Abstract
Objective
The objective of this study was to investigate the effect of modified Dioscorea pills (MDP) on microcirculatory remodeling in the hippocampus of rats with chronic cerebral hypoperfusion (CCH) through the angiopoietin (Ang)/tyrosine kinase receptor tyrosine kinase with immunoglobulin-like and EGF-like domains (Ang receptor) 2 (Tie-2) signaling pathways, which may underlie the cognitive improvement observed in CCH rats.
Methods
Forty male Sprague–Dawley rats raised under specific pathogen-free conditions were randomly divided into three groups: control group (10 rats), model group (15 rats), and MDP group (15 rats). The rats in the model group and MDP group underwent bilateral common carotid artery occlusion using the 2-vessel occlusion (2-VO) method to induce CCH. Rats in the control group underwent the same surgical procedures as those in the model group, except for ligation and occlusion of the carotid arteries. After 1 week of 2-VO, rats in the MDP group were administered MDP condensed decoction intragastrically at a dose of 1 ml/100 g body weight (prepared by the Preparation Room of Hubei Provincial Hospital of Traditional Chinese Medicine) for 45 days, while rats in the other two groups received normal saline intragastrically with the same dose and duration as the MDP group. After the intervention, all rats were euthanized, and brain perfusion was performed to obtain the hippocampal tissue for analysis. Immunohistochemical staining for CD43 was performed to assess microvessel density (MVD); western blot and the reverse transcription-polymerase chain reaction (RT-PCR) were used to analyze the expression of proteins and genes in angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), Tie-2, and vascular endothelial growth factor (VEGF) proteins and genes in the hippocampal tissue and compute the Ang-1/Ang-2 ratio.
Results
MDP treatment reduced neuronal loss and promoted restoration of the damaged hippocampal structure in CCH rats. The model group showed significantly higher MVD (14.93 ± 1.92) compared to the control group (5.78 ± 1.65) (P < 0.01), whereas MDP treatment further increased MVD (21.19 ± 2.62). Western blot and RT-PCR analysis revealed that CCH significantly increased the expression of Ang-1, Ang-2, Tie-2, and VEGF proteins and genes, while MDP treatment further significantly upregulated the expression of these proteins and genes. In addition, MDP significantly elevated the gene and protein expression of the Ang-1/Ang-2 ratio compared to the control group (P = 0.041, P = 0.029).
Conclusion
CCH induces microvascular neogenesis in the hippocampus, and MDP promotes angiogenesis and microcirculation remodeling in CCH rats via the Ang/Tie signaling pathway, which may be an important mechanism for its restorative effects on hippocampal perfusion and improvement of cognitive function in CCH rats.
1 Introduction
Chronic cerebral hypoperfusion (CCH) is a condition that is becoming more prevalent and severe in the elderly population, likely due to aging and the progression of atherosclerosis. CCH has been strongly associated with cognitive dysfunction and other neuropsychiatric diseases. One of the underlying mechanisms of CCH is the increased levels of reactive oxygen species, which can lead to neuronal damage and neuroinflammation [1]. This neuroinflammation has been implicated in various neuropsychiatric diseases such as vascular dementia [2], chronic epilepsy [3], and bipolar disorder [4]. Currently, the treatment for CCH primarily focuses on protecting neurons and improving cerebral perfusion, as these are important factors in mitigating the detrimental effects of CCH on brain health.
The rat model of CCH can be induced using the 2-vessel occlusion (2-VO) method, which has been shown to cause neuronal loss in the cerebral cortex and hippocampus [5]. The 2-VO model is commonly used to study and intervene in CCH. Previous research has demonstrated that modified Dioscorea pills (MDP), a well-known prescription for treating vascular dementia, can improve episodic memory and cognitive impairment in rats with CCH induced by 2-VO. The beneficial effects of MDP are attributed to its ability to inhibit inflammation, reduce neuronal damage, promote glial proliferation, enhance synaptic protein expression and synaptic plasticity, and upregulate the expression of key genes such as GAP-43 and vascular endothelial growth factor (VEGF) in the hippocampus [6]. In recent years, there has been a shift in focus from solely studying neurons to considering the comprehensive role of neurovascular units in neurological diseases [7,8]. Cerebral microvessels play a critical role in maintaining normal brain function, and further investigation is required to determine whether MDP can improve CCH while protecting and restoring the neural structure of the hippocampus.
Studies have shown that hypoxia or inflammation can lead to increased expression of angiogenic factors such as VEGF, bFGF, and PDGF, which bind to their corresponding receptors on the cell membrane of vascular endothelial cells, triggering downstream signaling cascades and promoting angiogenesis. This process involves the dissolution of the vascular basement membrane by extracellular matrix metalloproteinases, leading to the dissociation of vascular endothelial cells and the formation of new blood vessels [9]. Over time, these newly formed microvessels integrate into the existing vascular system and mature into stable blood vessels. The angiopoietin (Ang)/tyrosine kinase receptor tyrosine kinase with immunoglobulin-like and EGF-like domains (Ang receptor) 2 (Tie-2) and VEGF/vascular endothelial growth factor receptor 2 signaling pathways are known to be involved in angiogenesis and maintenance of vascular stability [10]. Generally, angiopoietin-1 (Ang-1) ligands act via the Tie-2 receptor, playing a stabilizing role in vascular endothelium. On the other hand, angiopoietin-2 (Ang-2) blocks Ang1 activation of Tie-2, leading to endothelial cell instability and promoting vascular remodeling [11,12]. Further investigation is required to explore how MDP may interfere with hippocampal vascular remodeling in the context of CCH and improve cognitive function via the Ang/Tie signaling pathway. This could shed light on the mechanism by which MDP exerts its beneficial effects on CCH and cognitive function, and potentially provide insights for developing novel therapeutic strategies for CCH and related neurovascular disorders.
2 Materials and methods
2.1 Animals
Male Sprague–Dawley rats (40 in total) with specific-pathogen-free status were purchased from the Hubei Provincial Academy of Preventive Medicine (License number: SCXK(Hubei)2015-0018). The rats had an average body weight of approximately 280 ± 20 g and were housed in the Experimental Animal House of Guizhou Medical University under standard laboratory conditions, including an ambient temperature of 23 ± 1°C, relative humidity of 50 ± 10%, and a 12 h light/dark cycle. They were provided with free access to food and water. The rats were acclimated to the housing conditions for 1 week prior to the experimental procedures.
