Abstract
A deeper understanding of the underlying biological mechanisms of secondary brain injury induced by traumatic brain injury (TBI) will greatly advance the development of effective treatments for patients with TBI. Hypoxia-inducible factor-1 alpha (HIF-1α) is a central regulator of cellular response to hypoxia. In addition, growing evidence shows that HIF-1α plays the important role in TBI-induced changes in biological processes; however, detailed functional mechanisms are not completely known. The aim of the present work was to further explore HIF-1α-mediated events after TBI. To this end, next-generation sequencing, coupled with cellular and molecular analysis, was adopted to interrogate vulnerable events in a rat controlled cortical impact model of TBI. The results demonstrated that TBI induced accumulation of HIF-1α at the peri-injury site at 24 h post-injury, which was associated with neuronal loss. Moreover, gene set enrichment analysis unveiled that neuroinflammation, especially an innate inflammatory response, was significantly evoked by TBI, which could be attenuated by the inhibition of HIF-1α. Furthermore, the inhibition of HIF-1α could mitigate the activation of microglia and astrocytes. Taken together, all these data implied that HIF-1α might contribute to secondary brain injury through regulating neuroinflammation.
1 Introduction
Traumatic brain injury (TBI) is the major cause of mortality and disability and poses an immense public health and economic burden to individuals and countries [1,2]. However, despite much effort, no effective medical treatment exists in large part due to the limited understanding of complex pathological processes after TBI [3,4]. TBI results from external mechanical force to the cerebral parenchyma followed by delayed secondary insults. Secondary brain injury deteriorates brain damage and causes progressive neuronal death and cognitive dysfunction [5]. Therefore, the protracted onset of secondary injury provides a promising window for reducing neurological deficits with TBI.
Hypoxia-inducible factor-1 (HIF-1) is the mast nuclear transcription factor, which orchestrates adaptive physiological and pathophysiological responses to hypoxia [6]. HIF-1 is composed of an oxygen-regulated alpha subunit (HIF-1α) and a constitutively expressed beta subunit (HIF-1β) [7]. Hypoxia-mediated post-translational modifications of HIF-1α are essential to maintain cellular and organismal homeostasis in response to internal and external stimuli [8]. It has been demonstrated that HIF-1 is involved in brain development [9], neurogenesis [10,11], and neuroprotection [12]. However, aberrant HIF-1 activation participates in multiple brain pathologies [13], including neurodegenerative diseases [14] and TBI [15].
Increasing evidence shows that HIF-1α is highly implicated in TBI [16]. However, the role of HIF-1α in TBI remains not to be completely understood. To further investigate HIF-1α-related biological processes after TBI, next-generation sequencing, coupled with cellular and molecular analysis, was utilized to interrogate vulnerable events in a rat controlled cortical impact (CCI) model of TBI treated with HIF-1α inhibitor 2-methoxyestradiol (2ME2). The CCI model is a well-established and commonly used animal model of TBI, and predominately produces a highly reproducible focal brain injury with better control over impact velocity, deformation depth, and dwell time [17,18]. The results revealed that HIF-1α inhibition could attenuate TBI-induced neuronal apoptosis, neuroinflammation, especially innate immunity responses, and glial activation, which indicated that targeting HIF-1α might be an alternative therapeutic strategy to treat TBI. Besides, it has been suggested that the complement system contributes to secondary brain injury [19]; however, its upstream regulatory pathways remain largely unknown. Our results suggested that HIF-1α could modulate the activity of the complement system.
2 Materials and methods
2.1 Animals
Adult male Sprague Dawley rats (8-week-old, 280–300 g) were provided by Beijing Vital River Experimental Animals Technology, Ltd, Beijing, China. All animals were housed under a controlled environment and allowed food and water ad libitum. Rats were randomly divided into three groups (n = 36 per group, total animal number n = 108): control, TBI, and 2ME2-treated. After the CCI procedure, 2ME2, dissolved in 10% dimethyl sulfoxide in Dulbecco’s phosphate-buffered saline (10 mg/kg, M6383-50 mg, sigma), was injected intraperitoneally.
-
Ethical approval: The research related to the use of animals has been complied with all the relevant national regulations and institutional policies for the care and use of animals. All the experimental processes were approved by the Beijing Neurosurgical Institute Animal Care and Use Committee (Approval No. 201802001) on June 6, 2018.
2.2 Controlled cortical impact model
Based on our previous experimental protocol, rats were anesthetized and maintained at a body temperature of 37.0 ± 0.5°C with a thermal pad [20]. The rat head was then fixed in a stereotaxic frame. After exposing the skull with a midline scalp, a craniotomy (diameter = 6 mm) was performed over the right parietal bone. Subsequently, CCI injury was induced with a PCI3000 PinPoint Precision Cortical Impactor (Hatteras Instruments, Cary, NC, USA) with the previous parameters (5 mm impactor tip diameter; 2 mm depth; 300 ms compression time; and 3 m/s velocity). Finally, the removed bone was replaced and fixed with wax. The rats of the control group underwent the same process without impact.
2.3 Tissue preparation
At 24 h post-injury, rats were anesthetized and perfused through the left cardiac ventricle with cold saline (0.9%). For RNA isolation, peri-injury cortices were collected and snap-frozen with liquid nitrogen immediately, and then stored at −80°C until RNA extraction. For immunofluorescence, mice were perfused with 0.9% cold saline and 4% paraformaldehyde (PFA) successively. Then, brains were post-fixed overnight in PFA. After 48-h cryoprotection in 30% sucrose at 4°C, 20 μm sections were acquired from brain tissues embedded in optimum cutting temperature compound.
