Abstract
A new NiII compound, [Ni2(tfpa)2(bipy)2(H2O)4] (1) with tetrafluorophthalate (tfpa2−) and 2,2′-bipyridine (abbreviated as bipy) ligands, has been synthesized and structurally characterized. The single-crystal X-ray diffraction analysis reveals that the tfpa2− anions act as bis-monodentate linkers connecting NiII centers to form the dinuclear structure of 1. The dimeric units are stabilized by intramolecular π–π stacking and are further connected into a layer through O–HLO hydrogen bonding. Variable-temperature magnetic susceptibility data in the 10–200 K temperature range indicate weak ferromagnetic coupling between the two NiII ions.
1 Introduction
Currently, the design, synthesis and potential application of polynuclear and polymeric metal-organic frameworks have been an active field of research by virtue of their distinctive structures and applications in a variety of fields like medicine, hydrogen storage, luminescence or magnetism [1], [2], [3], [4], [5], [6], [7], [8]. One research theme which plays an important part in this field is the synthesis of binuclear complexes with suitable bridging ligands together with proper chelate ligands for the central ions [9], [10], [11], [12]. On the one hand, binuclear compounds are much easier obtained compared with tri- or polynuclear species, since for the latter, the conformationally appropriate bridging ligands may be hard to acquire. On the other hand, they may be the most simple model to understand the magnetic interactions between two metal ions. High-spin nickel ions in binuclear complexes with carboxylate bridged ligands have received special attention [13], [14], [15]. However, to our knowledge, the synthesis of binuclear metal complexes containing tetrafluorophthalic acid (H2tfpa) has seldom been considered [16], [17]. Because of the steric and/or electronic effects of the tetrafluorophenyl groups, the H2tfpa component may afford new structure assemblies because of its special linking types and ligand-metal interactions [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. In addition, 2,2′-bipyridine (bipy) not only may provide supramolecular recognition sites for π–π stacking interactions to form interesting structures but also may be a strong chelating ligand inducing the formation of polynuclear compounds [6], [7], [11], [12], [15], [16], [17]. Based on the above considerations, in this contribution, the synthesis and crystal structure of a new binuclear compound [Ni2(tfpa)2(bipy)2(H2O)4], formed by H2tfpa and bipy (Scheme 1), are described. Variable-temperature magnetic susceptibility measurements show very weak ferromagnetic coupling between the two NiII ions at lower temperatures.

The formula for compound 1.
2 Experimental section
All reagents were of analytical reagent grade and used without further purification. Elemental analyses for C, H and N were carried out on an Elementar Vario EL elemental analyzer. Diffraction studies on the single crystal were conducted on a Bruker SMART APEX II charge-coupled device diffractometer applying graphite-monochromatized MoKα radiation (λ=0.71073 Å). Magnetic susceptibilities of solid 1 were recorded on an MPMS Multivu magnetometer from T=2 to 300 K with an applied field of 20 kOe (1 kOe=7.96×104 A m−1). Pascal’s constants were used to estimate the correction for the underlying diamagnetism of the sample. All data except for the magnetic data were collected at room temperature.
2.1 Preparation of bis(μ2-tetrafluorophthalato-κO,O′)-tetra-aqua-bis(2,2′-bipyridine-κN,N′)-dinickel (II), [Ni2(tfpa)2(bipy)2(H2O)4] (1)
Ni(CH3COO)2·4H2O (0.5 mmol, 0.126 g), H2tfpa (0.5 mmol, 0.119 g) and bipy (0.5 mmol, 0.088 g) were dissolved in 30 mL of ethanol-H2O (1:1, v/v), and the mixture was neutralized with KOH (0.2 mol L−1) to pH 7.5. The solution was filtered after being stirred for 4 h at 80°C. Three weeks later, green block-shaped crystals had grown from the filtrate by slow evaporation. Yield: 26 mg (11% based on Ni). –C36H24F8N4Ni2O12 (%): calcd. C 44.35, H 2.46, N 5.75; found C 44.39, H 2.40, N 5.69.