2.2 Preparation of the CCH model and intervention measures
A total of 40 rats were randomly assigned to three groups: control group (n = 10), model group (n = 15), and MDP group (n = 15), using a random number table. The CCH models, consisting of the model group and MDP group, were induced by bilateral common carotid artery occlusion (2-VO) wherein the rats’ left and right common carotid arteries were ligated separately, with a 1 week interval. Rats in the control group underwent the same surgical procedures as the model groups, except for the ligation and occlusion of the bilateral common carotid arteries. During surgery, the rectal temperature of the rats was maintained at 37.0–37.9°C using a heating pad to prevent brain injury due to low temperature. After approximately 2–4 min, all animals regained consciousness and were provided with free access to food and water. After 1 week of 2-VO surgery, rats in the MDP group were administered MDP condensed decoction intragastrically at a dose of 1 ml/100 g body weight (prepared by the Preparation Room of Hubei Provincial Hospital of Traditional Chinese Medicine) for 45 days, while rats in the other two groups were treated with normal saline intragastrically at the same dose and duration as the MDP group.
2.3 Sampling
After the gavage intervention, rats were euthanized, and brain tissues were obtained by perfusion sampling and fresh sampling. Perfusion sampling was conducted as follows: five rats from each group were deeply anesthetized with isoflurane inhalation, as previously described. Their hearts were exposed, and perfusion needles were inserted through the apex into the left ventricle and then into the aorta. The right atrial appendage was quickly cut, and 250 ml of normal saline was perfused through the perfusion needles. As clear perfusate flowed out, the rats’ tails straightened, trembling limbs ceased, and the livers gradually turned white. Subsequently, 250 ml of 4% paraformaldehyde was perfused successively. After approximately 40 min, the rats were decapitated, and the hippocampus was isolated and harvested. The harvested tissues were then fixed in 4% paraformaldehyde buffer solution for 12–24 h, followed by conventional dehydration, paraffin embedding, and staining with hematoxylin and eosin (HE) and immunohistochemical staining. Fresh sampling was performed for reverse transcription-polymerase chain reaction (RT-PCR) and western blot detection, involving five rats from each group. The steps for fresh sampling were as follows: rats were deeply anesthetized with isoflurane inhalation and then decapitated, and brain tissues were quickly stripped for RT-PCR detection.
2.4 Histopathological observation
Paraffin sections of the hippocampus were obtained for HE staining in order to observe the pathological characteristics of the CA1, CA3, and DG regions. The hippocampal tissue was dehydrated, rendered transparent, and embedded in paraffin. Subsequently, the tissue was cut into 4 µm slices, which were then baked, dewaxed, and stained with HE for subsequent microscopic observation using an Olympus biological microscope (Type BX53) at magnifications of 100× and 400×.
2.5 Immunohistochemistry and microvessel density (MVD) analysis
Immunohistochemistry and MVD detection were performed using the hippocampus of three rats from each group. Paraffin sections were prepared following the same procedure as for histopathological analysis. After antigen retrieval and blocking of endogenous peroxidase, the first antibodies (mouse anti-CD43) were added to the hippocampus paraffin sections. Subsequently, IgG polymers of horseradish peroxidase-labeled secondary antibody and freshly prepared DAB color solution were successively added to these sections. Finally, the sections were counterstained with Harris hematoxylin.
For the analysis of MVD, CD43-labeled images were collected. According to the corrected Weidner method, any single endothelial cell or cell clusters stained by anti-CD43, as long as they have a clear boundary with the surrounding microvessels or other connecting tissues, regardless of whether a lumen has formed, were counted as a microvessel, excluding vessels with smooth muscle walls or lumen diameters greater than eight times the diameter of erythrocytes. Each sample was first observed at 100× magnification to select three spots with the highest number of microvessels as “hot spots.” Then, the microvessels were counted at high magnification (400×) from the hot spots, and the average number of microvessels was calculated as MVD.
2.6 Western blot
To the hippocampus tissue was added RIPA lysate (Meilunbio, product no.: MA0151) to extract the total proteins of the hippocampus. By diluting and boiling, the extracted proteins were denatured prior to electrophoresis on the gel, and these proteins were transferred into the PVDF membrane (Genscript, product no. L00791C). They were sealed and reacted with primary antibodies (rabbit anti-GAPDH, 37 kD, Hangzhou Xianzhi Biological Co., Ltd., product no. AB-P-R 001, 1:10,000 dilution; rabbit anti-Ang-1 [70 kD], Wuhan Sanying, product no. 23302-1-AP, 1:10,000 dilution; rabbit anti-Ang-2, 75 kD, product number: ABclonal A0698, diluted 1:10,000; rabbit anti-tie2126 kD, ABclonal, product number A7222, diluted at 1:10,000; rabbit anti-VEGF, 45 kD, Wuhan Sanying, product number: 19003-1-AP, 1:10,000 dilution). Afterward, HRP-labeled secondary antibodies (HRP-labeled sheep antimouse secondary antibody, Wuhan Doctor Bioengineering Co., Ltd., product number: BA1051, 1:10,000 dilution) were added and the films were developed, fixed, and processed. Finally, the films were analyzed with ipp6.0 software to determine the film grayscale value using GAPDH as an inner reference.
2.7 RT-PCR
Hippocampal tissues weighing 100 mg were extracted to obtain RNA using the Trizol method. The extracted RNA was then reverse-transcribed into cDNA, followed by the addition of designed primers for real-time fluorescence quantitative reaction using the SYBR Green dye method. Amplification and melting curves were drawn, and the relative expression of target genes such as Ang-1, Ang-2, Tie-2, and VEGF was calculated using the QPCR algorithm (relative quantification, 2⁻ΔΔCt), with GAPDH as an internal reference (Table 1).
Primer sequence table
| Gene | Primer | Sequence (5′–3′) | PCR products (bp) |
|---|---|---|---|
| Rat GAPDH | Forward | ACAGCAACAGGGTGGTGGAC | 253 |
| Reverse | TTTGAGGGTGCAGCGAACTT | ||
| Rat Ang-1 | Forward | AATGTGCCTACACTTTCA | 345 |
| Reverse | GATTTAGTACCTGGGTCTC | ||
| Rat Ang-2 | Forward | GTGGCAGATTGTTTTCCT | 231 |
| Reverse | TGCACTGAGTCGTCGTAG | ||
| Rat Tie-2 | Forward | GCTCTGGGAGATCGTTAGCT | 151 |
| Reverse | CTCCCTCCAGCATTGTCTCA | ||
| Rat VEGF | Forward | CCCACGACAGAAGGGGAGCA | 161 |
| Reverse | CGCATTAGGGGCACACAGGAC |
2.8 Statistics
All data obtained in this experiment, except for HE staining, are presented as quantitative values expressed as mean ± standard deviation (mean ± SD). Statistical analysis was performed using SPSS 19.0 software. T-test was used for intergroup comparisons, and one-way ANOVA was used for comparisons among multiple groups. A significance level of P < 0.05 was considered statistically significant, while P < 0.01 was considered extremely statistically significant.
-
Ethical approval: The research related to animals’ use complied with all the relevant national regulations and institutional policies for the care and use of animals. All animal handling and experimental protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People’s Republic of China. The study was approved by the Committee on Ethics of the First People’s Hospital of Guiyang (Guizhou, China), with ethical approval number 2022007.