2.4 Immunofluorescence
After heat-induced retrieval in sodium citrate buffer, slides were treated with blocking and permeabilization buffer (5% bovine serum albumin, 0.3% Triton X-100 in phosphate-buffered saline) at room temperature for 1 h. Then, the primary antibodies were added: mouse anti-HIF-1A (ab1), rabbit anti-glial fibrillary acidic protein (ab7260), rabbit anti-NeuN (ab177487), rabbit anti-Iba1 (Wako, 019-19741), rabbit anti-C3 (Proteintech, 21337-1-AP), and rabbit anti-Stat3 (Proteintech, 10253-2-AP) and incubated overnight at 4°C. Subsequently, the sections were incubated with secondary antibodies. Finally, 4’,6-diamidino-2-phenylindole (DAPI) was utilized to counterstain nuclei. For each primary antibody, three sections per brain were used. Images surrounding 1 mm2 from the margin of peri-contusion were taken with Nikon Instruments A1 confocal laser microscope (Nikon, Tokyo, Japan). Fluorescence intensity was quantified by using the ImageJ software (National Institutes of Health, NIH).
2.5 RNA isolation and quantitative real-time PCR (qRT-PCR)
RNA was extracted from rat brain cortex with Trizol reagent (Invitrogen) as described in our previous publication [21]. cDNA was synthesized using total RNA with the superscript II reverse transcription system according to the manufacturer’s instructions. Then, the thermal cycling protocol of qRT-PCR was performed as follows: 95°C for 15 s, 95°C for 5 s, and 60°C for 31 s, which was repeated for 40 cycles. The sequences of HIF-1α primers are listed as follows: forward primer: 5′-GCGGCGAGAACGAGAAGAAA-3′; reverse primer: 5′- TGTCAAGATCACCAGCACCT-3′. The expression of mRNA level was normalized with glyceraldehyde-3-phosphate dehydrogenase and determined by a comparative CT method.
2.6 Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
TUNEL staining was used to detect apoptosis in the peri-injury cortex by commercial TUNEL kit (In Situ Cell Death Detection Kit, Roche, Germany). Briefly, sections were subjected to permeabilization (0.1% Triton X-100, 0.1% sodium citrate) on ice. Sections were then treated with TUNEL reaction buffer in a humidified condition at 37°C for 60 min in the dark. Finally, nuclei were stained with DAPI. Images surrounding 1 mm2 from the margin of peri-contusion were taken with Nikon Instruments A1 confocal laser microscope (Nikon, Tokyo, Japan). The number of TUNEL-positive cells was measured using ImageJ software (National Institutes of Health, NIH).
2.7 RNAseq and analysis
RNAseq and functional analysis were performed as described in our previous work [3]. Briefly, the HISAT (2.0) package was used to align reads to the rat reference genome. The differential expression was finished with DESeq2 package (1.26.0). Gene set enrichment analysis was obtained with gene set enrichment analysis (GSEA) software (4.0.3). Significantly enriched gene sets were identified using false discovery rate <0.25.
2.8 Statistical analysis
R statistical software was used to perform statistical analysis. All data were presented as mean ± standard error of mean (SEM). For normal distribution data, the difference was determined by one-way analysis of variance (ANOVA) followed by the Tukey’s honestly significant difference (HSD) post hoc test, while nonparametric test was conducted with Kruskal–Wallis test followed by Steel–Dwass multiple comparison test. A P-value <0.05 was considered to be statistically significant.
3 Results
3.1 Increased accumulation of HIF-1α at the peri-injury site after TBI
To determine the expression of HIF-1α after TBI, mRNA and protein levels of HIF-1α were measured using qRT-PCR and immunofluorescence, respectively. qRT-PCR results showed that mRNA levels of HIF-1α were significantly increased at 24 h post-injury (Figure 1a). 2ME2, an endogenous and naturally occurring metabolite of estradiol, can cross the blood–brain barrier (BBB) to exert their biological effects [22,23]. Although the bioavailability of 2ME2 is low (approximately 1.5%), efficient absorption of 2ME2 is observed in humans and rodent animals [24]. 2ME2 can reduce HIF-1α expression at the posttranscriptional level without affecting their transcription and stability through depolymerizing the microtubules, whereas there is no effect on HIF-1β levels and subcellular localization [25]. In the present work, 2ME2 could attenuate the mRNA expression of HIF-1α; however, the reduction did not reach statistical significance (Figure 1a). Consistently, the protein levels of HIF-1α were substantially upregulated after TBI and the expression was confined to peri-injury site (Figure 1b and c). Contrary to mRNA change, 2ME2 significantly reduced TBI-induced upregulation of HIF-1α (Figure 1b and c), which was in accordance with the post-translational effect of 2ME2 on HIF-1α. Collectively, TBI could increase HIF-1α expression, which was attenuated by HIF-1α inhibitor.

Increased accumulation of HIF-1α at the peri-injury site at 24 h post-injury. (a) qRT-PCR analysis of HIF-1α in Sham, TBI and 2ME2-treated rats (n = 3 rats per group). The difference was determined by one-way ANOVA (F(2,6) = 10.76, P = 0.0104) followed by the Tukey HSD post hoc test. (b) Representative photomicrographs of HIF-1α staining. (c) Quantification of HIF-1α fluorescence intensity (n = 4–9 rats per group). Significance was determined by nonparametric Kruskal–Wallis test (Kruskal–Wallis chi-squared = 17.221, df = 2, P = 0.0001822) followed by Steel–Dwass multiple comparison test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.
3.2 Inhibition of HIF-1α attenuated TBI-induced neuronal loss
Apoptosis largely accounts for TBI-induced cell death; therefore, the effect of HIF-1α on cell death was then investigated. TUNEL results demonstrated that TUNEL-positive cells at peri-injury site were significantly increased, which was dramatically mitigated by 2ME2 (Figure 2). Accordingly, it was observed that TBI caused massive neuron loss, which was also ameliorated by 2ME2 (Figure 3). These data suggested that HIF-1α mediated TBI-induced neuron loss.
