Abstract
Objective
Accumulating evidence has suggested that thyroid hormone levels affect the prognosis of acute ischemic stroke (AIS), but the results have been inconsistent.
Methods
Basic data, neural scale scores, thyroid hormone levels, and other laboratory examination data of AIS patients were collected. The patients were divided into excellent and poor prognosis group at discharge and 90 days after discharge. Logistic regression models were applied to evaluate the relationship between thyroid hormone levels and prognosis. A subgroup analysis was performed based on stroke severity.
Results
A number of 441 AIS patients were included in this study. Those in the poor prognosis group were older, with higher blood sugar levels, higher free thyroxine (FT4) levels, and severe stroke (all p < 0.05) at baseline. Free thyroxine (FT4) showed a predictive value (all p < 0.05) for prognosis in the model adjusted for age, gender, systolic pressure, and glucose level. However, after adjustment for types and severity of stroke, FT4 showed insignificant associations. In the severe subgroup at discharge, the change in FT4 was statistically significant (p = 0.015), odds ratio (95% confidence interval) = 1.394 (1.068–1.820) but not in the other subgroups.
Conclusions
High-normal FT4 serum levels in patients with severe stroke receiving conservative medical treatment at admission may indicate a worse short-term prognosis.
1 Introduction
Stroke was the second leading cause of death worldwide after ischemic heart disease. At the same time, 101 million people in the world suffered from stroke and 6.55 million people dead from stroke [1]. Stroke was also a major cause of death in China, with approximately 3.94 million new cases of stroke and 2.19 million stroke-related deaths each year. As of 2019, there have been about 28 million cases of stroke; 24 million of these were cases of acute ischemic stroke (AIS) [2]. From 2013 (2.28%) to 2019 (2.58%), the prevalence of stroke in China increased by 13.2%, with an annual increase rate of 2.2% [3].
With age, changes occur in all bodily systems, including the endocrine system. The thyroid function is particularly important due to its central role in metabolism, thermogenesis, and immunity, among others, as well as because of its contribution to most common chronic age-associated diseases, especially cardiovascular disorders [4,5,6]. The prevalence of thyroid dysfunction increases with the aging of the global population [6,7]. The relationship between thyroid hormones and cardiovascular health has attracted considerable research attention [8,9]. Observational findings have revealed that not only the abnormal range of thyroid hormones but also their normal range is involved in the occurrence and development of cardiovascular diseases [10,11]. However, no consensus has been achieved on the optimal target level of thyroid hormones in acute ischemic stroke patients. The results of previous studies on the association between acute thyroxine levels and the clinical outcomes in AIS patients are contradictory [12]. For example, a study including 129 patients with AIS indicated that thyroid hormone levels were not an independent predictor of stroke outcome [13]. Another study focused on the association of anterior pituitary hormones with stroke indicated that thyroid hormones were not associated with stroke prognosis [14]. The Rotterdam Study revealed that free thyroxine (FT4) levels in elderly subjects were positively associated with atherosclerosis throughout the whole disease spectrum, independently of cardiovascular risk factors [15]. On the other hand, subclinical hypothyroidism (SCH) and low FT4 levels have been associated with increased carotid intima-media thickness in recent studies [16,17,18]. In a retrospective study of 221 AIS cases and 182 non-AIS cases, detailed clinical data showed that lower triiodothyronine (FT3) concentrations measured at admission were predictive of poor neurological function within three months [19]. Another investigation prospectively recruited 563 intravenous thrombolysis (IVT) patients from five stroke centers in China and established that subclinical hyperthyroidism (SHyper) was an independent predictor of 3-month poor outcome and mortality [20]. We can thus speculate that low FT3, high FT4, and low thyroid-stimulating hormone (TSH) can predict a poor prognosis in patients with acute ischemic stroke. Thus, we conducted this study to investigate whether thyroid hormone levels within the normal reference range are associated with poorer functional outcome in AIS patients and to explain the underlying mechanisms.
2 Methods
2.1 Study design and population
This study was ambispective cohort study. Patients diagnosed with acute ischemic stroke with standardized treatment at Lianyungang Hospital affiliated to Xuzhou Medical University were consecutively included. The complete study cohort consisted of two parts. One part retrospectively was collected from September 1, 2019, to October 21, 2021, and the other prospectively was from October 21, 2021, to December 31, 2021.
Standardized treatment was given to patients according to guidelines [21] for respiratory support, blood pressure, temperature and glucose management, and antiplatelet therapy but does not include thrombolysis and endovascular therapy.
Patients were enrolled in this study if they met the following criteria: (1) age ≥18 years old; (2) onset within 48 h; (3) presence of acute ischemic lesions in anterior circulation, which were confirmed by imaging methods (magnetic resonance imaging or computed tomography); and (4) received standardized treatment.
Exclusion criteria for this study were: (1) intracranial hemorrhage or mass lesion; (2) with severe infection or septic shock; (3) liver or renal failure; (4) incomplete laboratory, clinical or follow-up data; (5) thyroid dysfunction (normal reference ranges were those routinely used at Lianyungang First People’s Hospital Laboratory: for TSH, 0.56–5.91 mIU/L; for FT4, 7.9–17.0 pmol/L; and for FT3, 3.8–6.47 pmol/L); and (6) received thrombolysis or mechanical thrombectomy.
2.2 Data collection
Baseline clinical information of all enrolled patients was collected from the database, including age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), the National Institutes of Health Stroke Scale (NIHSS), history of hypertension, diabetes (DM), atrial fibrillation (AF), stroke or coronary heart disease (CHD), current smoking (any usage of cigarette per day in the past 30 days), and drinking (drinking more than 100 mL [alcohol content >50% liquor] per day on average and drinking for more than 1 year; abstaining from drinking for more than 1 year is not). NIHSS score on admitted was determined by two trained neurologists. Laboratory findings at admission included TSH, FT4, FT3, blood glucose (BG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), homocysteine (HCY), blood urea nitrogen (BUN), and creatinine (Cr). We calculated BUN to creatinine (BUN/Cr) ratio, because BUN/Cr was identified as an potential predictor indicator for poor outcomes of patients with stroke [22]. The trial of Org 10172 in Acute Stroke Treatment (TOAST) classification was divided into large-artery atherosclerosis (LAA), cardioembolic (CE), small artery occlusion (SAO), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE) [23]. Patients were assessed for stroke severity by NIHSS score, with ≤4 being mild stroke and >4 being severe stroke [24].
