Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
Abstract
Objective
The aim of this study was to ascertain whether dynamic cerebral autoregulation (CA) in the middle cerebral artery (MCA) is disturbed by cerebral infarctions outside the MCA territory.
Methods
We estimated transfer function parameters gain and phase from simultaneous recordings of spontaneous oscillation in blood pressure and MCA cerebral blood flow velocity in 10 consecutive patients with isolated anterior cerebral artery (ACA) infarctions and in 22 consecutive patients with isolated posterior cerebral artery (PCA) infarctions. All ACA infarctions were in the motor, premotor, or supplementary motor cortex areas and presented with pronounced leg hemiparesis. Twenty-eight age- and sex-matched healthy subjects served as controls.
Results
Compared to controls, phase was significantly reduced in the MCA ipsilateral to the lesion site and in the contralateral MCA (unaffected hemisphere) in the very low (0.02–0.07 Hz) and low (0.07–0.15 Hz) frequency ranges in the ACA infarctions but not in the PCA infarctions. Gain was reduced only in the very low frequency range in the MCA contralateral to the ACA lesion site. Systemic factors were unrelated to phase and gain results.
Conclusion
Bilateral impairment of MCA dynamic CA in patients with a unilateral ACA infarction is frequent.
1 Introduction
Cerebral perfusion depends on various factors, such as the mean arterial pressure (MAP), cranial perfusion pressure, blood carbon dioxide and oxygen tension, blood pH value, metabolic demands, and remote influences from the brain’s functional connectivity networks (CNs) [1]. In a strict sense, the relationship between cerebral blood flow (CBF) and MAP determines cerebral autoregulation (CA); the other variables modulate this relationship, influencing overall CBF. The phenomenon of neurovascular coupling (NVC) is illustrated by cognitive tasks and arm movements that increase CBF or its velocity (CBFV, measured by transcranial Doppler [TCD] ultrasound), indicating increased perfusion [2,3,4,5,6,7]. Interestingly, such NVC studies showed additional effects in various other vascular territories, ultimately leading to the concept of brain CNs [2,3,6,7].
During an ischaemic stroke in the middle cerebral artery (MCA) territory, the dynamical aspects of CA (dCA) are frequently disturbed; interestingly, dCA is disturbed not only on the stroke-affected side but also on the unaffected side [8]. The reason for this behaviour is unclear, but systemic factors such as high blood pressure (BP) [9,10], diabetes mellitus [11], or the presence of cerebral microangiopathy [12,13] are discussed. CN considerations as a possible confounder of the dCA in the MCA have not yet been addressed. To gain a first impression on such a hypothesis, we investigated whether isolated infarctions in other supratentorial vascular territories (anterior cerebral artery [ACA]; posterior cerebral artery [PCA]) can affect dCA in the MCA. Given the body of knowledge about the motoric CNs derived from functional magnetic resonance imaging (fMRI; summarized in refs [2,3]), we hypothesize that infarctions in the motoric areas in the ACA territory should affect the dCA in the MCA, while infarctions in the PCA territory which usually do not have motor symptoms would not. Anatomically, the blood supply to the PCA is usually provided via the vertebral and the basilar arteries, and both are independent of the internal carotid artery (ICA) and the MCA. The ICA supplies both the MCA and the ACA. To avoid any contamination of our results by haemodynamically relevant ICA obstructions, we considered this possibility a priori and excluded patients with such relevant ICA pathologies.
2 Methods
The Lucerne Hospital is a large tertiary teaching hospital with a full-service stroke centre. All patients with stroke syndrome receive standardized care, first undergoing a focused clinical examination followed by multimodal cranial computed tomography. If indicated, intravenous thrombolysis and/or arterial thrombectomy immediately follows. All patients with stroke syndrome are transferred to the stroke unit for close clinical monitoring. Patients are scored on the National Institutes of Health Stroke Scale (NIHSS [14]) and modified Rankin scale [15] upon hospital admission and daily while in the stroke unit; they also undergo monitoring of MAP, heart rhythm, body temperature, blood glucose concentration, and oxygen saturation. An echocardiogram measuring the left ventricular ejection fraction, an extensive ultrasound examination with assessment of dCA, brain MRI with diffusion-weighted imaging (DWI), T2, fluid-attenuated inversion recovery, and susceptibility-weighted imaging sequences were performed within a maximum of 72 h after hospitalization. Summarizing all imaging modalities, each ischaemic event is classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification [16]. If the neurological deficit resolved within 24 h and DWI remained negative, these patients were classified as having suffered a transient ischaemic attack. Irrespective of whether the patient is symptomatic, any present cerebral microangiopathy is classified according to the Fazekas scale [17]. Infarct size is calculated by the ABC/2 method, which we evaluate manually and compare with the measurements made by automated software [18].