3 Results
3.1 Pathology observation
HE staining revealed that compared to the control group, the model group showed a significant loss of neurons with disordered arrangement in the CA1, CA3, and DG regions of the hippocampus. The radiation layer appeared loose with pale staining and showed multiple cracks in the CA1 region. Many disarranged cells infiltrated the white matter of the CA3 region, and a large blank gap was observed between the cortex and subcortical in the DG region. However, in the MDP group, these abnormal pathological findings were significantly restored, with evident regeneration of neurons in the CA1, CA3, and DG regions (Figure 1).
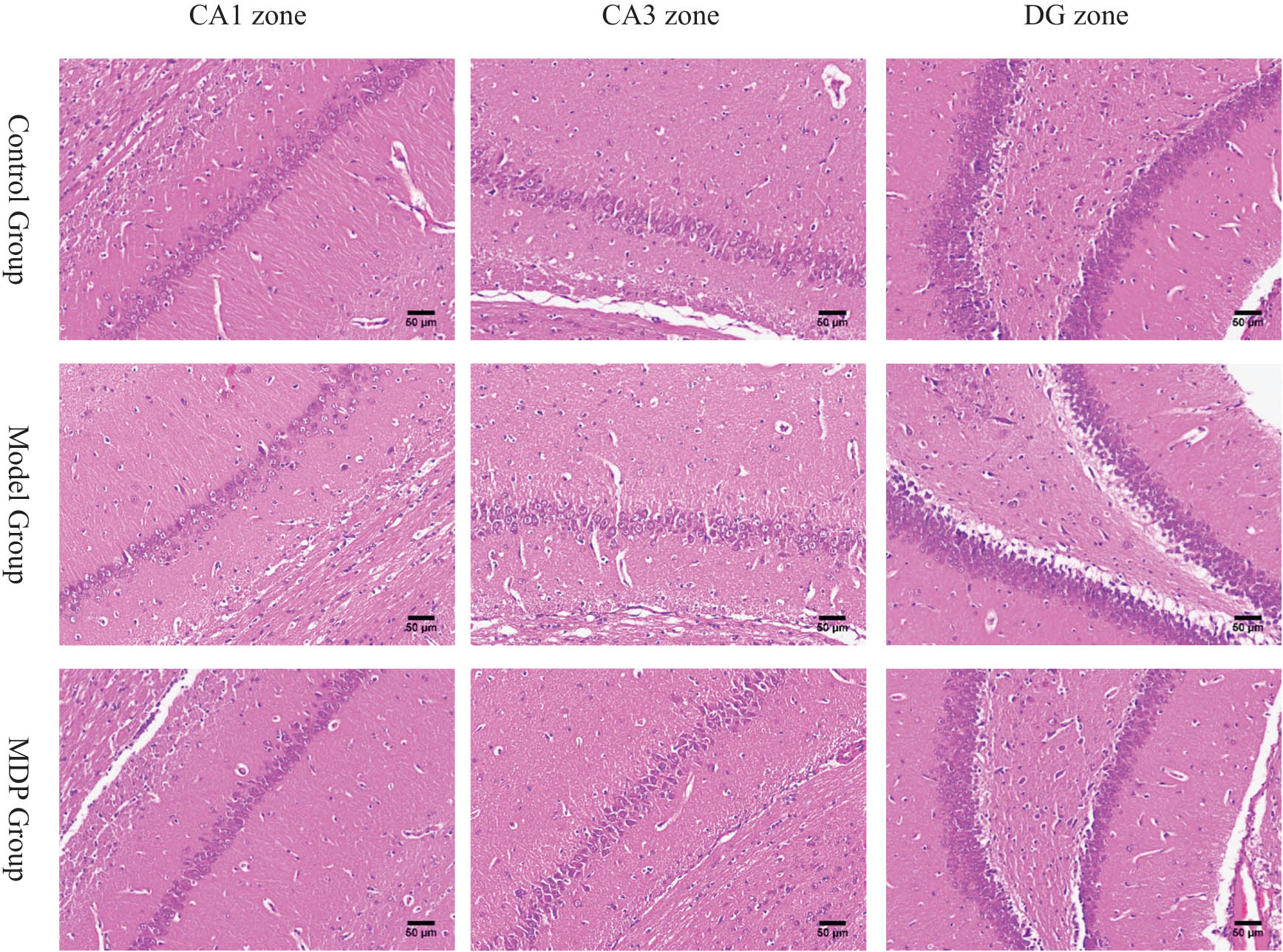
The pathology of CA1, CA3, and DG zones in the hippocampus among the three groups. CCH induced neurons loss, white matters loosen and disarrange in CA1, CA3, and DG zones in the hippocampus. MDP significantly restored them. Using Image J software, we calculated that the total neurons in the CA1 zone are, respectively, 155 ± 21, 110 ± 18, 182 ± 36 per slice (400 times magnetite). There are significant differences between the two groups (P < 0.05).
3.2 Immunohistochemistry
CCH induced by 2-VO resulted in evident neovascularization in the hippocampus. Compared with the control group, the MVD in the model group (14.93 ± 1.92) was significantly higher (P < 0.01) than that in the control group (5.78 ± 1.65). Furthermore, the MVD in the MDP group (21.19 ± 2.62) was significantly higher (P < 0.01, P < 0.01) than that of both the model group and control group, indicating that MDP further promotes microvascular regeneration (Figure 2).
