cl2⋯Cl6g–C12g (θ2=150.91°), corresponding to halogen⋯halogen interactions of Type I [38].
Complex 2 is the first crystal structure with two mononuclear ZnII units with different coordination numbers (tetrahedral four and octahedral six) comprising the ligand 3,5,6-trichloro-pyridine-2-oxyacetate (3,5,6-tcpa). A similar co-crystal with tetra- and hexa-coordinated mononuclear ZnII centers has been described before: [Zn(2,4-dcpa)2(H2O)4][Zn(2,4-dcpa)2(H2O)2] (2A) (2,4-dcpa=2,4-dichloro-phenoxyacetate) [37]. Their structures can be compared, and some interesting similarities and differences are obvious. (i) The corresponding equivalent Zn–Ocarboxy and Zn–Owater bond lengths equal to each other in 2, but differ significantly in 2A. (ii) For the octahedral complex unit, the trans-O–Zn–O bond angles all equal to 180° in 2 (by symmetry), whereas they range from 176. 8° to 178.7° in 2A. (iii) Because of the similar steric hindrance of three or two chloro substituents, none of the phenoxy oxygen atoms are involved in coordination, but they take part in extensive inter- and intra-molecular hydrogen bonds. (iv) 3,5,6-tcpa has one more chlorine atom in contrast to 2,4-dcpa, giving rise to the formation of weak Cl⋯Cl halogen bonds in the crystal structure of 2, which are absent in compound 2A.
3.2.1 Fluorescence spectra
The luminescence properties of compounds 1 and 2 in the solid state were investigated at room temperature (Figs. 5 and 6). The maximal emission peak appears at 421 nm (λex=366 nm) for 1 and at 407 nm (λex=305 nm) for 2. In contrast, free 3-H2cpa does not display any luminescence [15] and free 3,5,6-Htcpa has the emission peak maximum at 405 nm (λex=321 nm) [20], probably attributable to π*→n or π*→π transitions. The emission of 1 is assigned to ligand-to-metal charge transfer (LMCT) as was previously proposed for a related 1D polymer [15].

The excitation and emission spectra of complex 1 in the solid state at room temperature.
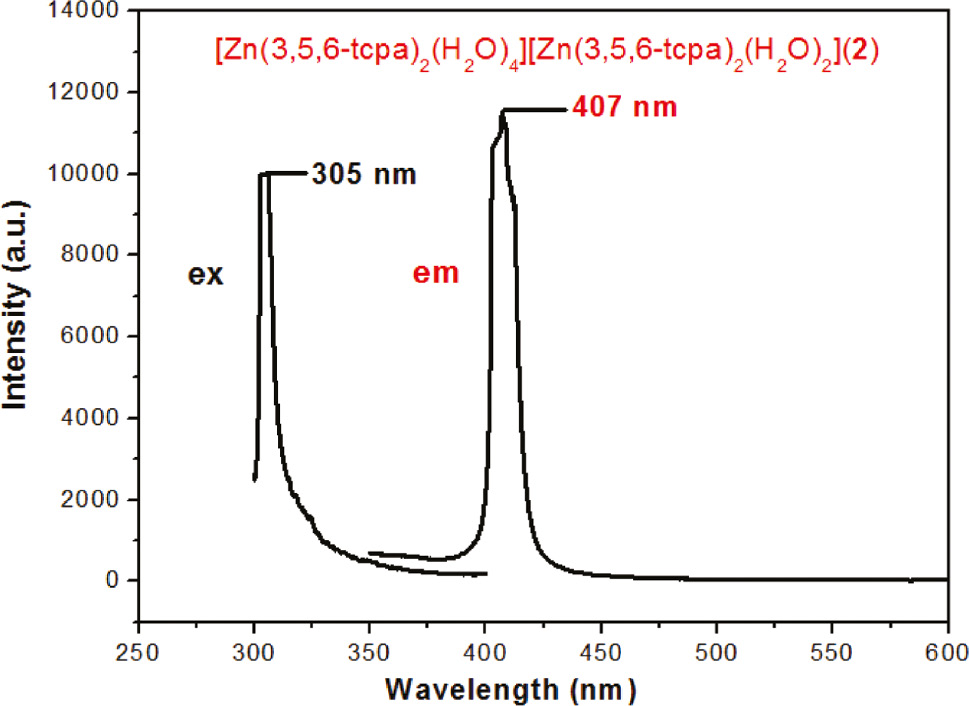
The excitation and emission spectra of complex 2 in the solid state at room temperature.