2.2 Single-crystal X-ray structure determination
One green crystal with dimensions of 0.25×0.22×0.18 mm3 was selected for measurement. A total of 6817 reflections were collected in the range of 2.51°≤θ≤25.50° by using the ω-2θ scan mode, of which 3295 were unique with Rint=0.0112. The structure of 1 was solved by Direct Methods [29], [30] with Shelxs-1997 and refined by full-matrix least-squares on F2 using the Shelxl-97 software [31], [32]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were generated geometrically and treated by a mixture of independent and constrained refinement. The crystal data and collection parameters for 1 are summarized in Table 1. Selected bond lengths and bond angles are given in Table 2. Hydrogen bond parameters are listed in Table 3.
Crystallographic data and data collection and structure refinement of 1.
| Formula | C36H24F8N4Ni2O12 |
| Mr | 973.97 |
| Temperature, K | 296.2(2) |
| Crystal size, mm3 | 0.25×0.22×0.18 |
| Crystal system | Triclinic |
| Space group | P1̅ (no. 2) |
| a, Å | 7.2085(8) |
| b, Å | 10.4793(12) |
| c, Å | 12.5255(14) |
| α, ° | 82.6020(10) |
| β, ° | 76.6900(10) |
| γ, ° | 76.3110(10) |
| V, Å3 | 891.84(17) |
| Z | 1 |
| Dcalcd, g cm−3 | 1.81 |
| μ(MoKα), mm−1 | 1.2 |
| F(000), e | 492 |
| hkl range | ±8, ±12, ±15 |
| ((sinθ)/λ)max, Å−1 | 0.6057 |
| Refl. measured | 6817 |
| Refl. unique | 3295 |
| Rint | 0.0112 |
| Param. refined | 286 |
| R(F)/wR(F2)a (all refl.) | 0.0232/0.0617 |
| GoF (F2)a | 1.096 |
| Δρfin (max/min), e Å−3 | 0.29/–0.44 |
aw=1/[σ2(Fo)2+(0.0339P)2+0.3675P], P=(Fo2+2Fc2)/3.
Selected bond lengths (Å) and bond angles (°) for 1 and the related isostructural complex 1-Co [16].a
| 1 | 1-Co | ||
|---|---|---|---|
| Ni1–N1 | 2.0743(10) | Co1–N1 | 2.1139(16) |
| Ni1–N2 | 2.0722(9) | Co1–N2 | 2.1173(15) |
| Ni1–O1 | 2.0645(8) | Co1–O1 | 2.0759(14) |
| Ni1–O4A | 2.0713(8) | Co1–O3A | 2.0735(14) |
| Ni1–O5 | 2.1112(8) | Co1–O5 | 2.1466(15) |
| Ni1–O6 | 2.0581(8) | Co1–O6 | 2.0664(14) |
| Ni1LNi1A | 5.107 | Co1LCo1A | 5.074 |
| N2–Ni1–N1 | 79.30(4) | N1–Co1–N2 | 77.31(6) |
| N1–Ni1–O5 | 91.61(3) | N2–Co1–O5 | 93.18(6) |
| O6–Ni1–O1 | 89.70(3) | O6–Co1–O3 | 91.85(6) |
| O1–Ni1–O4A | 86.78(3) | O1–Co1–O3A | 88.44(5) |
| O1–Ni1–N2 | 175.74(3) | O3–Co1–N1 | 173.04(5) |
| O6–Ni1–O5 | 90.15(3) | O6–Co1–O5 | 89.11(5) |
| O4A–Ni1–O5 | 176.53(3) | O1–Co1–O5 | 177.52(5) |
aSymmetry code for A: 1−x, −y, 1−z.
Hydrogen bond geometries (Å, °) for 1.a
| Donor–H⋯Acceptor | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| O5–H5ALO3A | 0.86 | 1.92 | 2.749 (2) | 160.7 |
| O5–H5BLO2 | 0.86 | 1.97 | 2.714(2) | 143.5 |
| O6–H6ALO3A | 0.85 | 2.12 | 2.844(2) | 143.1 |
| O6–H6BLO1B | 0.85 | 1.98 | 2.758(1) | 170.6 |
aSymmetry code: A −x, −y, 1−z; B 1−x, −y, 1−z.