2.3 Functional outcome and follow-up
The functional outcome was assessed by the modified Rankin Scale (mRS) at discharge and 90 days after onset. 90-day mRS was determined by two trained operators via telephone. An excellent outcome was defined as mRS 0–2.
2.4 Blood thyroid function tests measurement
Within 24 h after admission, all patients underwent venous blood drawing for routine laboratory after overnight fast. Serum TSH, FT4, and FT3 were measured by the chemiluminescence immunoassay (Beckman Coulter DXI800).
2.5 Statistical analysis
The analyses were done with R version 4.2.3 (R Core Team 2022). Kolomogorov–Simirnov test was used to identify the normality of data. The normal distribution data were analyzed by independent sample t test, and the non-normal distribution data were analyzed by Mann–Whitney U test. Fisher’s exact test or the chi-square test was used to compare categorical variables as appropriate. Statistical significance was determined as a bilateral test.
Univariate logistic regression analysis was used to explore the association between baseline information and prognosis. Multivariate logistic regression analysis model was used to determine the relationship between the thyroid hormone levels and functional outcome in acute ischemic stroke and controlled confounding factors. Three multivariate logistic regression models were developed. Model 1 was without adjusting any covariates; model 2 was adjusted for age, gender, and variables that were significant in the univariate regression except for neural scales. Model 3 added neural scale scores to model 2.
All subjects were divided into two subgroups: NIHSS ≤4 subgroup and NIHSS >4 subgroup. Multivariate logistic regression was used to evaluate the relationship between thyroid hormone levels and stroke prognosis in subgroups (the variables are the same as those in model 4 but do not include NIHSS). p < 0.05 (bilateral) is defined as a statistically significant difference.
-
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee. Ethical approval for this study was obtained from the ethics committees of Lianyungang Hospital affiliated to Xuzhou Medical University (No. KY-20210917001-01).
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
3 Results
3.1 Study population and clinical characteristics
The study population was 777 patients with acute ischemic stroke who received conventional medical therapy. After screening with exclusion criteria, 441 patients were finally determined as the population meeting the study criteria (Figure 1). Due to the small sample size of SOE and SUE in TOAST classification, they were combined into other group for easier statistical analysis. As shown in Table 1, compared with the group with poor functional outcomes, gender, DBP, smoking, drinking, atrial fibrillation, hypertension, CHD, TSH, FT3, HbAlc, TC, TG, HDL, LDL, Hcy, BUN, Cr, and BUN/Cr had a similar distribution in the group with excellent outcomes (all p ≥ 0.05) whether mRS was scored at discharge or 90 days later. People with higher mRS were older and had higher NIHSS scores, higher BG levels, higher FT4 levels, and higher proportion of DM and LAA (all p < 0.05).
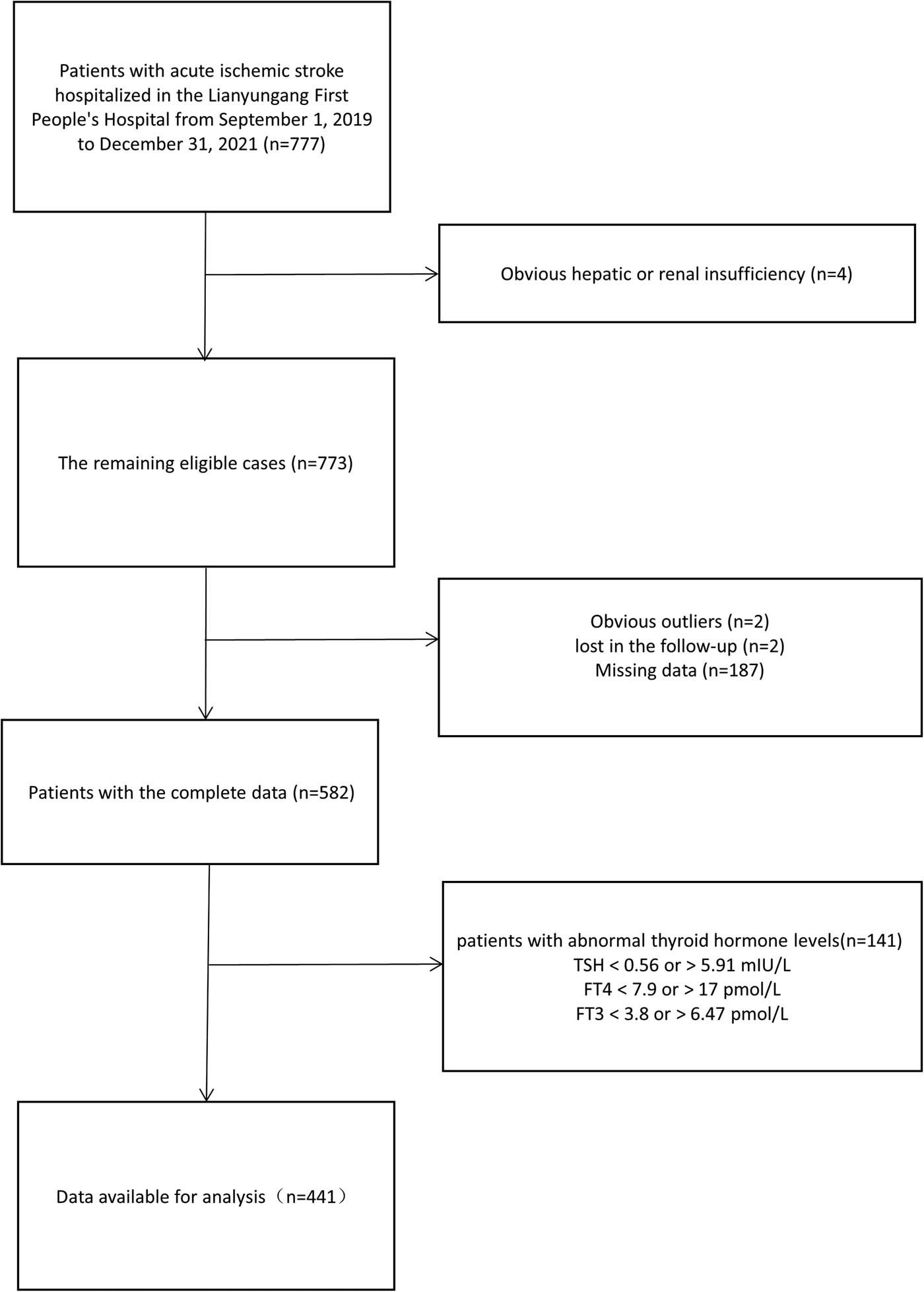
Subjects screening flowchart. Abbreviations: TSH, thyroid-stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine.