For this study, we recruited 10 consecutive patients with unilateral ACA infarction and 22 with PCA infarction who had received the abovementioned structured stroke care, who had temporal bone windows for TCD ultrasound examinations, and who did not have stenosis ≥50% in the extra- or intracranial arteries supplying the brain. All investigations were performed within 72 h after symptom onset. From our previously reported healthy population [19], we included 29 age- and sex-matched participants as controls and averaged the results of the bilateral MCAs as a reference value. All healthy persons were without known cardiocirculatory complains or anatomical variations.
3 dCA assessment
For details of the dCA assessment, see previous reports [3,19]. CBFV (MultidopX, DWL; Compumedics, Sipplingen, Germany; 2-MHz probes) and BP (Finometer Pro; Finapres Medical Systems, Amsterdam, The Netherlands) were simultaneously recorded over a minimum period of 6 min. The end-tidal pCO2 (ETCO2) was measured via nostril tubes using a capnography function built into the TCD device. The ETCO2 for each patient is reported as the mean ETCO2 over the total recording period. Cerebrovascular resistance was calculated by the mean BP over the mean CBFV. Patient recordings were performed at their stroke unit site with the patients in a resting position and the head slightly elevated. They were instructed not to make movements. Those of the controls (healthy subjects) were performed in a separate room with the same resting body position and the head slightly elevated. All recordings followed the recommendations of the CA network as much as possible [20].
The data were analysed using MATLAB (2020b; MathWorks Inc., Natick, MA, USA). After visual inspection for artefacts, only artefact-free data periods of 5 min were used. Each raw data time series was averaged over 1-s intervals (one example of a patient with an ACA infarction is given in Figure 1). The transfer function estimates between BP and CBFV time series were extracted from their respective power autospectra or cross-spectra using Welch’s averaged periodogram method, with a Hanning window length of 100 s, a window overlap of 50%, and a total fast Fourier transformation data length of 300 s. Coherence, phase (in radians), and gain (in cm/s/mmHg or as %gain in %cm/s/mmHg) were calculated over a frequency range of 0.02–0.50 Hz and are reported as their respective average in the very low (VLF, 0.02–0.07 Hz), low (LF, 0.07–0.15 Hz), and high (HF, 0.16–0.5 Hz) frequency ranges. For interpretation, gain and phase usually follow an opposite direction, and an impaired dCA is indicated by a low phase and/or a high gain.
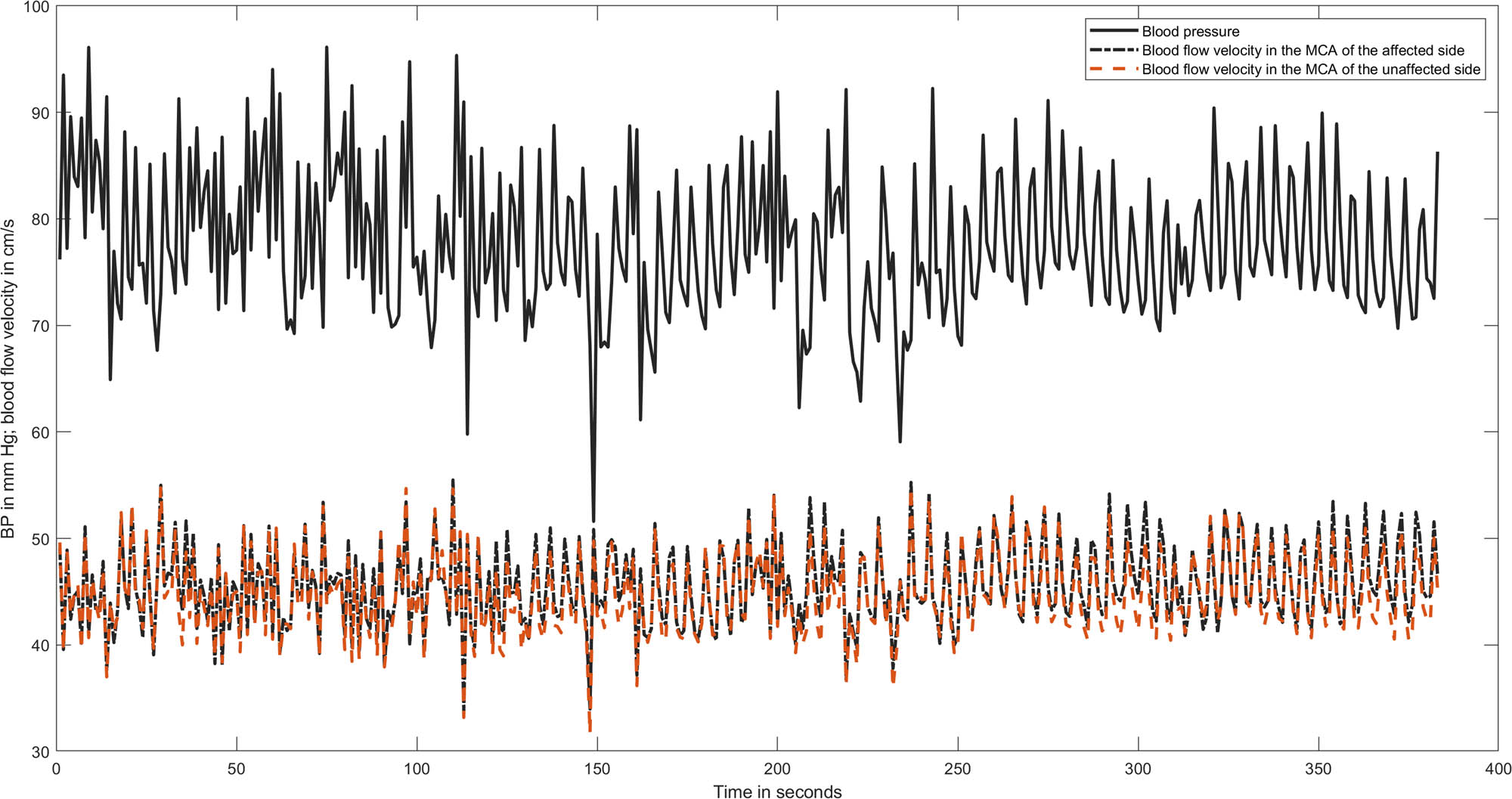
A representative recording of BP and MCA blood flow velocity in both hemispheres of a patient with an infarction in the ACA. All time series are averaged over 1 s to reduce the amount of data points. The calculated phase values were as follows: in the very low frequency 0.54 radian in the affected hemisphere and 0.64 in the unaffected one; in the low frequency phase 0.43 in both MCAs.