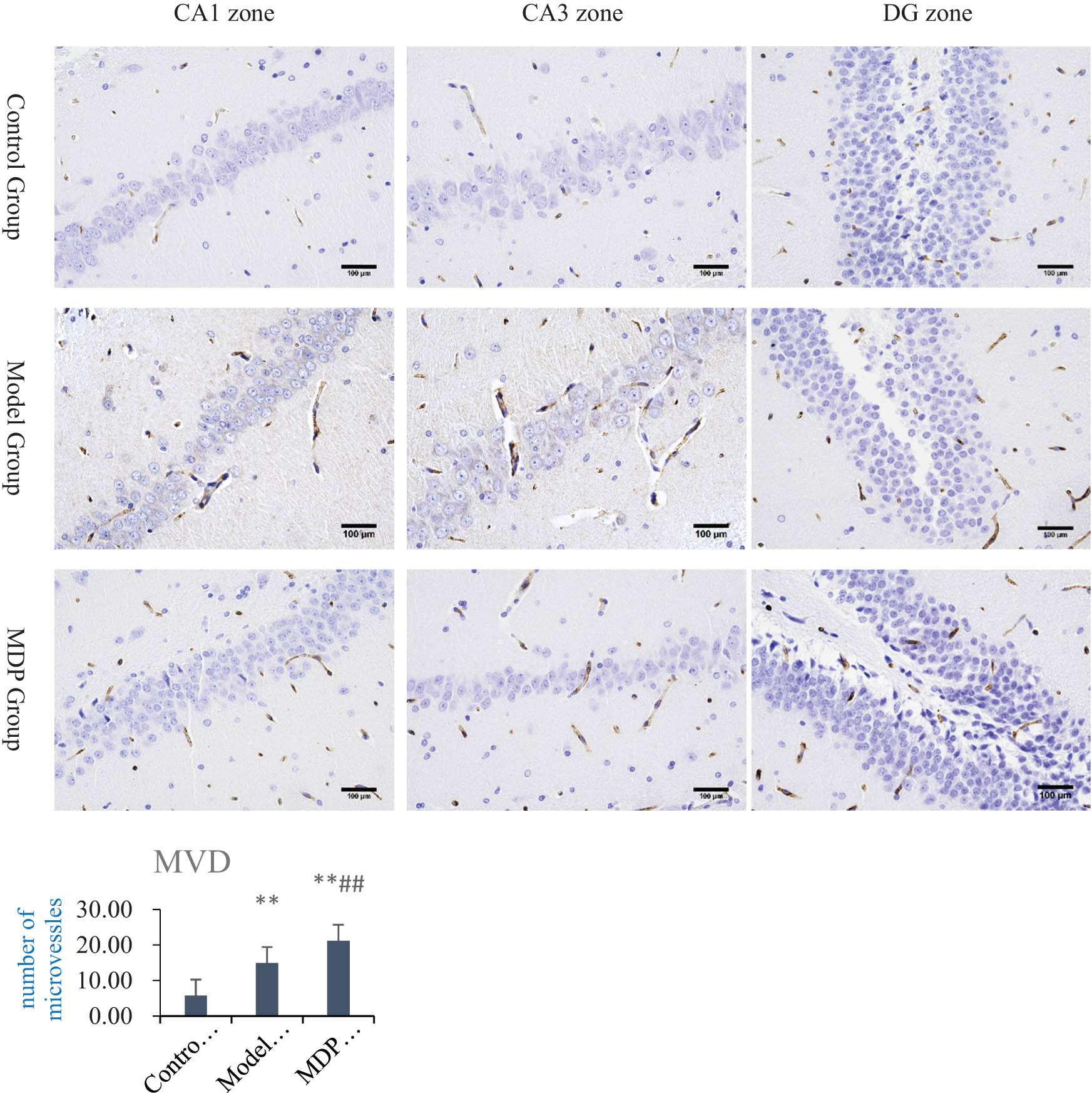
Immunohistochemistry CD43 staining in the hippocampus and MVD analysis. MVD in the model group (14.93 ± 1.92), control group (5.78 ± 1.65), and the MDP group (21.19 ± 2.62). **P < 0.01 vs control group; ## P < 0.01 vs the model group.
3.3 Western blot
The expression of Ang-1 in the model group was significantly increased compared to the control group (0.597 ± 0.090 vs 0.345 ± 0.058, P = 0.005), and MDP further upregulated the expression of Ang-1 (0.750 ± 0.060), with significant differences observed when compared to both the control group and the model group (P < 0.001, P = 0.039). Similarly, the expression of Ang-2 in the model group was significantly increased compared to the control group (0.592 ± 0.035 vs 0.442 ± 0.068, P = 0.007), and MDP further upregulated the expression of Ang-2 (0.771 ± 0.017), with significant differences observed when compared to both the control group and the model group (P < 0.001, P = 0.003). Furthermore, the expression of Tie-2 in the model group was significantly increased compared to the control group (0.703 ± 0.025 vs 0.524 ± 0.044, P < 0.001), and MDP further upregulated the expression of Tie-2 (0.909 ± 0.019), with significant differences observed when compared to both the control group and the model group (P < 0.001, P < 0.001). Finally, the expression of VEGF in the model group was significantly increased compared to the control group (0.706 ± 0.075 vs 0.486 ± 0.030, P = 0.002), and MDP further upregulated the expression of VEGF (0.844 ± 0.034), with significant differences observed when compared to both the control group and the model group (P < 0.001, P = 0.016) (Figure 3).
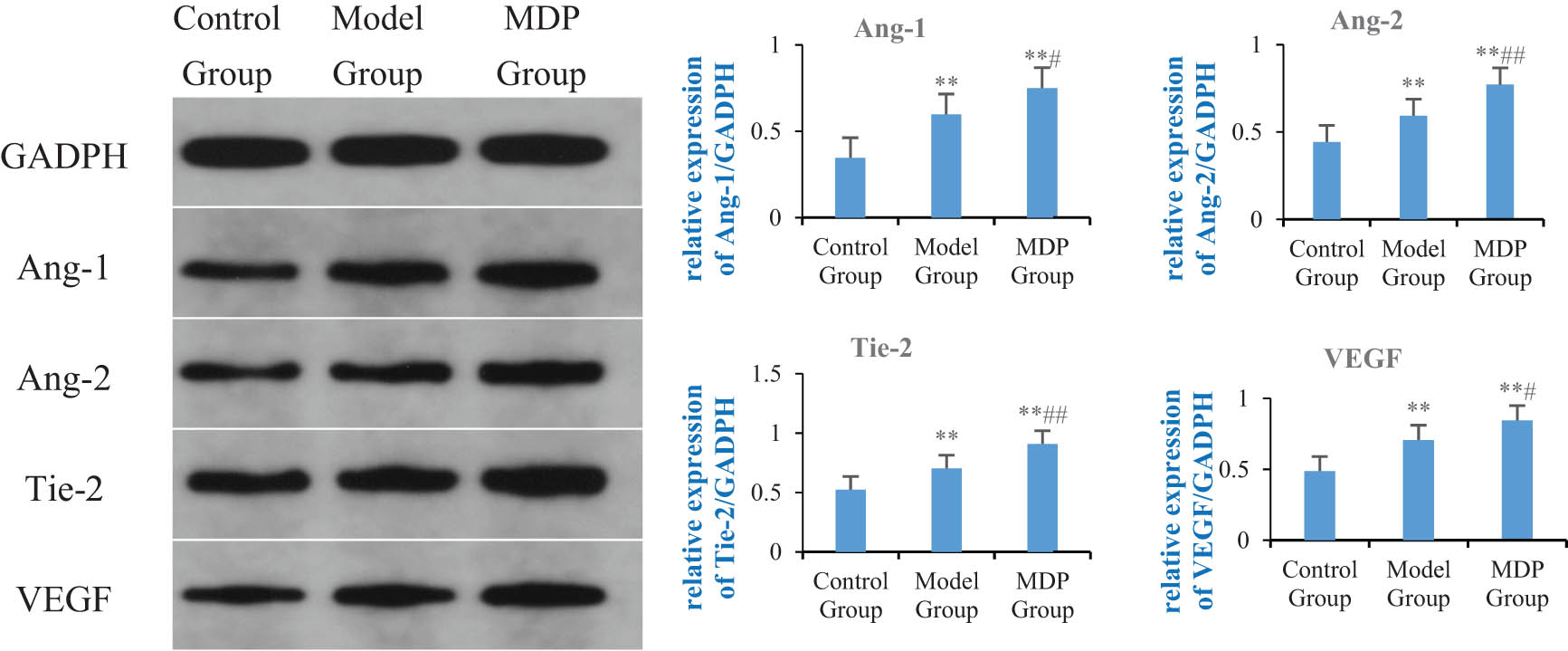
Expressions of Ang-1, Ang-2, Tie-2, and VEGF in the hippocampus among the three groups. Using GAPDH as the inner reference, the relative expression levels of Ang-1, Ang-2, Tie-2, and VEGF in the hippocampus form are calculated. The left image shows the immunoblotting of GAPDH, Ang-1, Ang-2, Tie-2, and VEGF, while the right bar graph, respectively, shows the relative expression levels of Ang-1, Ang-2, Tie-2, and VEGF in each group of rats. **P < 0.01 vs control group; # P < 0.05 vs model group; ## P < 0.01 vs model group.