Baseline characteristics of patients
| mRS at discharge | 90-Day mRS | ||||||
|---|---|---|---|---|---|---|---|
| Total (n = 441) | Excellent prognosis (n = 334) | Poor prognosis (n = 107) | p | Excellent prognosis (n = 343) | Poor prognosis (n = 98) | p | |
| Parameter | |||||||
| 163 (37.0) | 121 (36.2) | 42 (39.3) | 0.653 | 124 (36.2) | 39 (39.8) | 0.589 | |
| Age (years) | 66.00 [57.00, 72.00] | 65.50 [57.00, 71.00] | 68.00 [58.00, 77.00] | 0.038 | 66.00 [57.00, 71.50] | 67.50 [58.00, 77.00] | 0.061 |
| SBP (mmHg) | 146.00 [137.00, 156.00] | 145.00 [137.00, 154.75] | 150.00 [137.00, 164.00] | 0.017 | 146.00 [137.50, 155.00] | 150.00 [137.00, 162.75] | 0.054 |
| DBP (mmHg) | 85.00 [79.00, 92.00] | 85.00 [79.00, 92.00] | 85.00 [80.00, 92.50] | 0.841 | 85.00 [79.00, 92.00] | 84.50 [80.00, 92.75] | 0.797 |
| Medical history | |||||||
| Smoking | 132 (29.9) | 101 (30.2) | 31 (29.0) | 0.898 | 103 (30.0) | 29 (29.6) | 1.000 |
| Drinking | 117 (26.5) | 91 (27.2) | 26 (24.3) | 0.635 | 92 (26.8) | 25 (25.5) | 0.897 |
| AF | 35 (7.9) | 26 (7.8) | 9 (8.4) | 0.997 | 26 (7.6) | 9 (9.2) | 0.760 |
| DM | 114 (25.9) | 76 (22.8) | 38 (35.5) | 0.013 | 79 (23.0) | 35 (35.7) | 0.016 |
| Hypertension | 281 (63.7) | 212 (63.5) | 69 (64.5) | 0.941 | 220 (64.1) | 61 (62.2) | 0.822 |
| Stroke | 81 (18.4) | 56 (16.8) | 25 (23.4) | 0.164 | 59 (17.2) | 22 (22.4) | 0.301 |
| CHD | 34 (7.7) | 27 (8.1) | 7 (6.5) | 0.755 | 27 (7.9) | 7 (7.1) | 0.981 |
| Biochemical | |||||||
| TSH (mmol/L) | 1.71 [1.17, 2.49] | 1.71 [1.19, 2.50] | 1.67 [1.10, 2.46] | 0.482 | 1.72 [1.19, 2.51] | 1.60 [1.07, 2.40] | 0.327 |
| FT4 (mmol/L) | 12.19 [11.02, 13.40] | 12.04 [10.90, 13.18] | 12.58 [11.50, 13.91] | 0.001 | 12.07 [10.92, 13.28] | 12.54 [11.42, 13.82] | 0.007 |
| FT3 (mmol/L) | 4.65 [4.32, 5.00] | 4.64 [4.31, 5.00] | 4.66 [4.42, 4.98] | 0.818 | 4.65 [4.31, 5.00] | 4.65 [4.42, 4.96] | 0.965 |
| BG (mmol/L) | 5.33 [4.68, 7.07] | 5.19 [4.64, 6.28] | 5.97 [5.06, 7.82] | <0.001 | 5.20 [4.64, 6.30] | 5.97 [5.06, 8.18] | <0.001 |
| HbA1c (mmol/L) | 6.00 [5.60, 7.20] | 6.00 [5.60, 7.00] | 6.20 [5.60, 7.70] | 0.173 | 6.00 [5.60, 7.05] | 6.15 [5.60, 7.75] | 0.242 |
| TC (mmol/L) | 4.55 [3.89, 5.27] | 4.53 [3.90, 5.20] | 4.60 [3.86, 5.54] | 0.325 | 4.52 [3.89, 5.20] | 4.65 [3.87, 5.55] | 0.202 |
| TG (mmol/L) | 1.45 [1.04, 2.18] | 1.43 [1.05, 2.08] | 1.59 [1.00, 2.25] | 0.523 | 1.43 [1.06, 2.09] | 1.59 [0.97, 2.22] | 0.848 |
| HDL (mmol/L) | 1.05 [0.88, 1.19] | 1.04 [0.88, 1.19] | 1.07 [0.94, 1.20] | 0.183 | 1.04 [0.88, 1.19] | 1.09 [0.94, 1.22] | 0.092 |
| LDL (mmol/L) | 2.52 (0.69) | 2.49 (0.65) | 2.60 (0.79) | 0.169 | 2.49 (0.66) | 2.63 (0.77) | 0.074 |
| Hcy (μmol/L) | 9.80 [8.10, 12.30] | 9.70 [8.10, 12.30] | 10.00 [8.05, 12.15] | 1.000 | 9.70 [8.10, 12.30] | 10.00 [8.03, 12.23] | 0.997 |
| BUN (mmol/L) | 5.45 [4.50, 6.48] | 5.48 [4.59, 6.47] | 5.20 [4.30, 6.49] | 0.400 | 5.46 [4.57, 6.47] | 5.41 [4.32, 6.50] | 0.533 |
| Cr (mmol/L) | 59.00 [49.20, 70.00] | 59.20 [49.85, 70.25] | 57.50 [46.35, 69.45] | 0.269 | 59.20 [49.70, 70.20] | 57.05 [46.62, 69.38] | 0.279 |
| BUN/Cr | 0.09 [0.07, 0.11] | 0.09 [0.07, 0.11] | 0.09 [0.08, 0.11] | 0.836 | 0.09 [0.07, 0.11] | 0.09 [0.08, 0.11] | 0.739 |
| NIHSS > 4 | 113 (25.6) | 36 (10.8) | 77 (72.0) | <0.001 | 42 (12.2) | 71 (72.4) | <0.001 |
| TOAST | 0.002 | 0.005 | |||||
| LAA | 189 (42.9) | 126 (37.7) | 63 (58.9) | 132 (38.5) | 57 (58.2) | ||
| CE | 33 (7.5) | 26 (7.8) | 7 (6.5) | 26 (7.6) | 7 (7.1) | ||
| SAO | 206 (46.7) | 172 (51.5) | 34 (31.8) | 175 (51.0) | 31 (31.6) | ||
| Other | 13 (2.9) | 10 (3.0) | 3 (2.8) | 10 (2.9) | 3 (3.1) | ||