3.1 Statistics
MATLAB’s Statistical Toolbox was used. The Lilliefors test was used to test for the normal distribution of continuous data. Normally distributed data are reported as mean ± SD (standard deviation); nonnormally distributed data and discrete data are reported as the median [25th, 75th percentile]. To compare means, we used either a t test or one-way analysis of variance. To compare medians, we used the Wilcoxon rank-sum test or Kruskal‒Wallis test. To predict any parameter by regression analysis, univariate regression analysis was used. A p value ≤0.05 was considered indicative of statistical significance.
-
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee. The study was approved by the Ethics Committee of Northwest and Central Switzerland (PB_2016-01719). The research is a part of the larger trial registered at ClinicalTrials.gov NCT04611672.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
4 Results
The overall baseline characteristics of the patients and the controls are reported in Table 1. None of the presented baseline characteristics significantly predicted the dCA parameter by regression analysis or showed significant differences between the patients with PCA or ACA infarcts. Only infarct size (see below) was different between the PCA and ACA infarctions (p < 0.01). The allocations of stroke-related large artery disease were in the vertebral artery system as we excluded patients with large artery disease in the carotid artery system.
Patient baseline characteristics
| Patients n = 32 | Controls n = 28 | |
|---|---|---|
| Age (years) | 63 ± 14 | 63 ± 14 |
| Women/men | 11/21 | 8/21 |
| Body mass index (kg/m2) | 26 ± 4.1 | |
| Heart rate (beats per minute) | 68 ± 13 | 67 ± 12 |
| MAP (mmHg) | 94 ± 8 | 92 ± 14 |
| Mean ETCO2 (mmHg) | 40.0 ± 1.8 | 39.3 ± 4.5 |
| Mean CBFV (cm/s) | 50 ± 12 | 50 ± 13 |
| Arterial hypertension | 15 | |
| Diabetes mellitus | 6 | |
| Blood glucose concentration at entry (reference range 3.8–6.4 mmol/l) | 6.19 ± 1.15 | |
| Dyslipidaemia | 29 | |
| Atrial fibrillation | 3 | |
| Left ventricular ejection fraction (%) | 60 [55, 65] | |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 85 [70, 91] | |
| Infarct volume | 3.0 [0.31, 8.90] | |
| NIHSS at entry | 2 [1, 5] | |
| Cerebral microangiopathy on MRI | 23/1/3/5 | |
| Fazekas grade 0/1/2/3 | ||
| TOAST | Cardioembolic 13 | |
| Large artery disease 5 | ||
| Lacunar 2 | ||
| Unknown 12 |
ETCO2, end-tidal carbon dioxide concentration; CBFV, cerebral blood flow velocity; Ref., reference; NIHSS, National Institutes of Health Stroke Scale; MRI, magnetic resonance imaging; cerebral microangiopathy classified by the Fazekas scale (17) does not include normal brain imaging results; we classified normal brain imaging results as Fazekas grade 0 in our analysis. TOAST, Trial of Org 10172 in Acute Stroke Treatment (16).