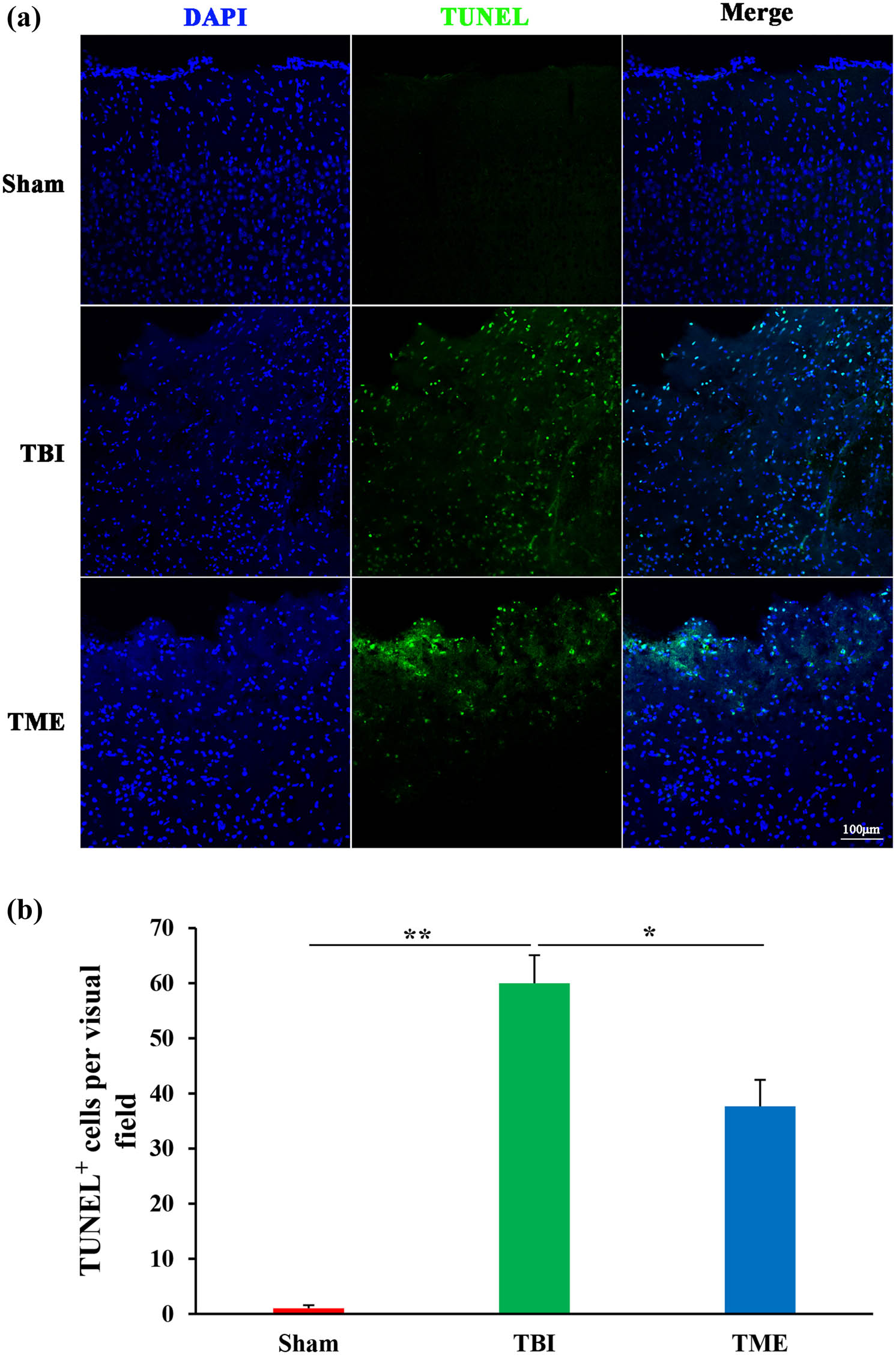
Inhibition of HIF-1α attenuated TBI-induced apoptosis. (a) Representative images of TUNEL staining. (b) Quantification of TUNEL-positive cells in Sham, TBI, and 2ME2-treated groups (n = 3–4 rats per group). The difference was determined by one-way ANOVA (F(2,7) = 46.49, P = 9.08 × 10−5) followed by the Tukey HSD post hoc test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.

Inhibition of HIF-1α reduced TBI-induced neuronal loss. (a) Representative images of NeuN staining. (b) Quantification of NeuN fluorescence intensity (n = 5–6 rats per group). The difference was determined by one-way ANOVA (F(2,14) = 6.936, P = 0.00807) followed by the Tukey HSD post hoc test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.
3.3 HIF-1α was involved in neuroinflammation-related biological processes
To deeply understand the underlying roles of HIF-1α in TBI, genome-wide transcriptome was adopted. In light of the powerful ability of GSEA in identifying the key events in health and disease states [26], GSEA was then used to annotate the biological processes after TBI. GSEA showed that inhibition of HIF-1α significantly attenuated innate immunity-related pathways activated by TBI, such as Jak-Stat signaling pathway, cytokine–cytokine receptor interaction, Toll-like and Nod-like receptor signaling pathway, and complement cascades (Figure 4a and b). Furthermore, the enrichment analysis was further confirmed by immunostaining of Stat3 and C3 (Figure 4c). Besides, apoptosis was also observed to be significantly inhibited by 2ME2, which was consistent with the aforementioned TUNEL results. Taken together, these data demonstrated that HIF-1α was involved in neuroinflammation-related biological processes.