3.4 RT-PCR
The expression of Ang-1 mRNA in the model group was significantly increased compared to the control group (1.691 ± 0.488 vs 0.992 ± 0.034, P = 0.032), and MDP further upregulated it (3.358 ± 0.596), with significant differences observed compared to both the control group and the model group (P <0.001, P <0.001). The expression of Ang-2 mRNA in the model group showed a tendency to increase compared to the control group but did not reach statistical significance (1.560 ± 0.491 vs 1.011 ± 0.016, P = 0.203). However, MDP further upregulated the expression of Ang-2 mRNA (2.673 ± 0.804), with significant differences observed compared to both the control group and the model group (P < 0.001, P = 0.002). Similarly, the expression of Tie-2 mRNA in the model group showed an increasing trend compared to the control group but did not reach statistical significance (1.362 ± 0.297 vs 1.001 ± 0.002, P = 0.095). Nevertheless, MDP further upregulated the expression of Tie-2 mRNA (2.294 ± 0.443), with significant differences observed compared to both the control group and the model group (P < 0.001, P < 0.001). The expression of VEGF mRNA in the model group also showed a tendency to increase compared to the control group but did not reach statistical significance (1.447 ± 0.264 vs 1.012 ± 0.036, P = 0.219). However, MDP further upregulated the expression of VEGF mRNA (2.754 ± 0.797), with significant differences observed compared to both the control group and the model group (P < 0.001, P < 0.001) (Figure 4).
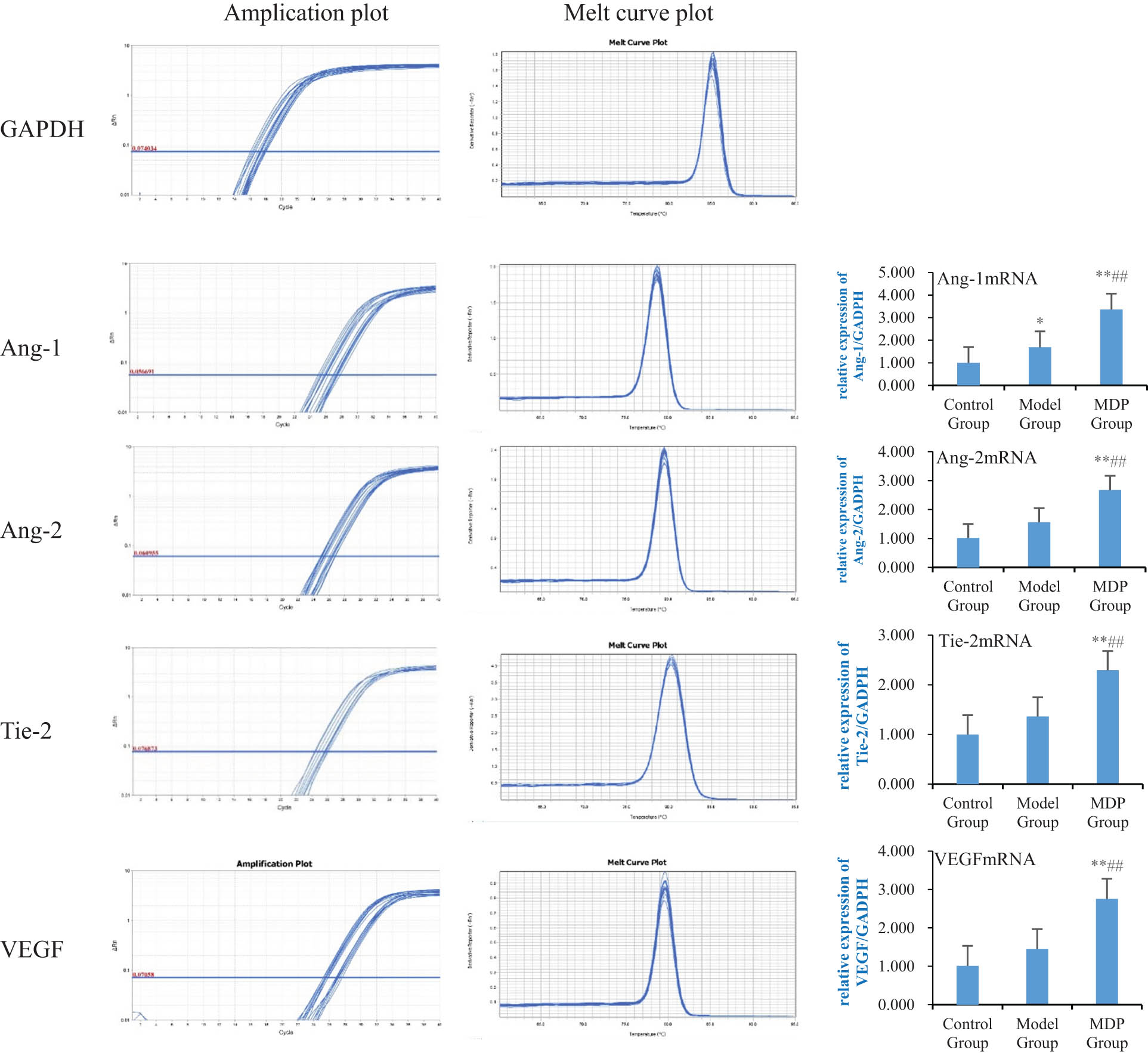
Expressions of Ang-1 mRNA, Ang-2 mRNA, Tie-2 mRNA, and VEGF mRNA in the hippocampus among the three groups. Using GAPDH as the inner reference, the relative expression levels of Ang-1 mRNA, Ang-2 mRNA, Tie-2 mRNA, and VEGF mRNA in the hippocampus form are calculated. The left image shows the amplification and fusion curves of GAPDH, Ang-1, Ang-2, Tie-2, and VEGF genes. The right bar graph shows the relative expression levels of Ang-1 mRNA, Ang-2 mRNA, Tie-2 mRNA, and VEGF mRNA in each group of rats. **P < 0.01 vs control group; *P < 0.05 vs control group; ## P < 0.01 vs model group.