In the patients with PCA infarctions, the median infarct volume was 4.10 ml [0.40, 12.75], with a maximum of 106 ml. Motor symptom was not present in any patient with PCA infarct. Considering the dCA parameters, there were no significant differences between the PCA infarct patients and the controls (Table 2). Thus, infarct size seems to be unrelated to dCA in the MCA.
Transfer function estimates of dynamic cerebral autoregulation in the MCA in patients with acute infarctions in the PCA or ACA
| Transfer function parameter | Controls (n = 28) | PCA infarctions (n = 22) | ACA infarctions (n = 10) | ||
|---|---|---|---|---|---|
| Affected hemisphere | Unaffected hemisphere | Affected hemisphere | Unaffected hemisphere | ||
| Coherence | |||||
| VLF | 0.50 ± 0.13 | 0.58 ± 0.12 | 0.59 ± 0.11 | 0.57 ± 0.12 | 0.62 ± 0.12 |
| LF | 0.72 ± 0.15 | 0.68 ± 0.13 | 0.68 ± 0.13 | 0.70 ± 0.17 | 0.70 ± 0.16 |
| HF | 0.65 ± 0.15 | 0.69 ± 0.14 | 0.69 ± 0.16 | 0.67 ± 0.16 | 0.69 ± 0.18 |
| Gain (cm/s/mmHg) | |||||
| VLF | 0.17 ± 0.20 | 0.24 ± 0.16 | 0.28 ± 0.20 | 0.27 ± 0.13 | 0.48 ± 0.14 |
| t −3.74, df 32, p = 0.007 | |||||
| LF | 0.51 ± 0.28 | 0.46 ± 0.28 | 0.45 ± 0.25 | 0.44 ± 0.14 | 0.49 ± 0.09 |
| HF | 0.57 ± 0.39 | 0.57 ± 0.28 | 0.60 ± 0.28 | 0.48 ± 0.15 | 0.59 ± 0.21 |
| Phase (radian) | |||||
| VLF | 1.09 ± 0.36 | 0.95 ± 0.43 | 0.90 ± 0.49 | 0.70 ± 0.30 | 0.42 ± 0.15 |
| t 3.00, df 36, p = 0.005 | t 4.70, df 33, p = 0.0005 | ||||
| LF | 0.75 ± 0.22 | 0.65 ± 0.27 | 0.68 ± 0.22 | 0.48 ± 0.19 | 0.47 ± 0.28 |
| t 3.34, df 36, p = 0.001 | t 2.74, df 34, p = 0.009 | ||||
| HF | 0.45 ± 0.43 | 0.35 ± 0.30 | 0.34 ± 0.32 | 0.11 ± 0.40 | 0.06 ± 0.42 |
PCA, posterior cerebral artery; ACA, anterior cerebral artery; VLF, very low frequency; LF, low frequency; HF, high frequency; for the respective frequency ranges, see the text. All statistical results refer to tests against the controls. t, t-statistic; df, degrees of freedom; p, level of significance.
In the patients with ACA infarctions, the median infarct size was 0.9 ml [0.04, 4.08], with a maximum of 29 ml. Regression analysis showed that dCA parameters were not related to infarct size. Compared to the controls (Figure 2), phase was significantly reduced in the VLF and the LF in the affected and unaffected hemispheres; the result that phase in the nonaffected side was lower than that in the affected side was mainly due to a very low outliner; phase was similarly low on both sides when this outliner was removed; therefore, our result regarding the unaffected side should not be overinterpreted given the small total number of patients. There was little difference in gain except for an increased gain in the unaffected hemisphere in the VLF range (due to the same outliner patients as in phase). Figure 3 demonstrates that nearly all infarct lesions were within the motor or premotor cortical areas. Clinically, all patients exhibited hemiparesis with the leg weaker than the arm (seven due to left-sided lesions and three due to right-sided lesions).