Note: abbreviations: systolic blood pressure (SBP), diastolic blood pressure (DBP), National Institutes of Health Stroke Scale (NIHSS), diabetes (DM), atrial fibrillation (AF), coronary heart disease (CHD), thyroid-stimulating hormone (TSH), free thyroxine (FT4), free triiodothyronine (FT3), blood glucose (BG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), homocysteine (Hcy), blood urea nitrogen (BUN) and creatinine (Cr), BUN to creatinine ratio (BUN/Cr), modified Rankin Scale (mRS), the Trial of Org 10172 in Acute Stroke Treatment (TOAST), large-artery atherosclerosis (LAA), cardioembolic (CE), small artery occlusion (SAO). Other defined as stroke of other determined etiology and stroke of undetermined etiology combined.
3.2 Univariable and multivariable analyses of functional outcomes at discharge and 90 days
A univariate analysis was conducted for each variable in the baseline table using a logistic regression. The resulting variables were defined as functional outcomes at discharge and 90 days, respectively. Variables with statistical significance are listed in Table 2. DM, FT4, BG, and NIHSS showed statistical significance at discharge and 90 days after discharge (all p < 0.05). When TOAST was used as a factor variable in the single-factor logistic regression model (LAA was used as a control), only SAO showed a correlation with the outcome variable (all p < 0.05). In addition, the SBP showed statistical significance at discharge (p = 0.029).
Univariate logistic regression for functional outcomes
| OR | 95% CI | p | |
|---|---|---|---|
| At discharge | |||
| Diabetes | 1.87 | 1.167–2.995 | 0.009 |
| SBP | 1.014 | 1.001–1.026 | 0.029 |
| BG | 1.101 | 1.029–1.179 | 0.006 |
| FT4 | 1.249 | 1.101–1.417 | 0.001 |
| NIHSS > 4 | 21.246 | 12.314–36.659 | <0.001 |
| TOAST | |||
| CE | 0.538 | 0.222–1.308 | 0.172 |
| SAO | 0.395 | 0.246–0.637 | <0.001 |
| Other | 0.6 | 0.159–2.258 | 0.45 |
| 90-Day mRS | |||
| Diabetes | 1.857 | 1.145–3.011 | 0.012 |
| BG | 1.107 | 1.032–1.186 | 0.004 |
| FT4 | 1.195 | 1.051–1.359 | 0.006 |
| NIHSS > 4 | 18.846 | 10.892–32.608 | <0.001 |
| TOAST | |||
| CE | 0.623 | 0.256–1.519 | 0.298 |
| SAO | 0.41 | 0.251–0.671 | <0.001 |
| Other | 0.695 | 0.184–2.619 | 0.591 |
Note: A univariate analysis was conducted for each variable in the baseline table using a logistic regression. The resulting variables were defined as functional outcomes at discharge and 90 days, respectively. Meaningful variables were displayed in Table 2.
Two logistic regression models were used to evaluate the relationships between functional outcomes and thyroid hormone levels. The resulting variables were defined as functional outcomes at discharge and 90 days, respectively. FT4 was statistical significance in model 1 (no adjust), model 2 (additionally adjusted for age, sex, SBP, BG; to avoid interactions, BG was included in model 2 but DM not), and model 3 (with the addition of TOAST and NIHSS in model 2), the relationship between FT4 and functional outcome became not significant (all p > 0.05) (Figure 2). In model 3, NIHSS was statistical significance (all p < 0.05), but TOAST was not significant (all p > 0.05) (Table 3). In the two regression models, FT3 and TSH were not statistically significant (all p > 0.05), no matter how to adjust the covariates.

Forest plot about four logistic regression. Note: model 1 was without adjusting any covariates; model 2, additionally adjusted for age, Gender, SBP, and BG; model 3 added NIHSS and TOAST.
Statistical value in model 3
| At discharge | After 90 days | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| NIHSS > 4 | 20.216 (11.339–36.041)) | <0.001 | 17.339 (9.8–30.68) | <0.001 |
| TOAST | ||||
| CE | 0.272 (0.085–0.872) | 0.028 | 0.399 (0.132–1.211) | 0.105 |
| SAO | 0.589 (0.325–1.07) | 0.083 | 0.617 (0.339–1.124) | 0.115 |
| Other | 0.842 (0.165–4.294) | 0.836 | 1.004 (0.203–4.969) | 0.996 |
Note: The statistical values of the newly added variables NIHSS and TOAST in two models 3 were displayed.