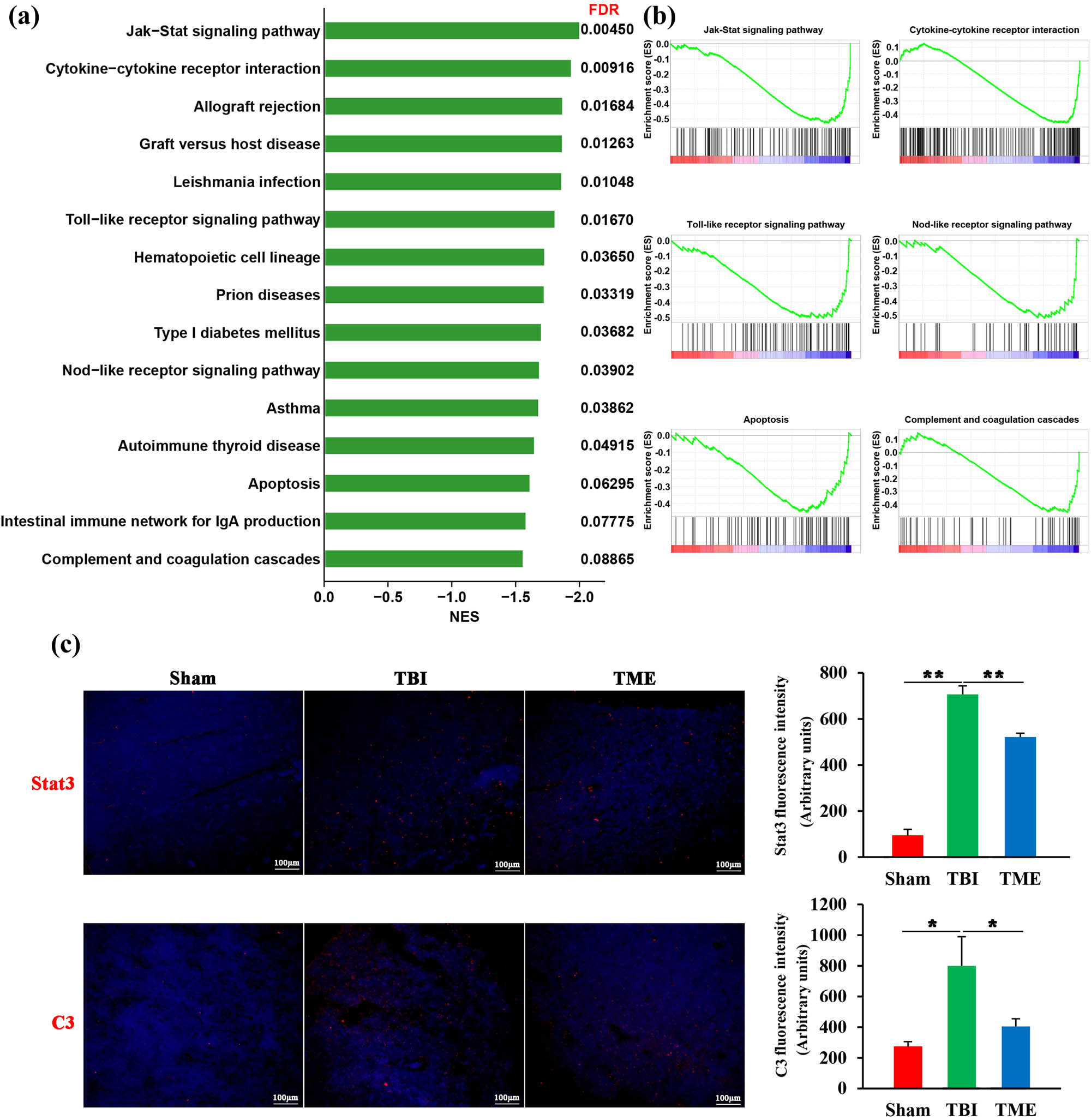
HIF-1α was involved in neuroinflammation-related biological processes. (a) Bar graph showing enriched kyoto encyclopedia of genes and genomes pathways obtained from GSEA. (b) Representative GSEA enrichment plots. (c) Representative images and quantification of Stat3 (n = 3 rats per group, F(2,6) = 148.2, P = 7.81 × 10−6) and C3 (n = 3–4 rats per group, F(2,8) = 7.655, P = 0.00139) staining. The difference was determined by one-way ANOVA followed by the Tukey HSD post hoc test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.
3.4 HIF-1α-mediated activation of glial cells after TBI
Glial cells, especially brain-resident microglia, are believed to be crucial players in evoking neuroinflammatory response after injury. Therefore, the effect of HIF-1α on glial cells, including astrocytes and microglia, was then evaluated. Iba1 immunostaining revealed that microglia were significantly activated following TBI. In contrast, the extent of microglia activation was enormously decreased by 2ME2 (Figure 5). The same phenomenon was also observed in astrocytes (Figure 6). Inhibition of HIF-1α after TBI could mitigate the activation of astrocytes. Collectively, these data implied that HIF-1α might promote neuroinflammation through glial cell activation.

Inhibition of HIF-1α mitigated TBI-induced microglial activation. (a) Representative images of Iba1 staining. (b) Quantification of Iba1 fluorescence intensity (n = 3–4 rats per group). The difference was determined by one-way ANOVA (F(2,7) = 18.29, P = 0.00166) followed by the Tukey HSD post hoc test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.