CCDC 1574809 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Structure description for [Ni2(tfpa)2(bipy)2 (H2O)4] (1)
Single-crystal X-ray diffraction analysis has revealed that the asymmetric unit of compound 1 consists of two NiII cations, two tetrafluorophthalate anions (tfpa2−), two bipy ligands as well as four coordinated water molecules.
The Ni1 center is coordinated by two carboxylate oxygen atoms (O1 and O4A) of two different tfpa2− dianions, two nitrogen atoms (N1 and N2) of a bipy ligand together with two H2O molecules (O5 and O6), exhibiting a distorted NiN2O4 octahedral geometry (Table 2 and Fig. 1). The N1, N2, O1 and O6 atoms define the equatorial plane, while the O5 and O4A atoms occupy the axial positions. Ni1 deviates from the equatorial least-squares plane N1/N2/O1/O6 by 0.0113 Å toward O4A, with an O4A–Ni1–O5 angle of 176.53(3)°. The tfpa2− dianions act as bis-monodentate linkers connecting two NiII cations related by crystallographic inversion symmetry forming the binuclear structure of 1.

The molecular structure of 1, showing the atom numbering scheme. Displacement ellipsoids are shown at the 30% probability level. The H atoms have been omitted for clarity. Dashed lines show the π–π stacking between the benzene rings of tfpa2− and the pyridine rings of bipy. Symmetry operations used to generate equivalent atoms: A 1−x, −y, 1−z.
In the structure of 1, there are offset face-to-face π–π stacking interactions between the benzene rings of tfpa2− and the pyridine rings of bipy (Fig. 1): C1/C2/C3/C4/C5/C6 and N2A/C13A/C14A/C15A/C16A/C17A, C1A/C2A/C3A/C4A/C5A/C6A and N2/C13/C14/C15/C16/C17. The dihedral angle, average ring-ring distance and ring-centroid separation are 4.36°, 3.303 Å and 4.542 Å, respectively.
The coordinated H2O ligands are involved as donors in intermolecular hydrogen bonding interactions with coordinated and uncoordinated carboxylate oxygen atoms of the tfpa2− anions (Table 3 and Fig. 2). The dimeric units of 1 are associated into a layer, stabilized by π–π stacking and O–HLO hydrogen bonds (Fig. 2).
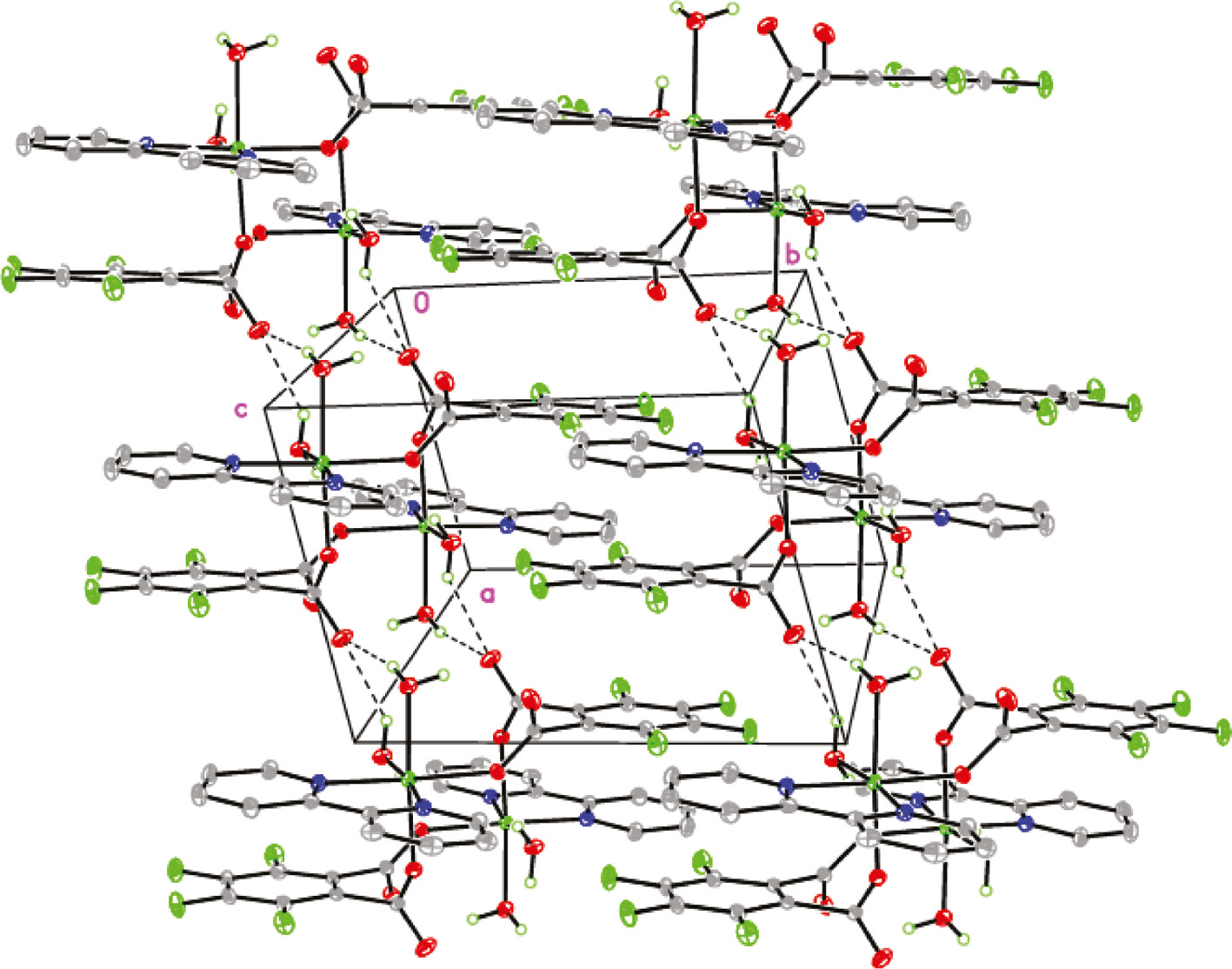