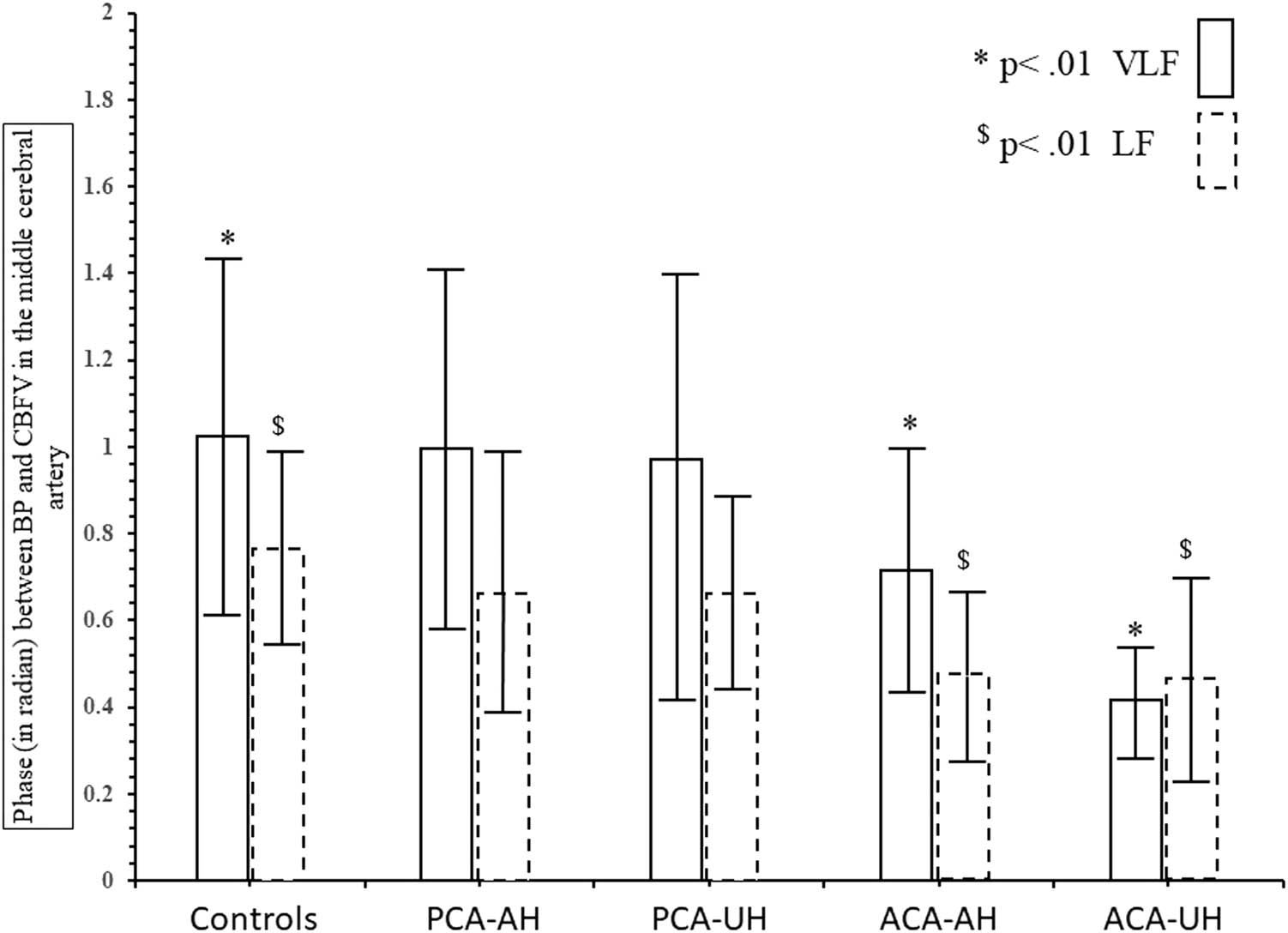
Comparison of MCA phase between controls and the patients with infarcts in the PCA or the ACA territories in the very low (VLF) and low frequency (LF) ranges.

Location (n = 10) and horizontal extent of the infarcts in the ACA territory. Each colour represents one patient; the infarctions were allocated in the right hemisphere in three patients and in the left hemisphere in seven patients. In one patient the coloured dashed line was chosen to indicate infarct size to allow us to visualize the other infarcts which are within the boundaries of this large infarct.
5 Discussion
Overall, PCA infarction does not affect CBF regulation in the MCA territory, while ACA infarction does with pronounced leg hemiparesis as the major motor finding. Because the main cardiovascular factors and ETCO2 were not different between the two stroke allocation groups, they cannot account for the different dCA findings. The sizes of infarcts in both territories were also unrelated to the dCA impairments in the MCA territory; large artery disease in the carotid artery distribution (as a common reason of dCA impairment) was excluded, and small vessel disease was rare. As Figure 1 shows, all our ACA lesions affected cortical areas relevant for motor function. We, therefore, interpret our findings as supportive for our hypothesis that motor CN contribute to MCA dCA disturbances [4,5,21,22,23].