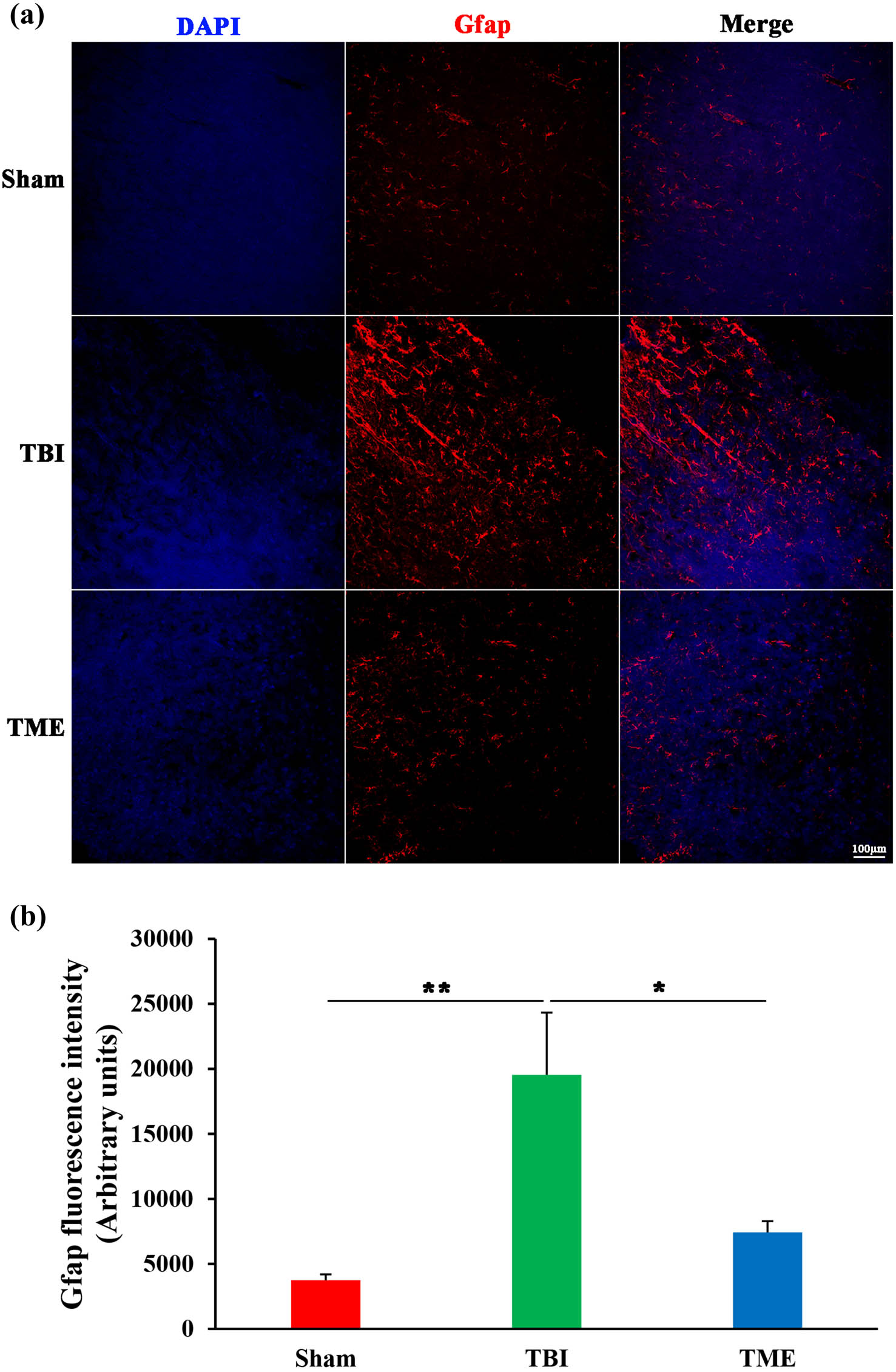
Inhibition of HIF-1α ameliorated TBI-induced activation of astrocytes. (a) Representative images of Iba1 staining. (b) Quantification of GFAP fluorescence intensity (n = 5–6 rats per group). The difference was determined by one-way ANOVA (F(2,13) = 6.997, P = 0.00866) followed by the Tukey HSD post hoc test. TBI, traumatic brain injury; 2ME2, 2-methoxyestradiol. Data are expressed as mean values ± SEM. *P < 0.05; **P < 0.01.
4 Discussion
Increasing evidence shows that HIF-1α is implicated in multiple processes of TBI [27], which promoted us to further investigate the role of HIF-1α in TBI based on a rat model of TBI. The results showed that TBI increased the expression of HIF-1α at the peri-injury site at 24 h post-impact. Moreover, high expression of HIF-1α was associated with neuronal loss. In order to get more insight into the mechanism of HIF-1α-mediated brain damages, a combination of next-generation sequencing and HIF-1α inhibitor was adopted. Functional enrichment analysis demonstrated that HIF-1α could evoke innate inflammatory response at the acute phase of TBI, which might be attributed to glial activation after TBI. Collectively, the present work revealed that HIF-1α could participate in secondary brain injury through the induction of neuroinflammation.
TBI can result in cerebral hypoxia, which exerts a protective or detrimental effect on the clinical outcome of patients [16,27]. For severe TBI, approximately 70% of TBI patients suffered from cerebral hypoxia, and serum HIF-1α concentration highly correlated with poor outcome after head trauma [28]. HIF-1α could induce secondary brain injury through multiple processes, such as BBB disruption [29,30], brain edema [31,32], neuronal apoptosis [15,33,34,35], and neuroinflammation [36]. Brain edema is associated with high disability and morbidity, which is influenced by aquaporin expression and the integrity of BBB [37]. Following TBI, HIF-1α drove aquaporin 4 and 9 upregulation, thereby inducing cerebral edema and functional deficits. Besides, except for primary mechanical impact, the integrity of BBB was broken by an enhanced expression of matrix metalloproteinase-9 mediated by HIF-1α, which also accounted for brain edema. Additionally, HIF-1α could cause neuronal apoptosis by increasing the expression of pro-apoptotic BNIP3 and p53, and mediated tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced neuronal apoptosis through modulating TRAIL decoy receptor 1 (DcR1). More recently, Qian et al. found that HIF-1α promoted neuronal injury via enhancing vascular endothelial growth factor (VEGF) A expression, which was attenuated by MicroRNA-31.