Layer formed by hydrogen bonding interactions in 1.
Compound 1 is isostructural with [Co2(tfpa)2(bpy)2 (H2O)4] (1-Co) [16]. As Ni and Co are neighboring 3d elements and the ionic radius of NiII (0.69 Å) is much smaller than that of CoII (0.75 Å), the corresponding bond lengths of 1 are significantly shorter than those in 1-Co (Table 2), but the NiLNi separation (5.107 Å) is longer than that of CoLCo (5.074 Å) in the cobalt analogue.
Comparing 1 with other binuclear species [Co2(tfpa)2 (bipy)2(H2O)4] (1-Co) [16], {[Cu2(tfpa)(Htfpa)2(bipy)2](H2O)2] (CH3OH)} (1-Cu) [17] and [Cd2(tfpa)2(bipy)2(H2 O)2] (1-Cd) [17] constructed with the same H2tfpa and bipy tectons (Scheme 2), some important features and differences between them can be found as follows: (i) the number of H2tfpa ligands. There are three H2tfpa in 1-Cu, but two in 1, 1-Co and 1-Cd. (ii) The degree of deprotonation of H2tfpa. In order to balance the corresponding positive charges of metal ions, two H2tfpa molecules have become mono-deprotonated as Htfpa−, while one is doubly deprotonated as tfpa2− in 1-Cu. By contrast, H2tfpa is fully deprotonated in 1, 1-Co and 1-Cd. (iii) The coordination number of the central metal ions. As the ionic radii of the 3d elements (NiII, CuII and CoII) are smaller than those of the 4d ones (CdII), the coordination numbers in 1-Cu (five), 1 and 1-Co (six) are smaller than in 1-Cd (seven), displaying square-pyramidal (1-Cu), octahedral (1 and 1-Co) and pentagonal-bipyramidal (1-Cd) coordination environments, respectively. (iv) The coordination mode of H2tfpa. Although the H2tfpa entities in the four structures all show similar μ2-bridging fashion, distinct coordination differences are observed. In 1-Cu, only one carboxyl group participates in coordination, and the other remains uncoordinate. In detail, one Htfpa− and tfpa2− adopt a μ2-η2:η0 pattern, and the other Htfpa− shows a μ2-η1:η1 connectivity. In contrast, the two carboxyl groups of tfpa2− are all involved in coordination in 1, 1-Co and 1-Cd. They are bis-monodentate in 1 and 1-Co, but bis-bidentate chelating in 1-Cd.