To our knowledge, our study is the first one that describes with a bed side test that cerebral perfusion regulation in the MCA territory is affected in patients with ACA infarctions, while CBFV, as an index of CBF, is unchanged. Thus, hypothetically, dCA assessment seems more sensitive for studying even fine perfusion regulation processes and could be used for a wider approach to study motor recovery predictability [23]. Compared to MRI or other CBF-measuring techniques, the advantages of assessing dCA by TCD are its robustness and its availability as a bedside test.
This study has limitations. The environments were different between the patients and the controls. This could have influenced the results as the stroke-unit setting is noisy and could produce more noisy recordings, making the recordings more sensitive to errors [24]. However, the clear distinction between the results in the PCA infarct patients and the ACA infarct patients can be used as an argument against the relevance of the suggested noisy surroundings. The number of patients in the ACA infarct group is small, but the results are very impressive. The mechanism behind the resulting phase decrease is not directly derivable from our data, but the fact that gain as an index of the vascular tone remained widely unchanged favours a metabolic stimulus.
-
Author contributions: Manuel Bolognese: writing, important intellectual content; Grzegorz Karwacki: data analysis; Mareike Österreich: study concept, data collection; Martin Müller: writing, data analysis, study concept; Lehel Lakatos: data analysis and data collection.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–559.10.1152/physrev.00022.2020Suche in Google Scholar PubMed PubMed Central
[2] Raimondo L, Oliveira ĹAF, Heij J, Priovoulos N, Kundu P, Leoni RF, et al. Advances in resting state fMRI acquisitions for functional connectomics. Neuroimage. 2021;243:118503. 10.1016/j.neuroimage.2021.118503. PMID: 34479041.Suche in Google Scholar PubMed
[3] Gaberova K, Pacheva I, Ivanov I. Task-related fMRI in hemiplegic cerebral palsy-A systematic review. J Eval Clin Pract. 2018;24:839–50. 10.1111/jep.12929. PMID: 29700896.Suche in Google Scholar PubMed
[4] Müller M, Österreich M. Cerebral microcirculatory blood flow dynamics during rest and a continuous motor task. Front Physiol. 2019;10:1355. 10.3389/fphys.2019.01355. PMID: 31708802; PMCID: PMC6821676.Suche in Google Scholar PubMed PubMed Central
[5] Müller M, Österreich M. Cerebrovascular dynamics during continuous motor task. Physiol Res. 2019;68:997–1004. 10.33549/physiolres.934147. PMID: 31647292.Suche in Google Scholar PubMed
[6] Khalaf A, Sybeldon M, Sejdic E, Akcakaya M. A brain-computer interface based on functional transcranial Doppler ultrasound using wavelet transform and support vector machines. J Neurosci Methods. 2018;293:174–82. 10.1016/j.jneumeth.2017.10.003. PMID: 29017899.Suche in Google Scholar PubMed
[7] Bleton H, Perera S, Sejdić E. Cognitive tasks and cerebral blood flow through anterior cerebral arteries: A study via functional transcranial Doppler ultrasound recordings. BMC Med Imaging. 2016;16:22. 10.1186/s12880-016-0125-0. PMID: 26969112; PMCID: PMC4788871.Suche in Google Scholar PubMed PubMed Central
[8] Nogueira RC, Aries M, Minhas JS, H Petersen N, Xiong L, Kainerstorfer JM, et al. Review of studies on dynamic cerebral autoregulation in the acute phase of stroke and the relationship with clinical outcome. J Cereb Blood Flow Metab. 2022;42:430–53. 10.1177/0271678X211045222. PMID: 34515547; PMCID: PMC8985432.Suche in Google Scholar PubMed PubMed Central
[9] Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: Transfer gain in different frequency domains. J Appl Physiol (1985). 2005;98:151–9.10.1152/japplphysiol.00471.2004Suche in Google Scholar PubMed
[10] Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–55.10.1161/HYPERTENSIONAHA.106.084939Suche in Google Scholar PubMed
[11] Vianna LC, Deo SH, Jensen AK, Holwerda SW, Zimmerman MC, Fadel PJ. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2015;308:H681–7.10.1152/ajpheart.00343.2014Suche in Google Scholar PubMed PubMed Central