3.3 Association between FT4 levels and functional outcomes in subgroups
On the group with NIHSS ≤4, none of the variables were found to be significant. In the population with severe stroke at admission, higher FT4 levels indicated higher mRS at discharge, p = 0.015, odds ratio (95% confidence interval) = 1.394 (1.068–1.820). But FT3 and TSH still were not significant (all p > 0.05) (Table 4).
Multiple logistic regression for functional outcomes in NIHSS subgroups
| NIHSS >4 | NIHSS ≤4 | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| At discharge | ||||
| TSH | 0.886 (0.596–1.316) | 0.547 | 0.953 (0.635–1.428) | 0.814 |
| FT4 | 1.394 (1.068–1.82) | 0.015 | 0.981 (0.775–1.242) | 0.873 |
| FT3 | 1.827 (0.654–5.103) | 0.250 | 1.011 (0.45–2.27) | 0.979 |
| After 90 days | ||||
| TSH | 0.817 (0.558–1.197) | 0.300 | 0.894 (0.586–1.364) | 0.604 |
| FT4 | 1.186 (0.933–1.508) | 0.164 | 0.923 (0.723–1.178) | 0.521 |
| FT3 | 1.552 (0.59–4.079) | 0.373 | 0.948 (0.405–2.221) | 0.903 |
Note: NIHSS was divided into two subgroups: NIHSS >4 group and NIHSS ≤4 group. TSH, FT4, and FT3 were evaluated again according to the method of building model 4. However, variables in model 4 no longer include NIHSS.
4 Discussion
Studies have shown that many systemic diseases, rather than thyroid-associated disorders, cause abnormal changes in serum thyroid hormone levels, in which thyroxine (T4) is not properly converted to triiodothyronine (T3), and inactive thyroid hormone T3 is accumulated in the body, known as euthyroid sick syndrome (ESS) [25]. ESS includes low-T3 syndrome, low-T3 and -T4 syndrome, high-T4 syndrome, and other abnormalities [26]. ESS has been observed in a variety of critical illnesses [27]. Common causes include severe infections, cranial trauma, respiratory failure, heart failure, starvation, surgery, and psychiatric disorders [28,29,30,31,32,33,34].
Thyroid hormones such as T3 and T4 have an irreplaceable role in the development, differentiation, and maturation processes of the brain tissue [35]. Thyroid hormones are secreted by the thyroid follicular epithelial cells. In peripheral blood, most T3 (80–90%) is converted from T4 by deiodinase [12]. In the blood circulation, some of the T4 is converted into T3 by deiodinase. T3 shows three- to five-fold greater activity than T4. Both hormones circulate in the peripheral blood in two forms: free and bound; the former enters the target cells and performs biological functions [36]. Interestingly, T3 and T4 have similar chemical structures and biological functions but have different associations with stroke prognosis [37,38]. In another investigation, patients with poor outcomes had significantly lower FT3 and serum total triiodothyronine (TT3) levels, and FT3/FT4 ratio, but higher FT4 levels [12]. A retrospective case–control study in patients with early-onset ischemic stroke without diabetes and hypertension revealed significantly lower levels of FT3 and FT4 in IS patients than in the controls [39]. In our research work, participants with higher mRS had higher FT4 levels. We further separated long-term and short-term prognoses and found that higher FT4 in patients with severe stroke at admission could indicate a worse short-term prognosis. No association between thyroid hormones and long-term prognosis of stroke was observed. Therefore, we speculated that the changes in FT4 in acute stroke are related mainly to the emergency response. We hypothesized that during emergency, increased synthesis of FT4 might have occurred, while no timely conversion of FT4 to FT3 was provided or was inhibited.
Thyroid hormones play a paradoxical role in AIS. The present research work on the relationship between thyroid hormone metabolic status and stroke prognosis is focused on the areas specified below.
4.1 SCH
SCH refers to a state in which TSH levels are higher than the normal, but those of free T3, T4, and total T3 and T4 are still in the normal range [27]. Acute ischemic stroke patients with SCH at admission were more likely to have excellent functional outcomes than those without SCH [40]. A significant protective association of SCH, with better outcomes and lower mortality after CIS, was found in another investigation [41]. Additionally, patients with acute ischemic stroke with high levels of TSH tended to have a better outcome [40,42]. Another study with 756 acute ischemic stroke patients recruited within seven days of onset established that the proportion of patients with excellent outcomes was significantly higher in the SCH group than in the control group on the 90th day [43]. Possible explanations for this association are ischemic preconditioning, reduced adrenergic tone, and hypometabolic state [30,36]. A study including 88 patients with cerebral infarction, most of which did not receive IVT, did not show a relationship between TSH and patient functional outcome [44], which is consistent with the conclusions of our work.
4.2 Subclinical hyperthyroidism
Subclinical hyperthyroidism is defined by reduced TSH levels in the presence of normal FT4 and FT3 values [45]. The low TSH levels of 199 patients in another previous investigation predicted poor clinical outcomes after endovascular thrombectomy in patients with anterior circulation ischemic stroke [46]. Furthermore, patients with subclinical hyperthyroidism had a higher risk of poor functional outcomes at 3 months after a stroke and a decreased rate of successful reperfusion after reperfusion therapy than patients in the euthyroid state [47]. Similar results were obtained in the present study. Although without a statistically significant difference, TSH in that earlier study was lower in the poor prognosis group (at discharge or at 90 days) than in the excellent prognosis group [42,47], which was not confirmed in our investigation.
4.3 Low-T3 syndrome
Low-T3 syndrome refers to decreased thyroid function and T3 level but normal T4 and TSH levels [48]. Reportedly, low FT3 values (<2.00 pg/mL) in 702 consecutive patients with acute stroke were independently associated with poor functional outcome and mortality at 3 months after stroke onset [49]. The results of 11 studies including a total number of 3936 enrolled patients with acute stroke showed that patients with a poor prognosis had lower T3 levels [12]. However, no similar results were obtained in our work. The median value of FT3 in our study population was 4.65 pmol/L, and the median value of FT3 in both the excellent and poor prognosis groups was close to this level.