3.2 Magnetic properties
The magnetic susceptibility of compound 1 was measured in the 2–300 K temperature range as shown in Fig. 3.

Temperature dependence of χMT (squares) and χM−1 (circles) for 1.
The experimental χMT value per (NiII)2 is 2.42 cm3 K mol−1 (μeff=4.40 μB) at 300 K, which is slightly larger than the theoretical value 2.00 cm3 K mol−1 (μeff=4.00 μB) for two spin-only uncoupled NiII ions with g=2.1–2.3 [33], which indicates that orbital contributions are involved. The χMT value increases gradually upon cooling from 300 K and reaches the maximum value of 2.72 cm3 K mol−1 at 10 K and finally drops quickly to 1.84 cm3 K mol−1 at 2 K. The increase in χMT with decreasing temperature clearly indicates ferromagnetic coupling interactions between the NiII centers.
The Heisenberg spin Hamiltonian model for the isotropic magnetic exchange interaction in the binuclear (NiII)2 unit has been given in the following expression, where the numbering 1 and 2 represent the marks of Ni1 and Ni1A as shown in Fig. 1.
where Ŝ1=Ŝ2=1. The exchange constant J presents magnetical coupling within the carboxylate-bridged dinuclear unit [34]. The experimental data were fitted based on the above Hamiltonian equation. The data in the temperature range of 10–300 K were considered in the fit, and the best-fit parameters obtained with this computing model yielded J=0.21 cm−1, g=2.09 and R=8.90×10−9. The small and positive J value suggests weak ferromagnetic exchange between NiII centers in 1. These parameters agree with the long NiLNi separation of 5.107 Å in complex 1 and are comparable to values reported in related cases [8], [35].
The cryomagnetic behavior of 1 obeys the Curie-Weiss law in the temperature range of 10–300 K, yielding Cm=2.42 cm3 K mol−1, θ=3.56 K. The positive θ value further reveals the presence of intramolecular ferromagnetic coupling.
4 Conclusions
We have presented a new binuclear NiII complex 1 formed by tfpa2− and bipy ligands. The magnetic properties of 1 indicate intramolecular ferromagnetic coupling. Our work here extends the coordination chemistry of H2tfpa and its anions with transition metal cations. The results may be valuable for the design and synthesis of other carboxylate-bridged discrete coordination compounds with interesting structural and magnetic properties.
Award Identifier / Grant number: 17A150040
Funding source: Science and Technology Innovation Talents in Henan Province
Award Identifier / Grant number: 164100510012
Funding source: Natural Science Foundation of China
Award Identifier / Grant number: 21671114 and U1804131
Award Identifier / Grant number: 182102310897
Funding statement: This work was supported by the key scientific research projects in colleges and universities of Henan Province (no. 17A150040), the Foundation for Science and Technology Innovation Talents in Henan Province (no. 164100510012), the Natural Science Foundation of China (nos. 21671114 and U1804131) as well as the Tackle Key Problem of Science and Technology Project of Henan Province, China (no. 182102310897).