[12] Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, et al. Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab. 2014;34:228–34.10.1038/jcbfm.2013.180Suche in Google Scholar PubMed PubMed Central
[13] Liu Z, Ma H, Guo ZN, Wang L, Qu Y, Fan L, et al. Impaired dynamic cerebral autoregulation is associated with the severity of neuroimaging features of cerebral small vessel disease. CNS Neurosci Ther. 2022;28:298–306.10.1111/cns.13778Suche in Google Scholar PubMed PubMed Central
[14] Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25:2220–6.10.1161/01.STR.25.11.2220Suche in Google Scholar
[15] Van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7.10.1161/01.STR.19.5.604Suche in Google Scholar PubMed
[16] Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.10.1161/01.STR.24.1.35Suche in Google Scholar PubMed
[17] Fazekas F, Barkhof F, Wahlund LO, Pantoni L, Erkinjuntti T, Scheltens P, et al. CT and MRI rating of white matter lesions. Cerebrovasc Dis. 2002;13(Suppl 2):31–6.10.1159/000049147Suche in Google Scholar PubMed
[18] Lakatos L, Bolognese M, Müller M, Österreich M, von Hessling A. Automated supra- and infratentorial brain infarct volume estimation on diffusion weighted imaging using the RAPID software. Front Neurol. 2022;13:907151. 10.3389/fneur.2022.907151. PMID: 35873774; PMCID: PMC9304979.Suche in Google Scholar PubMed PubMed Central
[19] Müller M, Österreich M, von Hessling A, Smith RS. Incomplete recovery of cerebral blood flow dynamics in sufficiently treated high blood pressure. J Hypertens. 2019;37:372–9.10.1097/HJH.0000000000001854Suche in Google Scholar PubMed PubMed Central
[20] Panerai RB, Brassard P, Burma JS, Castro P, Claassen JA, van Lieshout JJ, et al. Cerebrovascular Research Network (CARNet). Transfer function analysis of dynamic cerebral autoregulation: A CARNet white paper 2022 update. J Cereb Blood Flow Metab. 2023;43(1):3–25. 10.1177/0271678X221119760. PMID: 35962478.Suche in Google Scholar PubMed PubMed Central
[21] Havsteen I, Madsen KH, Christensen H, Christensen A, Siebner HR. Diagnostic approach to functional recovery: Functional magnetic resonance imaging after stroke. Front Neurol Neurosci. 2013;32:9–25. 10.1159/000346408. PMID: 23859959.Suche in Google Scholar PubMed
[22] Michely J, Volz LJ, Hoffstaedter F, Tittgemeyer M, Eickhoff SB, Fink GR, et al. Network connectivity of motor control in the ageing brain. Neuroimage Clin. 2018;18:443–55. 10.1016/j.nicl.2018.02.001. PMID: 29552486; PMCID: PMC5852391.Suche in Google Scholar PubMed PubMed Central
[23] Chi NF, Ku HL, Chen DY, Tseng YC, Chen CJ, Lin YC, et al. Cerebral motor functional connectivity at the acute stage: An outcome predictor of ischemic stroke. Sci Rep. 2018;8:16803. 10.1038/s41598-018-35192-y. PMID: 30429535; PMCID: PMC6235876.Suche in Google Scholar PubMed PubMed Central
[24] Liu J, Simpson DM, Panerai RB. Point-Counterpoint: Transfer function analysis of dynamic cerebral autoregulation: To band or not to band? J Cereb Blood Flow Metab. 2022. 10.1177/0271678X221098448.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science
Artikel in diesem Heft
- Research Articles
- HIF-1α participates in secondary brain injury through regulating neuroinflammation
- Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis
- The correlation between non-arteritic anterior ischemic optic neuropathy and cerebral infarction
- Enriched environment can reverse chronic sleep deprivation-induced damage to cellular plasticity in the dentate gyrus of the hippocampus
- Middle cerebral artery dynamic cerebral autoregulation is impaired by infarctions in the anterior but not the posterior cerebral artery territory in patients with mild strokes
- Leptin ameliorates Aβ1-42-induced Alzheimer’s disease by suppressing inflammation via activating p-Akt signaling pathway
- TIPE2 knockdown exacerbates isoflurane-induced postoperative cognitive impairment in mice by inducing activation of STAT3 and NF-κB signaling pathways
- Does the patellar tendon reflex affect the postural stability in stroke patients with blocked vision?