3.5 Ang-1/Ang-2 ratio
The Ang-1/Ang-2 ratio suggests that there are no significant differences in the gene expression ratio among the three groups (F = 2.576, P = 0.097) but MDP still significantly elevated the gene expression ratio of Ang-1/Ang-2 compared to the control group (P = 0.041). In terms of protein expression, in the physiological state (control group), the expression of Ang-1 is less than Ang-2, the ratio of Ang-1/Ang-2 is 0.779 ± 0.046, the interventions of CCH or MDP obviously increase the expression of Ang-1 much more than that of Ang-2, thus the ratios of Ang-1/Ang-2, respectively, reach 1.007 ± 0.113 and 0.972 ± 0.077. All reach significant differences when compared to that of the control group (P = 0.015, P = 0.029) but there is no significant difference between the model group and the MDP group (P = 0.627) (Table 2).
Ratio of Ang-1/Ang-2 in gene or protein expressions among the three groups
| Control group | Model group | MDP group | |
|---|---|---|---|
| Gene ratio | 1.015 ± 0.296 | 1.092 ± 0.196 | 1.356 ± 0.458* |
| Protein ratio | 0.779 ± 0.046 | 1.007 ± 0.113* | 0.972 ± 0.077* |
* P < 0.05 vs control group.
4 Discussion
In this study, the results of HE staining confirmed that MDP exerted neuroprotective and neural repair effects in the hippocampus, indicating its potential to improve blood supply in the hippocampus of CCH rats. This was further supported by CD43 immunohistochemistry staining and MVD analysis, which demonstrated that CCH induced spontaneous angiogenesis in the CA1, CA3, and DG regions of the hippocampus, and that MDP further enhanced the formation of microvessels. This suggests that the promotion of angiogenesis may be an important mechanism through which MDP improves blood supply in the hippocampus of CCH.
Angiogenesis and vascular remodeling are constant processes in the adult vascular system, adapting to the needs of blood supply and organ function by the communication between blood vessels and parenchymal cells [13]. Vascular integrity and remodeling are co-regulated by endothelial growth factors and inflammatory cytokines. The angiopoietin/tie (Ang/Tie) pathway is known to play a crucial role in regulating vascular stability and angiogenesis in physiological and pathological conditions, including improving blood supply to specific organs during inflammation [14]. Ang-1 is primarily produced by pericytes and platelets. Under physiological conditions, Ang-1 activates downstream signals by binding and phosphorylating Tie-2 receptors, promoting intercellular junctions and enhancing endothelial cell survival, thereby maintaining vascular stability and normal vascular function [15,16,17]. Ang-2, on the other hand, is mainly produced by endothelial cells and is physiologically expressed in vascular remodeling sites such as the ovaries, placenta, and uterus [18]. However, Ang-2 expression can be triggered by inflammatory mediators such as thrombin [19], hypoxia [20], and cancer [21] and is upregulated under pathological conditions such as macular edema, neuroinflammation, and sepsis [18,22]. Ang-2 acts as an antagonist of the Ang-1/Tie-2 axis, causing instability of blood vessels and rendering them susceptible to vascular endothelial growth factor A (VEGF). Ang-2 and VEGF synergistically drive vascular leakage, neovascularization, and inflammation [23,24]. Previous studies have shown that decreased Ang-2 expression and increased Ang-1 expression contribute to vascular remodeling in the ischemic brain [25]. In this study, regardless of gene or protein expression, Ang-1, Ang-2, Tie-2, and VEGF were upregulated in the model group, indicating that under CCH conditions, Ang-1, Tie-2, and VEGF were upregulated to meet the needs of the ischemic and inflammatory environments, promoting microvascular stability in the hippocampus. The upregulation of Ang-2 may be related to the remodeling of hippocampal blood vessels caused by hypoxia and inflammation. MDP further promotes significant upregulation of these modulating cytokines, especially Ang-1, to promote and maintain the stability of the existing blood vessels and the modification of removal of the defective blood vessels, thus improving blood supply in the hippocampus. In summary, Ang-1 plays a crucial role in maintaining the stability of the existing blood vessels and ensuring blood supply, while Ang-2 promotes the degradation of damaged blood vessels, creating conditions for the formation of new blood vessels. Together, they jointly contribute to improving blood supply under pathological conditions.
In terms of angiogenesis, Ang-1 activates Tie-2 to promote the migration of endothelial cells and facilitate the development of blood vessels in areas lacking blood vessels [26], thereby restoring cerebral perfusion in the ischemic area and preserving neurons in the ischemic penumbra from dying. Tie-2 also induces angiogenesis through independent mechanisms [27]. Additionally, in the presence of injury and hypoxia, upregulated VEGF enhances PI3K/AKT and MEK1/2/ERK1/2 signal transduction, leading to increased proliferation and migration of residual capillary endothelial cells in the ischemic penumbra [28]. VEGF is involved in almost all processes of angiogenesis, directly influencing the occurrence and development of angiogenesis and ultimately determining the outcomes of angiogenesis to some extent. In this study, Ang-1, Tie-2, and VEGF were found to be upregulated in the model group, indicating their important roles as regulatory factors in promoting angiogenesis. Consequently, the mean vessel density (MVD) in the model group was significantly higher than that in the control group. MDP treatment significantly increased the expression of these cytokines, suggesting that it is an important mechanism for promoting angiogenesis, improving hippocampal perfusion, and restoring cognitive function in CCH.
Tie-2 receptor is a tyrosine kinase receptor that is expressed in endothelial cells [29] and hematopoietic stem cells [30] and it binds to all four angiopoietins [31]. Oligomeric Ang-1 promotes the binding between Tie-2 and cells. Moreover, Tie-2 can also bind fibronectin, collagen, and vitronectin with high affinity, thereby anchoring Tie-2 to the extracellular matrix [32], which are important mechanisms through which Tie-2 promotes vascular stability. A high concentration (800 ng/mL) of Ang-2 can induce the phosphorylation of the Tie-2 receptor, leading to increased cell survival, proliferation, chemotaxis of brain capillary endothelial cells, and angiogenesis [33,34]. It can be observed that MDP promotes the upregulation of Ang-1, Ang-2, and Tie-2, which collectively enhance the stability of the existing blood vessels and the formation of new blood vessels. These mechanisms may explain why MDP significantly enhances MVD in CCH.