Neuroinflammation is the important contributor of secondary brain injury. Bae et al. demonstrated that leucine-rich repeat kinase 2 (LRRK2) was the direct target of HIF-1α and transcriptionally activated in HIF-1α-dependent manner by TBI, which in turn promoted neuroinflammation and exacerbated brain damage [30]. Likewise, in our work, we found that the inhibition of HIF-1α ameliorated innate inflammatory response and attenuated microglia activation. Microglia, the principal resident immune cell type in the central nervous system, maintain the cerebral homeostasis by phagocytosis of apoptotic cells and debris [38]. Besides, microglia are also the key players in synaptic pruning during development and neuronal plasticity in adult brain as well as trophic neuronal support [39,40]. However, microglia abnormality is highly involved in multiple neurological disorders, including Alzheimer’s disease [41,42], Parkinson’s disease [43], and TBI-induced neurological dysfunction [44]. Interestingly, Yuan et al. unveiled that HIF-1α activated nod-like receptor protein-3 (NLRP3) inflammasome-mediated pyroptosis in microglia after TBI and promoted TBI-induced behavioral and cognitive deficits, which further enhanced our understanding of the detailed role of HIF-1α in secondary brain injury [36]. Besides, increasing evidence shows that the complement system plays a critical role in microglia-mediated TBI-related neurodegeneration [45]. On the one hand, microglia-derived C1q could induce neuron loss and chronic neuroinflammation, thereby leading to abnormal sleep spindles and epileptic spikes after TBI [46]; on the other hand, C3 opsonin triggered microglial phagocytosis of synapses and chronic cognitive deficits [47,48]. In the current work, we observed that upregulation of C3 protein could be attenuated by the inhibition of HIF-1α, which implied that HIF-1α might evoke neuroinflammation by modulating the complement system pathways. Taken together, these findings suggest that HIF-1α may modulate the TBI-induced neuroinflammation through influencing multiple innate immune pathways, such as NLRP3 inflammasome and complement system.
Besides, it has been demonstrated that HIF-1α might play the neuroprotective role in TBI. Treatment with HIF-1α activator or stability with post-translational modification such as S-nitrosylation could increase the expression of VEGF, erythropoietin, and phosphoinositide-dependent kinase-1 and 4 (PDK1 and PDK4), thereby providing neuroprotection after TBI [49,50]. Furthermore, reduced expression of HIF-1α-related neuroprotective molecules might be related to poor outcome in the TBI-afflicted elderly [51]. Intriguingly, HIF-1α also mediated solute carrier family 12 member 2 (Slc12a2)-induced hippocampal neurogenesis after TBI through stimulation of VEGF expression [11]. The divergence about the role of HIF-1α in TBI might be attributed to injury severity, timing of inhibition, impact methods, and species. Therefore, further investigation will be required to explore in-depth the exact role of HIF-1α in different phases of TBI and the detailed functions in different cell types of brain. Fortunately, the rapid development of single-cell sequencing will enhance the understanding of HIF-1α in TBI. Besides, increasing evidence shows that 17β-estradiol (E2) could exert protective effects on TBI through dampening BBB disruption, attenuating neuroinflammation, and oxidative stress [52]. Given that 2ME2 is an endogenous and naturally occurring metabolite of estradiol, the role of estrogen receptors (ERs) in HIF-1α-related neuroprotection should be investigated although 2ME2 has a low affinity for ERs and in vivo 2ME2 treatment decreases levels of ERs [53]. Exclusion of the potential of ERs influences with pharmacological and genetic approaches would highlight the role of HIF-1α in TBI.
-
Funding information: This work was supported by grants from the National Natural Science Foundation of China (81771327), Construction of Central Nervous System Injury Basic Science and Clinical Translational Research Platform, and Budget of Beijing Municipal Health Commission 2020 (No. PXM2020_026280_000002).
-
Author contributions: XX and BL: research conceptualization, design, and drafting of the manuscript. XX, MY, BZ, JD, ZY, and QG: animal experiment, immunofluorescence, library construction, and data analysis. FN: performed qRT-PCR. All authors contributed to the manuscript and approved the final version.
-
Conflict of interest: All authors in this manuscript have declared no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Jiang JY, Gao GY, Feng JF, Mao Q, Chen LG, Yang XF, et al. Traumatic brain injury in China. Lancet Neurol. 2019;18(3):286–95.10.1016/S1474-4422(18)30469-1Search in Google Scholar PubMed
[2] Xu XJ, Yang MS, Zhang B, Niu F, Dong JQ, Liu BY. Glucose metabolism: A link between traumatic brain injury and Alzheimer’s disease. Chin J Traumatol. 2021;24(1):5–10.10.1016/j.cjtee.2020.10.001Search in Google Scholar PubMed PubMed Central
[3] Yang MS, Xu XJ, Zhang B, Niu F, Liu BY. Comparative transcriptomic analysis of rat versus mouse cerebral cortex after traumatic brain injury. Neural Regen Res. 2021;16(7):1235–43.10.4103/1673-5374.301028Search in Google Scholar PubMed PubMed Central
[4] Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048.10.1016/S1474-4422(17)30371-XSearch in Google Scholar PubMed