Compared with the spectra of 3,5,6-Htcpa, the co-crystal 2 has a similar emission band shape and location, which indicates that the intra-ligand transition is responsible for the emission of 2. The 2-nm red shift and enhancement of the luminescence emissions of 2 may be attributed to the increased rigidity of the ligands due to their complexation with ZnII, resulting in a decrease in energy loss by radiationless decay [39]. The result is consistent with those for the related ZnII complexes with the 2,4-dichloro-phenoxyacetic acid ligand [28], [29], [30]. These results suggest that complexes 1 and 2 have potential as new purple luminescence materials.
4 Conclusions
In summary, we have presented two mononuclear ZnII complexes: an octahedral coordination for 1 and co-crystals 2 with both octahedral and tetrahedral coordination geometries derived from two kinds of phenoxy/pyridineoxyacetic acid ligands. Our work not only enriches the coordination chemistry of 3-H2cpa and 3,5,6-Htcpa, but also maybe a useful reference for designing other monomeric materials with luminescence properties.
Acknowledgments
This work was supported by the key scientific research projects in Colleges and Universities of Henan province (No. 17A150040), the Foundation for Science and Technology Innovation Talents in Henan province (No. 164100510012), Natural Science Foundation of China (Nos. 21671114 and U1804131) as well as the Tackle Key Problem of Science and Technology Project of Henan Province, China (no. 182102310897).
References
[1] Z.-J. Lin, J. Lv, M. Hong, R. Cao, Chem. Soc. Rev.2014, 43, 5867.10.1039/C3CS60483GSearch in Google Scholar PubMed
[2] Z.-X. Du, G.-Y. Zhang, Z. Kristallogr. NCS2011, 226, 33.Search in Google Scholar
[3] J.-X. Li, Z.-X. Du, Z. Kristallogr. NCS2015, 230, 339.Search in Google Scholar
[4] Z.-X. Du, J.-X. Li, Z. Kristallogr. NCS2015, 230, 321.Search in Google Scholar
[5] J. Li, Z. Du, W. Huang, Synth. React. Inorg. Met.-Org. Chem.2014, 44, 352.10.1080/15533174.2013.769588Search in Google Scholar
[6] J.-X. Li, W.-B Guo, Z.-X. Du, W.-P. Huang, Inorg. Chim. Acta2011, 375, 290.10.1016/j.ica.2011.05.018Search in Google Scholar
[7] J.-X. Li, Z.-X. Du, B.-L. Zhu, H.-Q. An, J.-X. Dong, X.-J. Hu, W.-P. Huang, Inorg. Chem. Commun.2011, 14, 522.10.1016/j.inoche.2011.01.012Search in Google Scholar
[8] J.-X. Li, Z.-X. Du, W.-P. Huang, Z. Naturforsch.2011, 66b, 1029.10.1515/znb-2011-1007Search in Google Scholar
[9] J.-X. Li, Z.-X. Du, J. Coord. Chem.2016, 69, 2563.10.1080/00958972.2016.1216106Search in Google Scholar
[10] J.-X. Li, Z.-X. Du, Z. Naturforsch.2015, 70b, 505.10.1515/znb-2015-0010Search in Google Scholar
[11] S. Gao, J.-R. Li, J.-W. Liu, C.-S. Gu, L.-H. Huo, Acta Crystallogr.2004, E60, m22.10.1107/S1600536803027442Search in Google Scholar
[12] S.-J. Li, C.-S. Gu, S. Gao, H. Zhao, J.-G. Zhao, L.-H. Huo, Chin. J. Struct. Chem.2004, 23, 835.10.1002/jccs.200400125Search in Google Scholar
[13] Z.-P. Deng, L.-H. Huo, S. Gao, H. Zhao, Z. Anorg. Allg. Chem.2010, 636, 835.10.1002/zaac.200900374Search in Google Scholar
[14] S. Gao, C.-S. Gu, L.-H. Huo, J.-W. Liu, J.-G. Zhao, Acta Crystallogr.2004, E60, m1933.10.1107/S1600536804029976Search in Google Scholar
[15] Z.-Q. Gao, H.-J. Li, J.-Z. Gu, Chin. J. Inorg. Chem.2012, 28, 2655.Search in Google Scholar
[16] J.-Z. Gu, Y.-L. Shao, K.-Y. Zhu, Chin. J. Inorg. Chem.2013, 29, 1480.Search in Google Scholar
[17] J.-G. Zhao, C.-S. Gu, L.-H. Huo, J.-W. Liu, S. Gao, Acta Crystallogr.2005, E61, m76.10.1107/S1600536804031344Search in Google Scholar
[18] X.-F. Zhang, S. Gao, L.-H. Huo, J.-G. Zhao, Acta Crystallogr.2005, E61, m2286.10.1107/S1600536805032204Search in Google Scholar