The increased levels of Ang-2 induced by hypoxia activate Tie-2, which leads to the detachment and migration of pericytes from the basement membrane [35], resulting in increased vascular permeability and leakage. Additionally, Ang-2 can interact with integrin β1 [36] or αvβ [37], disrupting endothelial stability and promoting vascular leakage independently of Tie-2. In the first 3 days after stroke, elevated Ang-2 levels were associated with detrimental vascular permeability, while high Ang-2 levels after 7 days were associated with microvascular stability and maturation [38]. Although long-term overexpression of Ang-2 alone can promote vascular degeneration [39], in this study, when Ang-2 levels were upregulated along with VEGF in CCH, Ang-2 actually promoted angiogenesis [40]. Moreover, Ang-2 promotes the differentiation of neural progenitor cells and independently mediates their migration through Tie-2 [41], indicating that Ang-2 not only promotes vascular remodeling but also facilitates neural remodeling in CCH. Therefore, Ang-2 is an important target for MDP to improve hippocampal blood supply and promote neural recovery in CCH.
From the analysis of the Ang-1/Ang-2 ratio, we can see that MDP not only greatly increases the expressions of Ang1/2, VEGF, and Tie-2 compared to the control group and the model group, it also increased the ratio of Ang-1/Ang-2 compared to the control group, with the increase in ratio of Ang-1.
Ang-2 can alleviate cerebral ischemia/reperfusion injury by maintaining the integrity of blood vessels and promoting perfusion [42]. Therefore, the high-level expressions of Ang1/2, VEGF, and Tie-2 may all contribute to the high MVD and hippocampal perfusion in CCH.
5 Conclusion
The Ang-Tie-2 signaling pathway is a crucial pathway that regulates vascular stability and angiogenesis, with its regulatory effects varying depending on its concentration and interactions with other cytokines. In CCH, MDP induces significant upregulation of Ang-1, Ang-2, Tie-2, and VEGF, promoting both the stability of the original hippocampal blood vessels and the regeneration of microvessels. This leads to the reconstruction of the hippocampal microcirculation network, increased hippocampal perfusion, and improved cognitive function in rats with CCH.
-
Funding information: This study was supported by the Science Foundation of Guizhou Provincial Administration of Traditional Chinese Medicine (QZYY-2021-026), the Science and Technology Fund of Guizhou Provincial Health Commission (No. gzwkj2021-001), and the Key Projects of Guizhou Provincial Science and Technology Foundation (No. ZK[2023]key-001).
-
Author contributions: Guiying Kuang: review and editing (equal). Zhigang Shu: conceptualization (lead); writing – original draft (lead); formal analysis (lead); writing – review and editing (equal). Chunli Zhu: software (lead); writing – review and editing (equal). Hongbing Li: methodology (lead); writing – review and editing (equal). Cheng Zhang: Conceptualization (supporting); writing – original draft (supporting); writing – review and editing (equal).
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Therefore, we declare that all the datasets used and/or analyzed during the current study are available from the authors upon reasonable request. The corresponding email address for requesting data is mrbright789@sina.com.
References
[1] Mai C, Mankoo H, Wei L, An X, Li C, Li D, et al. TRPM2 channel: A novel target for alleviating ischaemia-reperfusion, chronic cerebral hypo-perfusion and neonatal hypoxic-ischaemic brain damage. J Cell Mol Med. 2020 Jan;24(1):4–12. 10.1111/jcmm.14679.Suche in Google Scholar PubMed PubMed Central
[2] Li HB, Liang WB, Zhou L. The experimental research on neuroplasticity in rats’ hippocampus subjected to chronic cerebral hypoperfusion and interfered by Modified Dioscorea Pills. Heliyon. 2019 Dec 28;6(1):e02897. 10.1016/j.heliyon.2019.e02897.Suche in Google Scholar PubMed PubMed Central
[3] Bascuñana P, Wolf BJ, Jahreis I, Brackhan M, García-García L, Ross TL, et al. 99mTc-HMPAO SPECT imaging reveals brain hypoperfusion during status epilepticus. Metab Brain Dis. 2021 Dec;36(8):2597–602. 10.1007/s11011-021-00843-z.Suche in Google Scholar PubMed PubMed Central
[4] Delvecchio G, Gritti D, Squarcina L, Brambilla P. Neurovascular alterations in bipolar disorder: A review of perfusion weighted magnetic resonance imaging studies. J Affect Disord. 2022 Nov 1;316:254–72. 10.1016/j.jad.2022.07.059.Suche in Google Scholar PubMed
[5] He H, Chen X. Luteolin attenuates cognitive dysfunction induced by chronic cerebral hypoperfusion through the modulation of the PI3K/Akt pathway in rats. J Vet Res. 2021 Jul 5;65(3):341–9. 10.2478/jvetres-2021-0037.Suche in Google Scholar PubMed PubMed Central
[6] Li HB. Restorative effect of modified dioscorea pills on the structure of hippocampal neurovascular unit in an animal model of chronic cerebral hypoperfusion. Heliyon. 2019 Apr 28;5(4):e01567. 10.1016/j.heliyon.2019.e01567.Suche in Google Scholar PubMed PubMed Central
[7] Tiedt S, Buchan AM, Dichgans M, Lizasoain I, Moro MA, Lo EH. The neurovascular unit and systemic biology in stroke - implications for translation and treatment. Nat Rev Neurol. 2022 Oct;18(10):597–612. 10.1038/s41582-022-00703-z.Suche in Google Scholar PubMed
[8] Dion-Albert L, Dudek KA, Russo SJ, Campbell M, Menard C. Neurovascular adaptations modulating cognition, mood, and stress responses. Trends Neurosci. 2023 Apr;46(4):276–92. 10.1016/j.tins.2023.01.005.Suche in Google Scholar PubMed
[9] Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. 2013 Jan;19(1):31–9. 10.1016/j.molmed.2012.10.010.Suche in Google Scholar PubMed
[10] Zhang Y, Liu J, Zou T, Qi Y, Yi B, Dissanayaka WL, et al. DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res Ther. 2021 May 10;12(1):281. 10.1186/s13287-021-02349-y.Suche in Google Scholar PubMed PubMed Central
[11] Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019 May 17;8(5):471. 10.3390/cells8050471.Suche in Google Scholar PubMed PubMed Central
[12] Khan KA, Wu FT, Cruz-Munoz W, Kerbel RS. Ang2 inhibitors and Tie2 activators: potential therapeutics in perioperative treatment of early stage cancer. EMBO Mol Med. 2021 Jul 7;13(7):e08253. 10.15252/emmm.201708253.Suche in Google Scholar PubMed PubMed Central