References
[1] P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris, C. Serre, Chem. Rev. 2012, 112, 1232–1268.10.1021/cr200256vSuche in Google Scholar PubMed
[2] M. P. Suh, H. J. Park, T. K. Prasad, D.-W. Lim, Chem. Rev. 2012, 112, 782–835.10.1021/cr200274sSuche in Google Scholar PubMed
[3] Y. Cui, Y. Yue, G. Qian, B. Chen, Chem. Rev. 2012, 112, 1126–1162.10.1021/cr200101dSuche in Google Scholar PubMed
[4] M. Kurmoo, Chem. Soc. Rev. 2009, 38, 1353–1379.10.1039/b804757jSuche in Google Scholar PubMed
[5] X. Feng, L.-F. Ma, L.-Y. Wang, J.-S. Zhao, Inorg. Chem. Commun. 2011, 14, 584–589.10.1016/j.inoche.2011.01.030Suche in Google Scholar
[6] R.-F. Jin, S.-Y. Yang, H.-M. Li, L.-S. Long, R.-B. Huang, L.-S. Zheng, CrystEngComm2012, 14, 1301–1316.10.1039/C1CE05959ASuche in Google Scholar
[7] Z.-F. Xu, D.-Z. Kuang, M.-Q. Liu, F.-X. Zhang, J.-Q. Wang, Chin. J. Struct. Chem. 2010, 29, 977–981.Suche in Google Scholar
[8] M. He, Q.-F. Li, T. Xie, G.-M. Xu, J. Yu, W. Li, Chin. J. Struct. Chem. 2010, 29, 582–586.Suche in Google Scholar
[9] R.-S. Zhu, J.-F. Song, Acta Crystallogr. 2009, E65, m1523–m1523.10.1107/S1600536809045747Suche in Google Scholar
[10] E. Escribano, M. Font-Bardia, T. Calvet, J. Lorenzo, P. Gamez, V. Moreno, Inorg. Chim. Acta2013, 394, 65–76.10.1016/j.ica.2012.07.018Suche in Google Scholar
[11] Y.-Y. Yang, Z.-J. Lin, T.-T. Liu, J. Liang, R. Cao, CrystEngComm2015, 17, 1381–1388.10.1039/C4CE02163KSuche in Google Scholar
[12] D. Tian, Y. Pang, Y.-H. Zhou, L. Guan, H. Zhang, CrystEngComm2011, 13, 957–966.10.1039/C0CE00281JSuche in Google Scholar
[13] F. Zhang, Q.-Y. Lin, W.-L. Hu, W.-J. Song, S.-T. Shen, P. Gui, Spectrochim. Acta A2013, 110, 100–107.10.1016/j.saa.2013.03.015Suche in Google Scholar PubMed
[14] Z. Lian, K. Jiang, T. Lou, N. Zhao, P. Liu, C. An, J. Cluster Sci. 2017, 28, 1509–1521.10.1007/s10876-017-1161-9Suche in Google Scholar
[15] B. Tao, H. Xia, C.-X. Huang, X.-W. Li, Z. Anorg. Allg. Chem. 2011, 637, 703–707.10.1002/zaac.201000393Suche in Google Scholar
[16] S.-Y. Zhang, W. Shi, J.-G. Ma, Y.-Q. Zhang, Z.-J. Zhang, P. Cheng, Dalton Trans. 2013, 42, 4313–4318.10.1039/c2dt32468gSuche in Google Scholar PubMed
[17] Z.-H. Zhang, X.-S. Yang, We. Guo, S.-C. Chen, M.-Y. He, Q. Chen, Polyhedron2016, 117, 695–702.10.1016/j.poly.2016.07.015Suche in Google Scholar
[18] Y. Shang, X. Feng, J. Li, Y. Wang, L. Wang, Z. Li, Inorg. Chem. Commun. 2019, 105, 47–54.10.1016/j.inoche.2019.04.003Suche in Google Scholar
[19] J. Zhang, J.-X. Li, Z. Naturforsch. 2016, 71b, 45–49.10.1515/znb-2015-0135Suche in Google Scholar
[20] J. Chai, Y. Liu, B. Liu, B. Yang, J. Mol. Struct. 2017, 1150, 307–315.10.1016/j.molstruc.2017.08.099Suche in Google Scholar
[21] D.-S. Zhang, Z. Chang, Y.-F. Li, Z.-Y. Jiang, Z.-H. Xuan, Y.-H. Zhang, J.-R. Li, Q. Chen, T.-L. Hu, X.-H. Bu, Sci. Rep. 2013, 3, 3312–3318.10.1038/srep03312Suche in Google Scholar PubMed PubMed Central
[22] A.-A. Al-Terkawi, G. Scholz, F. Emmerling, E. Kemnitz, Dalton Trans. 2017, 46, 12574–12578.10.1039/C7DT02564ESuche in Google Scholar PubMed