[5] Ng SY, Lee AYW. Traumatic brain injuries: Pathophysiology and potential therapeutic targets. Front Cell Neurosci. 2019;13:528.10.3389/fncel.2019.00528Search in Google Scholar PubMed PubMed Central
[6] Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408.10.1016/j.cell.2012.01.021Search in Google Scholar PubMed PubMed Central
[7] Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469–80.10.1124/mol.106.027029Search in Google Scholar PubMed
[8] Albanese A, Daly LA, Mennerich D, Kietzmann T, See V. The role of hypoxia-inducible factor post-translational modifications in regulating its localisation, stability, and activity. Int J Mol Sci. 2020;22(1).10.3390/ijms22010268Search in Google Scholar PubMed PubMed Central
[9] Kleszka K, Leu T, Quinting T, Jastrow H, Pechlivanis S, Fandrey J, et al. Hypoxia-inducible factor-2alpha is crucial for proper brain development. Sci Rep. 2020;10(1):19146.10.1038/s41598-020-75838-4Search in Google Scholar PubMed PubMed Central
[10] Li G, Zhao M, Cheng X, Zhao T, Feng Z, Zhao Y, et al. FG-4592 Improves Depressive-Like Behaviors through HIF-1-Mediated Neurogenesis and Synapse Plasticity in Rats. Neurotherapeutics. 2020;17(2):664–75.10.1007/s13311-019-00807-3Search in Google Scholar PubMed PubMed Central
[11] Lu KT, Huang TC, Wang JY, You YS, Chou JL, Chan MW, et al. NKCC1 mediates traumatic brain injury-induced hippocampal neurogenesis through CREB phosphorylation and HIF-1alpha expression. Pflug Arch. 2015;467(8):1651–61.10.1007/s00424-014-1588-xSearch in Google Scholar PubMed
[12] Mitroshina EV, Savyuk MO, Ponimaskin E, Vedunova MV. Hypoxia-Inducible Factor (HIF) in Ischemic Stroke and Neurodegenerative Disease. Front Cell Dev Biol. 2021;9:703084.10.3389/fcell.2021.703084Search in Google Scholar PubMed PubMed Central
[13] Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–48.10.1038/nrn1408Search in Google Scholar PubMed
[14] Zhang Z, Yan J, Chang Y, ShiDu Yan S, Shi H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr Med Chem. 2011;18(28):4335–43.10.2174/092986711797200426Search in Google Scholar PubMed PubMed Central
[15] Fang Y, Lu J, Wang X, Wu H, Mei S, Zheng J, et al. HIF-1alpha mediates TRAIL-induced neuronal apoptosis via regulating DcR1 expression following traumatic brain injury. Front Cell Neurosci. 2020;14:192.10.3389/fncel.2020.00192Search in Google Scholar PubMed PubMed Central
[16] Khan M, Khan H, Singh I, Singh AK. Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury. Neural Regen Res. 2017;12(5):696–701.10.4103/1673-5374.206632Search in Google Scholar PubMed PubMed Central
[17] Cernak I. Animal models of head trauma. NeuroRx. 2005;2(3):410–22.10.1602/neurorx.2.3.410Search in Google Scholar PubMed PubMed Central
[18] Shah EJ, Gurdziel K, Ruden DM. Mammalian models of traumatic brain injury and a place for drosophila in TBI research. Front Neurosci. 2019;13:409.10.3389/fnins.2019.00409Search in Google Scholar PubMed PubMed Central
[19] Hammad A, Westacott L, Zaben M. The role of the complement system in traumatic brain injury: a review. J Neuroinflammation. 2018;15(1):24.10.1186/s12974-018-1066-zSearch in Google Scholar PubMed PubMed Central
[20] Zhang B, Xu X, Niu F, Mao X, Dong J, Yang M, et al. Corticosterone replacement alleviates hippocampal neuronal apoptosis and spatial memory impairment induced by dexamethasone via promoting brain corticosteroid receptor rebalance after traumatic brain injury. J Neurotrauma. 2020;37(2):262–72.10.1089/neu.2019.6556Search in Google Scholar PubMed
[21] Xu XJ, Wang SM, Jin Y, Hu YT, Feng K, Ma ZZ. Melatonin delays photoreceptor degeneration in a mouse model of autosomal recessive retinitis pigmentosa. J Pineal Res. 2017;63(3).10.1111/jpi.12428Search in Google Scholar PubMed
[22] Kang SH, Cho HT, Devi S, Zhang Z, Escuin D, Liang Z, et al. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66(24):11991–7.10.1158/0008-5472.CAN-06-1320Search in Google Scholar PubMed
[23] Gorska M, Kuban-Jankowska A, Milczarek R, Wozniak M. Nitro-oxidative stress is involved in anticancer activity of 17beta-estradiol derivative in neuroblastoma cells. Anticancer Res. 2016;36(4):1693–8.Search in Google Scholar
[24] Duncan GS, Brenner D, Tusche MW, Brustle A, Knobbe CB, Elia AJ, et al. 2-Methoxyestradiol inhibits experimental autoimmune encephalomyelitis through suppression of immune cell activation. Proc Natl Acad Sci U S A. 2012;109(51):21034–39.10.1073/pnas.1215558110Search in Google Scholar PubMed PubMed Central
[25] Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3(4):363–75.10.1016/S1535-6108(03)00077-1Search in Google Scholar
[26] Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50.10.1073/pnas.0506580102Search in Google Scholar PubMed PubMed Central
[27] Seo DE, Shin SD, Song KJ, Ro YS, Hong KJ, Park JH. Effect of hypoxia on mortality and disability in traumatic brain injury according to shock status: A cross-sectional analysis. Am J Emerg Med. 2019;37(9):1709–15.10.1016/j.ajem.2018.12.022Search in Google Scholar PubMed
[28] Lv QW, Zheng ZQ, Zhang H, Guo M, Shen LJ. Serum hypoxia-inducible factor 1alpha emerges as a prognostic factor for severe traumatic brain injury. Clin Chim Acta. 2021;522:77–82.10.1016/j.cca.2021.08.017Search in Google Scholar PubMed
[29] Higashida T, Kreipke CW, Rafols JA, Peng C, Schafer S, Schafer P, et al. The role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury. J Neurosurg. 2011;114(1):92–101.10.3171/2010.6.JNS10207Search in Google Scholar PubMed
[30] Bae YH, Joo H, Bae J, Hyeon SJ, Her S, Ko E, et al. Brain injury induces HIF-1alpha-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death Dis. 2018;9(11):1125.10.1038/s41419-018-1180-ySearch in Google Scholar PubMed PubMed Central