- Inactivation of CACNA1H induces cell apoptosis by initiating endoplasmic reticulum stress in glioma
- miR-101-3p improves neuronal morphology and attenuates neuronal apoptosis in ischemic stroke in young mice by downregulating HDAC9
- A custom-made weight-drop impactor to produce consistent spinal cord injury outcomes in a rat model
- Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method
- Thyroid hormone levels paradox in acute ischemic stroke
- Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the NF-κB signaling pathway
- The clinical characteristics of acute cerebral infarction patients with thalassemia in a tropic area in China
- Comprehensive behavioral study of C57BL/6.KOR-ApoEshl mice
- Incomplete circle of Willis as a risk factor for intraoperative ischemic events during carotid endarterectomies performed under regional anesthesia – A prospective case-series
- HOTAIRM1 knockdown reduces MPP+-induced oxidative stress injury of SH-SY5Y cells by activating the Nrf2/HO-1 pathway
- Esmolol inhibits cognitive impairment and neuronal inflammation in mice with sepsis-induced brain injury
- EHMT2 affects microglia polarization and aggravates neuronal damage and inflammatory response via regulating HMOX1
- Hematoma evacuation based on active strategies versus conservative treatment in the management of moderate basal ganglia hemorrhage: A retrospective study
- Knockdown of circEXOC6 inhibits cell progression and glycolysis by sponging miR-433-3p and mediating FZD6 in glioma
- CircYIPF6 regulates glioma cell proliferation, apoptosis, and glycolysis through targeting miR-760 to modulate PTBP1 expression
- Relationship between serum HIF-1α and VEGF levels and prognosis in patients with acute cerebral infarction combined with cerebral-cardiac syndrome
- The promoting effect of modified Dioscorea pills on vascular remodeling in chronic cerebral hypoperfusion via the Ang/Tie signaling pathway
- Effects of enriched environment on the expression of β-amyloid and transport-related proteins LRP1 and RAGE in chronic sleep-deprived mice
- An interventional study of baicalin on neuronal pentraxin-1, neuronal pentraxin-2, and C-reactive protein in Alzheimer’s disease rat model
- PD98059 protects SH-SY5Y cells against oxidative stress in oxygen–glucose deprivation/reperfusion
- TPVB and general anesthesia affects postoperative functional recovery in elderly patients with thoracoscopic pulmonary resections based on ERAS pathway
- Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy
- Association between RS3763040 polymorphism of the AQP4 and idiopathic intracranial hypertension in a Spanish Caucasian population
- Effects of γ-oryzanol on motor function in a spinal cord injury model
- Electroacupuncture inhibits the expression of HMGB1/RAGE and alleviates injury to the primary motor cortex in rats with cerebral ischemia
- Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke
- CST3 alleviates bilirubin-induced neurocytes’ damage by promoting autophagy
- Excessive MALAT1 promotes the immunologic process of neuromyelitis optica spectrum disorder by upregulating BAFF expression
- Evaluation of cholinergic enzymes and selected biochemical parameters in the serum of patients with a diagnosis of acute subarachnoid hemorrhage
- 7-Day National Institutes of Health Stroke Scale as a surrogate marker predicting ischemic stroke patients’ outcome following endovascular therapy
- Cdk5 activation promotes Cos-7 cells transition towards neuronal-like cells
- 10.1515/tnsci-2022-0313
- PPARα agonist fenofibrate prevents postoperative cognitive dysfunction by enhancing fatty acid oxidation in mice
- Predicting functional outcome in acute ischemic stroke patients after endovascular treatment by machine learning
- EGCG promotes the sensory function recovery in rats after dorsal root crush injury by upregulating KAT6A and inhibiting pyroptosis
- Preoperatively administered single dose of dexketoprofen decreases pain intensity on the first 5 days after craniotomy: A single-centre placebo-controlled, randomized trial
- Myeloarchitectonic maps of the human cerebral cortex registered to surface and sections of a standard atlas brain
- The BET inhibitor apabetalone decreases neuroendothelial proinflammatory activation in vitro and in a mouse model of systemic inflammation
- Carthamin yellow attenuates brain injury in a neonatal rat model of ischemic–hypoxic encephalopathy by inhibiting neuronal ferroptosis in the hippocampus
- Functional connectivity in ADHD children doing Go/No-Go tasks: An fMRI systematic review and meta-analysis
- Review Articles
- Human prion diseases and the prion protein – what is the current state of knowledge?
- Nanopharmacology as a new approach to treat neuroinflammatory disorders
- Case Report
- Deletion as novel variants in VPS13B gene in Cohen syndrome: Case series
- Commentary
- Translation of surface electromyography to clinical and motor rehabilitation applications: The need for new clinical figures
- Revealing key role of T cells in neurodegenerative diseases, with potential to develop new targeted therapies
- Retraction
- Retraction of “Eriodictyol corrects functional recovery and myelin loss in SCI rats”
- Special Issue “Advances in multimedia-based emerging technologies...”
- Evaluation of the improvement of walking ability in patients with spinal cord injury using lower limb rehabilitation robots based on data science