4.4 High-T4 state
High-T4 state serum T4 levels may actually be elevated early in acute illness due to either the acute inhibition of type 1 iodothyronine deiodinases or an increase in thyroxine-binding globulin (TBG) levels [50,51]. This state is most often observed in the elderly and in patients with psychiatric disorders [52]. As the duration of illness increases, nondeiodinative pathways of T4 degradation increase, and serum T4 levels return to the normal range [53]. This may explain why higher FT4 in patients with severe stroke at admission is associated with a worse short-term prognosis in our study. A study in 2017 restricted to the LAA population showed an association between FT4 and 14-day mRS, but the NIHSS was not included in the analysis. In this study, we further explored the relationship between stroke severity and short-term prognosis. We speculated that the elevated FT4 was consistent with the elevated BG at the onset, which may be related to a short-term stress state.
The results of this study suggest that higher FT4 is associated with worse short-term prognosis of stroke but not with long-term prognosis. We speculate that it may be that fluctuations in thyroid hormones in stroke accelerate disease progression or have other adverse effects. Subsequently, the hormone levels slowly return to normal and the adverse effects disappear or are reduced. By measuring FT4 levels in patients, doctors earlier predicted the development of stroke severity. And this phenomenon may provide new ideas for treatment to delay the progression of stroke. We speculate that future studies could find new thresholds with AIS for thyroid hormone levels, doctors intervene in thyroid hormone levels in AIS patients who in the short term of the attack for the delay of stroke progression and improve prognosis.
4.5 Limitations
Nevertheless, the present study has some limitations. This was a single-center retrospective study, which might have selection bias. Since the population in this study was of Asian descent, the results may not be applicable to other ethnic groups. Also, the secondary outcomes, including mortality and hemorrhage, need to be evaluated in future large-scale studies.
5 Conclusions
High-normal FT4 serum levels in patients with severe stroke receiving conservative medical treatment at admission may indicate a worse short-term prognosis.
-
Funding information: This work was supported by the Science and Technology Project of Lianyungang Health Commission (202024), Jiangsu Province six one project top talent to be funded project (LGY2019062), Scientific research project of Bengbu Medical College (2020byzd341), Jiangsu Provincial Geriatric Health Research Grant Project (LD2021034; LR2021049), and Jiangsu Province Postgraduate Practice Innovation Program (SJCX21_1726).
-
Author contributions: GZ and XS conceived and designed the research. YJ and CX analyzed the data and drafted the manuscript. YX, ML, and ND collected the data and performed the research. All authors reviewed and edited the article and approved the final version of the article.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] GBD. Stroke collaborators. global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2019;20(10):795–820.Search in Google Scholar
[2] Ma Q, Li R, Wang L, Yin P, Wang Y, Yan C, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: An analysis for the global burden of disease study 2019. Lancet Public Health. 2021 Dec;6(12):e897–906.10.1016/S2468-2667(21)00228-0Search in Google Scholar PubMed PubMed Central
[3] Tu WJ, Hua Y, Yan F, Bian H, Yang Y, Lou M, et al. Prevalence of stroke in China, 2013-2019: A population-based study. Lancet Reg Health West Pac. 2022 Nov;28:100550.10.1016/j.lanwpc.2022.100550Search in Google Scholar PubMed PubMed Central
[4] Franceschi C, Ostan R, Mariotti S, Monti D, Vitale G. The aging thyroid: A reappraisal within the geroscience integrated perspective. Endocr Rev. 2019 Oct 1;40(5):1250–70.10.1210/er.2018-00170Search in Google Scholar PubMed
[5] Duntas LH. Aging and the hypothalamic-pituitary-thyroid axis. Vitam Horm. 2021;115:1–14.10.1016/bs.vh.2020.12.001Search in Google Scholar PubMed
[6] Chen J, Lippo L, Labella R, Tan SL, Marsden BD, Dustin ML, et al. Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. EMBO J. 2021 Jan 4;40(1):e105242.10.15252/embj.2020105242Search in Google Scholar PubMed PubMed Central
[7] Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002 Feb;87(2):489–99.10.1210/jcem.87.2.8182Search in Google Scholar PubMed
[8] Baqui M, Botero D, Gereben B, Curcio C, Harney JW, Salvatore D, et al. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J Biol Chem. 2003 Jan 10;278(2):1206–11.10.1074/jbc.M210266200Search in Google Scholar PubMed
[9] Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, et al. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab. 2014 Jul;99(7):2372–82.10.1210/jc.2013-4184Search in Google Scholar PubMed
[10] Chaker L, Baumgartner C, den Elzen WPJ, Collet TH, Ikram MA, Blum MR, et al. Thyroid function within the reference range and the risk of stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2016 Nov;101(11):4270–82.10.1210/jc.2016-2255Search in Google Scholar PubMed PubMed Central
[11] Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012 Aug 28;126(9):1040–9.10.1161/CIRCULATIONAHA.112.096024Search in Google Scholar PubMed PubMed Central