[23] J.-J. Li, T.-T. Fan, X.-L. Qu, H.-L. Han, X. Li, Dalton Trans. 2016, 45, 2924–2935.10.1039/C5DT04262CSuche in Google Scholar
[24] G.-Z. Liu, S.-H. Li, X.-L. Li, L.-Y. Xin, L.-Y. Wang, CrystEngComm2013, 15, 4571–4580.10.1039/c3ce40109jSuche in Google Scholar
[25] S.-C. Chen, J. Qin, Z.-H. Zhang, X.-X. Cai, J. Gao, L. Liu, M.-Y. He, Q. Chen, Z. Naturforsch.2013, 68b, 277–283.10.5560/znb.2013-3022Suche in Google Scholar
[26] J. Zhang, Y.-Y. Liu, K. Ying, Z. Kristallogr. NCS2012, 227, 410–412.Suche in Google Scholar
[27] J. Zhang, Y.-Y. Liu, K. Ying, Z. Kristallogr. NCS2012, 227, 568–570.Suche in Google Scholar
[28] J. Zhang, J.-C. Zhu, K. Ying, Z. Kristallogr. NCS2011, 226, 557–558.10.1524/zkri.2011.1376Suche in Google Scholar
[29] G. M. Sheldrick, Shelxs-2014, Program for Crystal Structure Solution, University of Göttingen, Göttingen (Germany) 2014.Suche in Google Scholar
[30] G. M. Sheldrick, Acta Crystallogr. 1990, A46, 467–473.10.1107/S0108767390000277Suche in Google Scholar
[31] G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
[32] G. M. Sheldrick, Shelxl-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen (Germany) 1997.Suche in Google Scholar
[33] R. L. Carlin, Magnetochemistry, Springer-Verlag, Berlin, 1986.10.1007/978-3-642-70733-9Suche in Google Scholar
[34] K. K. Nanda, A. W. Addison, N. Paterson, E. Sinn, L. K. Thompson, U. Sakaguchi, Inorg. Chem. 1998, 37, 1028–1036.10.1021/ic9712434Suche in Google Scholar
[35] C. Ma, W. Wang, X. Zhang, C. Chen, Q. Liu, H. Zhu, D. Liao, L. Li, Eur. J. Inorg. Chem.2004, 2004, 3522–3532.10.1002/ejic.200400166Suche in Google Scholar
©2019 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Research Articles
- Electron densities of two cyclononapeptides from invariom application
- Crystal structures, Hirshfeld surface analysis and Pixel energy calculations of three trifluoromethylquinoline derivatives: further analyses of fluorine close contacts in trifluoromethylated derivatives
- Synthesis and antifungal activities of 3-substituted phthalide derivatives
- Unexpected isolation of a cyclohexenone derivative
- Preparation and structure of 4-(dimethylamino)thiopivalophenone – intermolecular interactions in the crystal
- A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: synthesis, crystal structure and magnetic properties
- Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: syntheses, crystal structures and fluorescence properties
- Investigation of the reactivity of 4-amino-5-hydrazineyl-4H-1,2, 4-triazole-3-thiol towards some selected carbonyl compounds: synthesis of novel triazolotriazine-, triazolotetrazine-, and triazolopthalazine derivatives
- Synthesis and structural characterization of a Ni(II) coordination polymer with a tripodal 4-imidazolyl-functional ligand
- Crystal structure and photocatalytic degradation properties of a new two-dimensional zinc coordination polymer based on 4,4ʹ-oxy-bis(benzoic acid)
- Intermetallics of the types REPd3X2 and REPt3X2 (RE=La–Nd, Sm, Gd, Tb; X=In, Sn) with substructures featuring tin and In atoms in distorted square-planar coordination