[19] S. Gao, L.-H. Huo, J.-W. Liu, C.-S. Gu, Acta Crystallogr.2005, E61, m494.10.1107/S1600536805003661Search in Google Scholar
[20] J.-X. Li, Z.-X. Du, J. Clust. Sci.2019, DOI: https://doi.org/10.1007/s10876-019-01666-w.10.1007/s10876-019-01666-wSearch in Google Scholar
[21] Z.-X. Du, J.-X. Li, R.-F. Bai, Z. Kristallogr. NCS2019, DOI: https://doi.org/10.1515/ncrs-2019-0470.10.1515/ncrs-2019-0470Search in Google Scholar
[22] Z.-X. Du, J.-X. Li, R.-F. Bai, Z. Kristallogr. NCS2019, DOI: https://doi.org/10.1515/ncrs-2019-0434.10.1515/ncrs-2019-0434Search in Google Scholar
[23] A. Dimitrakopoulou, C. Dendrinou-Samara, A. A. Pantazaki, M. Alexiou, E. Nordlander, D. P. Kessissoglou, J. Inorg. Biochem.2008, 102, 618.10.1016/j.jinorgbio.2007.10.005Search in Google Scholar PubMed
[24] R. P. Sharma, A. Saini, J. Kumar, S. Kumar, P. Venugopalan, V. Ferretti, Inorg. Chim. Acta2017, 457, 59.10.1016/j.ica.2016.12.008Search in Google Scholar
[25] T. Afrati, C. Dendrinou-Samara, C. Raptopoulou, A. Terzis, V. Tangoulis, A. Tsipis, D. P. Kessissoglou, Inorg. Chem.2008, 47, 7545.10.1021/ic8003257Search in Google Scholar PubMed
[26] A. Dimitrakopoulou, C. Dendrinou-Samara, A. A. Pantazaki, C. Raptopoulou, A. Terzis, E. Samaras, D. P. Kessissoglou, Inorg. Chim. Acta2007, 360, 546.10.1016/j.ica.2006.07.102Search in Google Scholar
[27] C. Dendrinou-Samara, S. Katsamakas, C. Raptopoulou, A. Terzis, V. Tangoulis, D. P. Kessissoglou, Polyhedron2007, 26, 763.10.1016/j.poly.2006.09.002Search in Google Scholar
[28] X. Feng, S.-B. Miao, T.-F. Li, L.-Y. Wang, Russ. J. Coord. Chem.2011, 37, 572.10.1134/S1070328411070050Search in Google Scholar
[29] Y.-H. Zhou, Z.-Y. Wang, Trans. Met. Chem.2015, 40, 89.10.1007/s11243-014-9893-ySearch in Google Scholar
[30] Y.-Q. Yang, Z.-M. Chen, Y.-F. Kuang, Chin. J. Inorg. Chem.2013, 29, 185.Search in Google Scholar
[31] D.-Y. Ma, H.-F. Guo, J. Dong, J. Xu. J. Mol. Struct.2013, 1054, 46.10.1016/j.molstruc.2013.09.029Search in Google Scholar
[32] C.-S. Gu, J.-W. Liu, L.-H. Huo, H. Zhao, J.-G. Zhao, S. Gao, Acta Crystallogr.2004, E60, o760.10.1107/S1600536804008062Search in Google Scholar
[33] CrysAlis Pro, Rigaku Oxford Diffraction, Yarnton, Oxfordshire (U.K.) 2016.Search in Google Scholar
[34] O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann. J. Appl. Crystallogr.2009, 42, 339.10.1107/S0021889808042726Search in Google Scholar
[35] G. M. Sheldrick, Acta Crystallogr.2015, A71, 3.10.1107/S2053273314026370Search in Google Scholar
[36] G. M. Sheldrick, Acta Crystallogr.2015, C71, 3.Search in Google Scholar
[37] C. H. L. Kennard, G. Smith, E. J. O’Reilly, K. M. Stadnicka, B. J. Oleksyn, Inorg. Chim. Acta1982, 59, 241.10.1016/S0020-1693(00)87338-7Search in Google Scholar
[38] S. Biju, N. Gopakumar, J. C. Bunzli, R. Scopelliti, H. K. Kim, M. L. Reddy, Inorg. Chem.2013, 52, 8750.10.1021/ic400913fSearch in Google Scholar PubMed
[39] A. Vogler, H. Kunkely, Coord. Chem. Rev.2006, 250, 1622.10.1016/j.ccr.2005.10.009Search in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this Issue
- Research Articles
- Electron densities of two cyclononapeptides from invariom application
- Crystal structures, Hirshfeld surface analysis and Pixel energy calculations of three trifluoromethylquinoline derivatives: further analyses of fluorine close contacts in trifluoromethylated derivatives
- Synthesis and antifungal activities of 3-substituted phthalide derivatives
- Unexpected isolation of a cyclohexenone derivative
- Preparation and structure of 4-(dimethylamino)thiopivalophenone – intermolecular interactions in the crystal
- A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: synthesis, crystal structure and magnetic properties
- Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: syntheses, crystal structures and fluorescence properties
- Investigation of the reactivity of 4-amino-5-hydrazineyl-4H-1,2, 4-triazole-3-thiol towards some selected carbonyl compounds: synthesis of novel triazolotriazine-, triazolotetrazine-, and triazolopthalazine derivatives
- Synthesis and structural characterization of a Ni(II) coordination polymer with a tripodal 4-imidazolyl-functional ligand
- Crystal structure and photocatalytic degradation properties of a new two-dimensional zinc coordination polymer based on 4,4ʹ-oxy-bis(benzoic acid)
- Intermetallics of the types REPd3X2 and REPt3X2 (RE=La–Nd, Sm, Gd, Tb; X=In, Sn) with substructures featuring tin and In atoms in distorted square-planar coordination
- A 119Sn Mössbauer-spectroscopic characterization of the diamagnetic birefringence material Sn2B5O9Cl
- Synthesis, crystal structure and photoluminescence of the salts Cation+ [M(caffeine)Cl]− with Cation+=NnBu4+, AsPh4+ and M==Zn(II), Pt(II)
- Synthesis and characterization of two bifunctional pyrazole-phosphonic acid ligands
- A β-ketoiminato palladium(II) complex for palladium deposition
- Orthoamide und Iminiumsalze, XCVIa. Push-pull-substituierte 1,3,5-Hexatriene aus Orthoamiden von Alkincarbonsäuren und Birckenbach-analogen Acetophenonen
- Orthoamide und Iminiumsalze, IIICa. Weitere Ergebnisse bei der Umsetzung von Orthoamiden der Alkincarbonsäuren mit CH2- und CH2/NH-aciden Verbindungen
Articles in the same Issue
- Frontmatter
- In this Issue
- Research Articles
- Electron densities of two cyclononapeptides from invariom application
- Crystal structures, Hirshfeld surface analysis and Pixel energy calculations of three trifluoromethylquinoline derivatives: further analyses of fluorine close contacts in trifluoromethylated derivatives
- Synthesis and antifungal activities of 3-substituted phthalide derivatives
- Unexpected isolation of a cyclohexenone derivative
- Preparation and structure of 4-(dimethylamino)thiopivalophenone – intermolecular interactions in the crystal
- A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: synthesis, crystal structure and magnetic properties
- Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: syntheses, crystal structures and fluorescence properties
- Investigation of the reactivity of 4-amino-5-hydrazineyl-4H-1,2, 4-triazole-3-thiol towards some selected carbonyl compounds: synthesis of novel triazolotriazine-, triazolotetrazine-, and triazolopthalazine derivatives
- Synthesis and structural characterization of a Ni(II) coordination polymer with a tripodal 4-imidazolyl-functional ligand
- Crystal structure and photocatalytic degradation properties of a new two-dimensional zinc coordination polymer based on 4,4ʹ-oxy-bis(benzoic acid)
- Intermetallics of the types REPd3X2 and REPt3X2 (RE=La–Nd, Sm, Gd, Tb; X=In, Sn) with substructures featuring tin and In atoms in distorted square-planar coordination
- A 119Sn Mössbauer-spectroscopic characterization of the diamagnetic birefringence material Sn2B5O9Cl
- Synthesis, crystal structure and photoluminescence of the salts Cation+ [M(caffeine)Cl]− with Cation+=NnBu4+, AsPh4+ and M==Zn(II), Pt(II)
- Synthesis and characterization of two bifunctional pyrazole-phosphonic acid ligands
- A β-ketoiminato palladium(II) complex for palladium deposition
- Orthoamide und Iminiumsalze, XCVIa. Push-pull-substituierte 1,3,5-Hexatriene aus Orthoamiden von Alkincarbonsäuren und Birckenbach-analogen Acetophenonen
- Orthoamide und Iminiumsalze, IIICa. Weitere Ergebnisse bei der Umsetzung von Orthoamiden der Alkincarbonsäuren mit CH2- und CH2/NH-aciden Verbindungen