[12] Jiang X, Xing H, Wu J, Du R, Liu H, Chen J, et al. Prognostic value of thyroid hormones in acute ischemic stroke - a meta analysis. Sci Rep. 2017 Nov 24;7(1):16256.10.1038/s41598-017-16564-2Search in Google Scholar PubMed PubMed Central
[13] O’Keefe LM, Conway SE, Czap A, Malchoff CD, Benashski S, Fortunato G, et al. Thyroid hormones and functional outcomes after ischemic stroke. Thyroid Res. 2015 Jul 4;8(1):9.10.1186/s13044-015-0021-7Search in Google Scholar PubMed PubMed Central
[14] Neidert S, Katan M, Schuetz P, Fluri F, Ernst A, Bingisser R, et al. Anterior pituitary axis hormones and outcome in acute ischaemic stroke. J Intern Med. 2011 Apr;269(4):420–32.10.1111/j.1365-2796.2010.02327.xSearch in Google Scholar PubMed
[15] Bano A, Chaker L, Mattace-Raso FUS, van der Lugt A, Ikram MA, Franco OH, et al. Thyroid function and the risk of atherosclerotic cardiovascular morbidity and mortality: the rotterdam study. Circ Res. 2017 Dec 8;121(12):1392–400.10.1161/CIRCRESAHA.117.311603Search in Google Scholar PubMed
[16] Liu J, Cui X, Wang D, Wu S, Xiong Y, Zhang S, et al. Relationship of thyroid function with intracranial arterial stenosis and carotid atheromatous plaques in ischemic stroke patients with euthyroidism. Oncotarget. 2017 Jul 11;8(28):46532–9.10.18632/oncotarget.14883Search in Google Scholar PubMed PubMed Central
[17] Aziz M, Kandimalla Y, Machavarapu A, Saxena A, Das S, Younus A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017 Jul 1;24(7):643–59.10.5551/jat.39917Search in Google Scholar PubMed PubMed Central
[18] Zhao T, Chen B, Zhou Y, Wang X, Zhang Y, Wang H, et al. Effect of levothyroxine on the progression of carotid intima-media thickness in subclinical hypothyroidism patients: a meta-analysis. BMJ Open. 2017 Oct 22;7(10):e016053.10.1136/bmjopen-2017-016053Search in Google Scholar PubMed PubMed Central
[19] Zhang S, Zhao X, Xu S, Yuan J, Si Z, Yang Y, et al. Low free triiodothyronineis predicts worsen neurological outcome of patients with acute ischemic stroke: a retrospective study with bioinformatics analysis. BMC Neurol. 2019 Nov 5;19(1):272.10.1186/s12883-019-1509-xSearch in Google Scholar PubMed PubMed Central
[20] Zhang X, Gong P, Sheng L, Lin Y, Fan Q, Zhang Y, et al. Prognostic value of subclinical thyroid dysfunction in ischemic stroke patients treated with intravenous thrombolysis. Aging. 2019 Sep 3;11(17):6839–50.10.18632/aging.102215Search in Google Scholar PubMed PubMed Central
[21] Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019 Dec;50(12):e344–418.10.1161/STR.0000000000000211Search in Google Scholar PubMed
[22] Chen T, Li AP, Gong Q, Zhou L, Zhao YX, Zhou ZW, et al. The association of blood urea nitrogen to creatinine ratio and the prognosis of critically ill patients with cerebral infarction: a cohort study. Mediators Inflamm. 2022;2022:2151840.10.21203/rs.3.rs-1462345/v2Search in Google Scholar
[23] Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35–41.10.1161/01.STR.24.1.35Search in Google Scholar PubMed
[24] Reale G, Iacovelli C, Rabuffetti M, Manganotti P, Marinelli L, Sacco S, et al. Actigraphic sensors describe stroke severity in the acute phase: implementing multi-parametric monitoring in stroke unit. J Clin Med. 2023 Feb 2;12(3):1178.10.3390/jcm12031178Search in Google Scholar PubMed PubMed Central
[25] Lee S, Farwell AP. Euthyroid sick syndrome. Compr Physiol. 2016 Mar 15;6(2):1071–80.10.1002/cphy.c150017Search in Google Scholar PubMed
[26] Wang YF, Heng JF, Yan J, Dong L. Relationship between disease severity and thyroid function in Chinese patients with euthyroid sick syndrome. Med (Baltim). 2018 Aug;97(31):e11756.10.1097/MD.0000000000011756Search in Google Scholar PubMed PubMed Central
[27] Štěpánek L, Horáková D, Štěpánek L, Janout V, Janoutová J, Bouchalová K, et al. Free triiodothyronine/free thyroxine (FT3/FT4) ratio is strongly associated with insulin resistance in euthyroid and hypothyroid adults: a cross-sectional study. Endokrynol Pol. 2021;72(1):8–13.10.5603/EP.a2020.0066Search in Google Scholar PubMed
[28] Guo J, Hong Y, Wang Z, Li Y. Analysis of the incidence of euthyroid sick syndrome in comprehensive intensive care units and related risk factors. Front Endocrinol. 2021;12:656641.10.3389/fendo.2021.656641Search in Google Scholar PubMed PubMed Central
[29] Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev. 1996 Oct;17(5):518–32.10.1210/edrv-17-5-518Search in Google Scholar PubMed
[30] Cappola AR, Desai AS, Medici M, Cooper LS, Egan D, Sopko G, et al. Thyroid and cardiovascular disease research agenda for enhancing knowledge, prevention, and treatment. Circulation. 2019 May 13;139(25):2892–909.10.1161/CIRCULATIONAHA.118.036859Search in Google Scholar PubMed PubMed Central
[31] Douyon L, Schteingart DE. Effect of obesity and starvation on thyroid hormone, growth hormone, and cortisol secretion. Endocrinol Metab Clin North Am. 2002 Mar;31(1):173–89.10.1016/S0889-8529(01)00023-8Search in Google Scholar
[32] Nader S, Warner MD, Doyle S, Peabody CA. Euthyroid sick syndrome in psychiatric inpatients. Biol Psychiatry. 1996 Dec 15;40(12):1288–93.10.1016/0006-3223(95)00626-5Search in Google Scholar PubMed
[33] Powner DJ, Boccalandro C, Alp MS, Vollmer DG. Endocrine failure after traumatic brain injury in adults. Neurocrit Care. 2006;5(1):61–70.10.1385/NCC:5:1:61Search in Google Scholar
[34] Krug N, Bercker S, Busch T, Friese S, Jahn N, Voelker MT. Non-thyroidal Illness Syndrome (NTIS) is no independent predictor for mortality in ICU patients. BMC Anesthesiol. 2023 Mar 31;23(1):103.10.1186/s12871-023-02015-1Search in Google Scholar PubMed PubMed Central