- A 119Sn Mössbauer-spectroscopic characterization of the diamagnetic birefringence material Sn2B5O9Cl
- Synthesis, crystal structure and photoluminescence of the salts Cation+ [M(caffeine)Cl]− with Cation+=NnBu4+, AsPh4+ and M==Zn(II), Pt(II)
- Synthesis and characterization of two bifunctional pyrazole-phosphonic acid ligands
- A β-ketoiminato palladium(II) complex for palladium deposition
- Orthoamide und Iminiumsalze, XCVIa. Push-pull-substituierte 1,3,5-Hexatriene aus Orthoamiden von Alkincarbonsäuren und Birckenbach-analogen Acetophenonen
- Orthoamide und Iminiumsalze, IIICa. Weitere Ergebnisse bei der Umsetzung von Orthoamiden der Alkincarbonsäuren mit CH2- und CH2/NH-aciden Verbindungen
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Research Articles
- Electron densities of two cyclononapeptides from invariom application
- Crystal structures, Hirshfeld surface analysis and Pixel energy calculations of three trifluoromethylquinoline derivatives: further analyses of fluorine close contacts in trifluoromethylated derivatives
- Synthesis and antifungal activities of 3-substituted phthalide derivatives
- Unexpected isolation of a cyclohexenone derivative
- Preparation and structure of 4-(dimethylamino)thiopivalophenone – intermolecular interactions in the crystal
- A new binuclear NiII complex with tetrafluorophthalate and 2,2′-bipyridine ligands: synthesis, crystal structure and magnetic properties
- Two mononuclear zinc(II) complexes constructed by two types of phenoxyacetic acid ligands: syntheses, crystal structures and fluorescence properties
- Investigation of the reactivity of 4-amino-5-hydrazineyl-4H-1,2, 4-triazole-3-thiol towards some selected carbonyl compounds: synthesis of novel triazolotriazine-, triazolotetrazine-, and triazolopthalazine derivatives
- Synthesis and structural characterization of a Ni(II) coordination polymer with a tripodal 4-imidazolyl-functional ligand
- Crystal structure and photocatalytic degradation properties of a new two-dimensional zinc coordination polymer based on 4,4ʹ-oxy-bis(benzoic acid)
- Intermetallics of the types REPd3X2 and REPt3X2 (RE=La–Nd, Sm, Gd, Tb; X=In, Sn) with substructures featuring tin and In atoms in distorted square-planar coordination
- A 119Sn Mössbauer-spectroscopic characterization of the diamagnetic birefringence material Sn2B5O9Cl
- Synthesis, crystal structure and photoluminescence of the salts Cation+ [M(caffeine)Cl]− with Cation+=NnBu4+, AsPh4+ and M==Zn(II), Pt(II)
- Synthesis and characterization of two bifunctional pyrazole-phosphonic acid ligands
- A β-ketoiminato palladium(II) complex for palladium deposition
- Orthoamide und Iminiumsalze, XCVIa. Push-pull-substituierte 1,3,5-Hexatriene aus Orthoamiden von Alkincarbonsäuren und Birckenbach-analogen Acetophenonen
- Orthoamide und Iminiumsalze, IIICa. Weitere Ergebnisse bei der Umsetzung von Orthoamiden der Alkincarbonsäuren mit CH2- und CH2/NH-aciden Verbindungen

![Scheme 2: The formulae for 1-Co [16], 1-Cu and 1-Cd [17] as compared to compound 1.](/document/doi/10.1515/znb-2019-0128/asset/graphic/j_znb-2019-0128_scheme_002.jpg)