[13] Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014 Jan 24;343(6169):416–9. 10.1126/science.1244880.Suche in Google Scholar PubMed
[14] Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. 10.1016/S0092-8674(00)81813-9.Suche in Google Scholar
[15] Hansen TM, Singh H, Tahir TA, Brindle NP. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal. 2010 Mar;22(3):527–32. 10.1016/j.cellsig.2009.11.007.Suche in Google Scholar PubMed PubMed Central
[16] Zhao YT, Fallas JA, Saini S, Ueda G, Somasundaram L, Zhou Z, et al. F-domain valency determines outcome of signaling through the angiopoietin pathway. EMBO Rep. 2021 Dec 6;22(12):e53471. 10.15252/embr.202153471.Suche in Google Scholar PubMed PubMed Central
[17] Zhu J, Li J, Chung CS, Lomas-Neira JL, Ayala A. Patho-mechanisms for hemorrhage/sepsis-induced indirect acute respiratory distress syndrome: A role for lung TIE1 and its regulation by neutrophils. Shock. 2022 Apr 1;57(4):608–15. 10.1097/SHK.0000000000001902.Suche in Google Scholar PubMed PubMed Central
[18] Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997 Jul 4;277(5322):55–60. 10.1126/science.277.5322.55.Suche in Google Scholar PubMed
[19] Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002 Mar 1;99(5):1646–50. 10.1182/blood.v99.5.1646.Suche in Google Scholar PubMed
[20] Wang Q, Lash GE. Angiopoietin 2 in placentation and tumor biology: The yin and yang of vascular biology. Placenta. 2017 Aug;56:73–8. 10.1016/j.placenta.2017.03.021.Suche in Google Scholar PubMed
[21] Sfiligoi C, de Luca A, Cascone I, Sorbello V, Fuso L, Ponzone R, et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003 Feb 10;103(4):466–74. 10.1002/ijc.10851.Suche in Google Scholar PubMed
[22] Bate N, Lodge J, Brindle NPJ. Intrinsic differences in the mechanisms of Tie2 binding to angiopoietins exploited by directed evolution to create an Ang2-selective ligand trap. J Biol Chem. 2021 Aug;297(2):100888. 10.1016/j.jbc.2021.100888.Suche in Google Scholar PubMed PubMed Central
[23] Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005 Feb 15;118(Pt 4):771–80. 10.1242/jcs.01653.Suche in Google Scholar PubMed
[24] Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye (Lond). 2021 May;35(5):1305–16. 10.1038/s41433-020-01377-x.Suche in Google Scholar PubMed PubMed Central
[25] Zhao Q, Hu J, Xiang J, Gu Y, Jin P, Hua F, et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res. 2015 Oct 22;1624:489–96. 10.1016/j.brainres.2015.08.003.Suche in Google Scholar PubMed
[26] Thakkar AB, Ma Y, Dela Cruz M, Wu Y, Arechiga V, Swaminathan S, et al. Effect of HIV-1 Infection on Angiopoietin 1 and 2 Levels and Measures of Microvascular and Macrovascular Endothelial Dysfunction. J Am Heart Assoc. 2021 Nov 16;10(22):e021397. 10.1161/JAHA.121.021397.Suche in Google Scholar PubMed PubMed Central
[27] Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012 Jun;122(6):1991–2005. 10.1172/JCI58832.Suche in Google Scholar PubMed PubMed Central
[28] Wang HJ, Ran HF, Yin Y, Xu XG, Jiang BX, Yu SQ, et al. Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol Sin. 2022 Jul;43(7):1670–85. 10.1038/s41401-021-00803-4.Suche in Google Scholar PubMed PubMed Central
[29] Partanen J, Armstrong E, Mäkelä TP, Korhonen J, Sandberg M, Renkonen R, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992 Apr;12(4):1698–707. 10.1128/mcb.12.4.1698-1707.1992.Suche in Google Scholar PubMed PubMed Central
[30] Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterization of mouse TIE and TEK receptor tyrosine kinase genes and their expression in hematopoietic stem cells. Biochem Biophys Res Commun. 1993 Aug 31;195(1):301–9. 10.1006/bbrc.1993.2045.Suche in Google Scholar PubMed
[31] Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT, Ahn SY, et al. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004 Aug;18(11):1200–8. 10.1096/fj.03-1466com.Suche in Google Scholar PubMed
[32] Leligdowicz A, Richard-Greenblatt M, Wright J, Crowley VM, Kain KC. Endothelial activation: The Ang/Tie axis in Sepsis. Front Immunol. 2018 Apr 24;9:838. 10.3389/fimmu.2018.00838.Suche in Google Scholar PubMed PubMed Central
[33] Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene. 2000 Sep 14;19(39):4549–52. 10.1038/sj.onc.1203800.Suche in Google Scholar PubMed
[34] Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci. 2002 Jan 1;115(Pt 1):175–83. 10.1242/jcs.115.1.175.Suche in Google Scholar PubMed
[35] Geranmayeh MH, Rahbarghazi R, Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun Signal. 2019 Mar 20;17(1):26. 10.1186/s12964-019-0340-8.Suche in Google Scholar PubMed PubMed Central
[36] Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, et al. Endothelial destabilization by angiopoietin-2 via integrin β1 activation. Nat Commun. 2015 Jan 30;6:5962. 10.1038/ncomms6962.Suche in Google Scholar PubMed PubMed Central
[37] Yun JH, Park SW, Kim JH, Park YJ, Cho CH, Kim JH. Angiopoietin 2 induces astrocyte apoptosis via αvβ5-integrin signaling in diabetic retinopathy. Cell Death Dis. 2016 Feb 18;7(2):e2101. 10.1038/cddis.2015.347.Suche in Google Scholar PubMed PubMed Central
[38] Moisan A, Favre IM, Rome C, Grillon E, Naegele B, Barbieux M, et al. Microvascular plasticity after experimental stroke: a molecular and MRI study. Cerebrovasc Dis. 2014;38(5):344–53. 10.1159/000368597.Suche in Google Scholar PubMed
[39] Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002 Aug 20;99(17):11205–10. 10.1073/pnas.172161899.Suche in Google Scholar PubMed PubMed Central
[40] Duran CL, Borriello L, Karagiannis GS, Entenberg D, Oktay MH, Condeelis JS. Targeting Tie2 in the tumor microenvironment: From angiogenesis to dissemination. Cancers (Basel). 2021 Nov 16;13(22):5730. 10.3390/cancers13225730.Suche in Google Scholar PubMed PubMed Central
[41] Liu XS, Chopp M, Zhang RL, Hozeska-Solgot A, Gregg SC, Buller B, et al. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009 Aug 21;284(34):22680–9. 10.1074/jbc.M109.006551.Suche in Google Scholar PubMed PubMed Central
[42] Yu Y, Fang H, Qiu Z, Xia Z, Zhou B. DHA attenuates hypoxia/reoxygenation injury by activating SSeCKS in human cerebrovascular pericytes. Neurochem Res. 2020 Feb;45(2):310–21. 10.1007/s11064-019-02915-0.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science
Artikel in diesem Heft
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science