[35] Calzà L, Fernández M, Giardino L. Role of the thyroid system in myelination and neural connectivity. Compr Physiol. 2015 Jul 1;5(3):1405–21.10.1002/cphy.c140035Search in Google Scholar PubMed
[36] Scicluna BP, Klein Klouwenberg PMC, van Vught LA, Wiewel MA, Ong DSY, Zwinderman AH, et al. A molecular biomarker to diagnose community-acquired pneumonia on intensive care unit admission. Am J Respir Crit Care Med. 2015 Oct 1;192(7):826–35.10.1164/rccm.201502-0355OCSearch in Google Scholar PubMed
[37] Wang Y, Zhou S, Bao J, Pan S, Zhang X. Low T3 levels as a predictor marker predict the prognosis of patients with acute ischemic stroke. Int J Neurosci. 2017 Jul;127(7):559–66.10.1080/00207454.2016.1211649Search in Google Scholar PubMed
[38] Lee YM, Ki YJ, Choi DH, Kim BB, Shin BC, Song H, et al. Value of low triiodothyronine and subclinical myocardial injury for clinical outcomes in chest pain. Am J Med Sci. 2015 Nov;350(5):393–7.10.1097/MAJ.0000000000000573Search in Google Scholar PubMed
[39] Zhang N, Zhang L, Wang Q, Zhao J, Liu J, Wang G. Cerebrovascular risk factors associated with ischemic stroke in a young non-diabetic and non-hypertensive population: a retrospective case-control study. BMC Neurol. 2020 Nov 23;20(1):424.10.1186/s12883-020-02005-7Search in Google Scholar PubMed PubMed Central
[40] Dhital R, Poudel DR, Tachamo N, Gyawali B, Basnet S, Shrestha P, et al. Ischemic stroke and impact of thyroid profile at presentation: a systematic review and meta-analysis of observational studies. J Stroke Cerebrovasc Dis J Natl Stroke Assoc. 2017 Dec;26(12):2926–34.10.1016/j.jstrokecerebrovasdis.2017.07.015Search in Google Scholar PubMed
[41] Akhoundi FH, Ghorbani A, Soltani A, Meysamie A. Favorable functional outcomes in acute ischemic stroke patients with subclinical hypothyroidism. Neurology. 2011 Jul 26;77(4):349–54.10.1212/WNL.0b013e3182267ba0Search in Google Scholar PubMed
[42] Delpont B, Aboa-Eboulé C, Durier J, Petit JM, Daumas A, Legris N, et al. Associations between thyroid stimulating hormone levels and both severity and early outcome of patients with ischemic stroke. Eur Neurol. 2016;76(3–4):125–31.10.1159/000449055Search in Google Scholar PubMed
[43] Baek JH, Chung PW, Kim YB, Moon HS, Suh BC, Jin DK, et al. Favorable influence of subclinical hypothyroidism on the functional outcomes in stroke patients. Endocr J. 2010;57(1):23–9.10.1507/endocrj.K09E-206Search in Google Scholar
[44] Bunevicius A, Kazlauskas H, Raskauskiene N, Janusonis V, Bunevicius R. Ischemic stroke functional outcomes are independently associated with C-reactive protein concentrations and cognitive outcomes with triiodothyronine concentrations: a pilot study. Endocrine. 2014 Mar;45(2):213.10.1007/s12020-013-9958-2Search in Google Scholar PubMed
[45] Delitala AP. Subclinical hyperthyroidism and the cardiovascular disease. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2017 Oct;49(10):723–31.10.1055/s-0043-117893Search in Google Scholar PubMed
[46] Chen Z, Sun Y, Zhang Y, He Y, Chen H, Su Y, et al. level predicts a poor clinical outcome in patients with anterior circulation ischemic stroke after endovascular thrombectomy. Neurol Sci J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2020 Jul;41(7):1821–8.10.1007/s10072-020-04281-0Search in Google Scholar PubMed
[47] Lee SH, Jang MU, Kim Y, Park SY, Kim C, Kim YJ, et al. Subclinical hyperthyroidism could predict poor outcomes in patients with acute ischemic stroke treated with reperfusion therapy. Front Neurol. 2019;10:782.10.3389/fneur.2019.00782Search in Google Scholar PubMed PubMed Central
[48] Ross DS. Treating hypothyroidism is not always easy: When to treat subclinical hypothyroidism, TSH goals in the elderly, and alternatives to levothyroxine monotherapy. J Intern Med. 2022 Feb;291(2):128–40.10.1111/joim.13410Search in Google Scholar PubMed
[49] Suda S, Shimoyama T, Nagai K, Arakawa M, Aoki J, Kanamaru T, et al. Low free triiodothyronine predicts 3-month poor outcome after acute stroke. J Stroke Cerebrovasc Dis J Natl Stroke Assoc. 2018 Oct;27(10):2804–9.10.1016/j.jstrokecerebrovasdis.2018.06.009Search in Google Scholar PubMed
[50] Ganesan K, Wadud K. Euthyroid sick syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 Sep 26]. http://www.ncbi.nlm.nih.gov/books/NBK482219/.Search in Google Scholar
[51] Akman T, Topaloglu O, Altunoglu A, Neselioglu S, Erel O. Frequency of Euthyroid Sick Syndrome before and after renal transplantation in patients with end stage renal disease and its association with oxidative stress. Postgrad Med. 2022 Jan;134(1):52–7.10.1080/00325481.2021.1994267Search in Google Scholar PubMed
[52] Świstek M, Broncel M, Gorzelak-Pabiś P, Morawski P, Fabiś M, Woźniak E. Euthyroid sick syndrome as a prognostic indicator of COVID-19 pulmonary involvement, associated with poorer disease prognosis and increased mortality. Endocr Pract J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2022 May;28(5):494–501.10.1016/j.eprac.2022.02.006Search in Google Scholar PubMed PubMed Central
[53] Nistal-Nuño B. Euthyroid sick syndrome in paediatric and adult patients requiring extracorporeal circulatory support and the role of thyroid hormone supplementation: a review. Perfusion. 2021 Jan;36(1):21–33.10.1177/0267659120914136Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science
Articles in the same Issue
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science