[31] Ding JY, Kreipke CW, Speirs SL, Schafer P, Schafer S, Rafols JA. Hypoxia-inducible factor-1alpha signaling in aquaporin upregulation after traumatic brain injury. Neurosci Lett. 2009;453(1):68–72.10.1016/j.neulet.2009.01.077Search in Google Scholar PubMed PubMed Central
[32] Shenaq M, Kassem H, Peng C, Schafer S, Ding JY, Fredrickson V, et al. Neuronal damage and functional deficits are ameliorated by inhibition of aquaporin and HIF1alpha after traumatic brain injury (TBI). J Neurol Sci. 2012;323(1–2):134–40.10.1016/j.jns.2012.08.036Search in Google Scholar PubMed
[33] Li A, Sun X, Ni Y, Chen X, Guo A. HIF-1alpha involves in neuronal apoptosis after traumatic brain injury in adult rats. J Mol Neurosci. 2013;51(3):1052–62.10.1007/s12031-013-0084-7Search in Google Scholar PubMed
[34] Schaible EV, Windschugl J, Bobkiewicz W, Kaburov Y, Dangel L, Kramer T, et al. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1alpha response after traumatic brain injury in mice. J Neurochem. 2014;129(6):940–54.10.1111/jnc.12708Search in Google Scholar PubMed
[35] Qian Y, Li X, Fan R, Li Q, Zhang Y, He X, et al. MicroRNA-31 inhibits traumatic brain injury-triggered neuronal cell apoptosis by regulating hypoxia-inducible factor-1A/vascular endothelial growth factor A axis. Neuroreport. 2022;33(1):1–12.10.1097/WNR.0000000000001741Search in Google Scholar PubMed
[36] Yuan D, Guan S, Wang Z, Ni H, Ding D, Xu W, et al. HIF-1alpha aggravated traumatic brain injury by NLRP3 inflammasome-mediated pyroptosis and activation of microglia. J Chem Neuroanat. 2021;116:101994.10.1016/j.jchemneu.2021.101994Search in Google Scholar PubMed
[37] Szczygielski J, Kopanska M, Wysocka A, Oertel J. Cerebral Microcirculation, Perivascular Unit, and Glymphatic System: Role of Aquaporin-4 as the Gatekeeper for Water Homeostasis. Front Neurol. 2021;12:767470.10.3389/fneur.2021.767470Search in Google Scholar PubMed PubMed Central
[38] Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19(10):622–35.10.1038/s41583-018-0057-5Search in Google Scholar PubMed PubMed Central
[39] Wolf SA, Boddeke HW, Kettenmann H. Microglia in Physiology and Disease. Annu Rev Physiol. 2017;79:619–43.10.1146/annurev-physiol-022516-034406Search in Google Scholar PubMed
[40] Mosser CA, Baptista S, Arnoux I, Audinat E. Microglia in CNS development: Shaping the brain for the future. Prog Neurobiol. 2017;149-150:1–20.10.1016/j.pneurobio.2017.01.002Search in Google Scholar PubMed
[41] Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157–72.10.1038/s41582-020-00435-ySearch in Google Scholar PubMed
[42] Sobue A, Komine O, Hara Y, Endo F, Mizoguchi H, Watanabe S, et al. Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):1.10.1186/s40478-020-01099-xSearch in Google Scholar PubMed PubMed Central
[43] Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931–8.10.1038/s41591-018-0051-5Search in Google Scholar PubMed PubMed Central
[44] Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, et al. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020;40(14):2960–74.10.1523/JNEUROSCI.2402-19.2020Search in Google Scholar PubMed PubMed Central
[45] Schartz ND, Tenner AJ. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J Neuroinflammation. 2020;17(1):354.10.1186/s12974-020-02024-8Search in Google Scholar PubMed PubMed Central
[46] Holden SS, Grandi FC, Aboubakr O, Higashikubo B, Cho FS, Chang AH, et al. Complement factor C1q mediates sleep spindle loss and epileptic spikes after mild brain injury. Science. 2021;373(6560):eabj2685.10.1126/science.abj2685Search in Google Scholar PubMed PubMed Central
[47] Alawieh A, Langley EF, Weber S, Adkins D, Tomlinson S. Identifying the role of complement in triggering neuroinflammation after traumatic brain injury. J Neurosci. 2018;38(10):2519–32.10.1523/JNEUROSCI.2197-17.2018Search in Google Scholar PubMed PubMed Central
[48] Alawieh A, Chalhoub RM, Mallah K, Langley EF, York M, Broome H, et al. Complement drives synaptic degeneration and progressive cognitive decline in the chronic phase after traumatic brain injury. J Neurosci. 2021;41(8):1830–43.10.1523/JNEUROSCI.1734-20.2020Search in Google Scholar PubMed PubMed Central
[49] Khan M, Dhammu TS, Baarine M, Kim J, Paintlia MK, Singh I, et al. GSNO promotes functional recovery in experimental TBI by stabilizing HIF-1alpha. Behav Brain Res. 2018;340:63–70.10.1016/j.bbr.2016.10.037Search in Google Scholar PubMed PubMed Central
[50] Sen T, Sen N. Treatment with an activator of hypoxia-inducible factor 1, DMOG provides neuroprotection after traumatic brain injury. Neuropharmacology. 2016;107:79–88.10.1016/j.neuropharm.2016.03.009Search in Google Scholar PubMed PubMed Central
[51] Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma. 2009;26(9):1557–66.10.1089/neu.2008.0765Search in Google Scholar PubMed PubMed Central
[52] Kovesdi E, Szabo-Meleg E, Abraham IM. The role of estradiol in traumatic brain injury: Mechanism and treatment potential. Int J Mol Sci. 2020;22(1):1110.3390/ijms22010011Search in Google Scholar PubMed PubMed Central
[53] Lee JS, Kim YK, Yang H, Kang HY, Ahn C, Jeung EB. Two faces of the estrogen metabolite 2-methoxyestradiol in vitro and in vivo. Mol Med Rep. 2015;12(4):5375–82.10.3892/mmr.2015.4073Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science
Articles in the same Issue
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science

