Abstract
Objectives
Polycystic ovarian syndrome (PCOS) is a heterogeneous endocrine condition that influences 15–20 % of women at their fertile age. In this academic work, we aimed to research serum anti-mullerian hormone (AMH) levels using the Vidas® and Atellica® methods and other hormone parameters in PCOS.
Methods
This study included 55 controls and 55 PCOS women diagnosed by Rotterdam criteria. AMH levels were measured by Vidas® and the Atellica® devices, and the other parameters were obtained from Ankara Bilkent City Hospital’s Laboratory Information System.
Results
The AMH levels utilized by the Vidas® (p<0.001) and the Atellica® (p=0.001) were both more elevated in PCOS women. The Vidas® and the Atellica® methods were well correlated, and the correlation coefficient value was 0.899. The AMH cut-off value was 3.23 μg/L with a sensitivity of 65.5 % and a specificity of 34.5 % by utilizing the Vidas® method and 3.66 μg/L with a sensitivity of 65.4 % and a specificity of 36.4 % by utilizing the Atellica® method. PCOS women’s serum AMH levels were positively correlated with fasting glucose and luteinizing hormone (LH) and negatively correlated with age and progesterone. The PCOS group’s levels of serum estradiol (E2) were notably more elevated and levels of serum prolactin were notably lower than the controls.
Conclusions
This study showed that serum AMH levels measured with both the Atellica® and the Vidas® present significant differences between PCOS patients and the controls but still, AMH alone is not diagnostic enough to distinguish PCOS patients from controls.
Introduction
Gynecological health issues include diseases of the female reproductive system or estrogen-controlled organs, such as polycystic ovarian syndrome (PCOS), an endocrine status that influences 15–20 % of women of fertile age [1], [2], [3]. PCOS, a heterogeneous condition, is a multifactorial, polygenic, inflammatory, dysregulated steroid state that can lead to obesity, insulin resistance, cardiovascular problems, and infertility [2], 4]. Endocrine, reproductive, metabolic, and psychosocial symptoms in women with PCOS might fluctuate throughout a lifetime [5]. According to the 2003 Rotterdam consensus, at least two of the following criteria must present for PCOS diagnosis: oligomenorrhea (OA), hyperandrogenism (HA), and polycystic ovary morphology on ultrasound [6].
Anti-mullerian hormone (AMH), also defined as the mullerian-inhibiting substance, belongs to the transforming growth factor-beta family. AMH contains homodimer disulfide-linked glycoprotein [7]. Male testicular Sertoli cells and female ovarian granulosa cells both produce it. One of PCOS’ characteristic features is the increment of ovarian follicles at their growing stage. The increment can be seen in both pre-antral follicles and small antral follicles that produce AMH, which is crucial for regulating folliculogenesis [8], 9]. AMH prevents primordial follicle reinforcement and modulates follicle-stimulating hormone (FSH) activity in early follicular development [10], 11].
AMH measurement is useful in determining gender, gonadal function, and fertility, as well as a gonadal tumor marker, due to the variations in AMH levels between genders, differences in circulating levels together with sexual feature development, and AMH’s specificity for Sertoli cells and granulosa cells. In women, AMH is known as an ovarian reserve marker, and there is a relationship between AMH and the primordial follicles and an inverse relationship with the chronological age, predicting the ovarian response to in vitro fertilization. It may even be able to predict when menopause will start. Serum AMH concentrations are generally steady during the menstrual cycle, unlike other ovarian reserve indicators that exhibit considerable changes. Serum AMH concentrations, which are correlated with HA and ovarian antral follicle count, are 2–4 times higher in women with PCOS due to increased stock of pre-antral follicles and small antral follicles of polycystic ovaries, and increased levels of AMH appear to be related to predicting anovulatory and irregular cycles and the severity of PCOS [12], [13], [14]. Some researchers have suggested that measuring serum AMH levels may have a role in the diagnosis of PCOS [15], 16]. Since there is no standardization for AMH immunoassays, values obtained using various assay techniques or kits may vary and cannot be used interchangeably. AMH levels and cut-off values depend on the method and, there is no international standard for AMH measurement [17], 18].
The aims of the present study are as follows: firstly, to measure AMH levels in two different devices that measure AMH levels by immunoassay method, secondly, to detect the measurement difference in these measurements, and thirdly, to evaluate the clinical meaning of this difference in PCOS diagnosed patient group and the control group. For the present research, the measurements of serum AMH and evaluation of the results for PCOS diagnosis were performed using two different AMH measurement methods: the Vidas® (bioMérieux SA, Marcy l’Etoile, France) and the Atellica® (Siemens Healthineers, Erlangen, Germany) methods.
To our knowledge, no research has been conducted to compare these AMH techniques. We also looked at the hormonal characteristics of women with PCOS and controls.
Materials and methods
Subjects
This study included volunteers who applied to Ankara Bilkent City Hospital’s obstetrics and gynecology outpatient clinic for 3 months. Individuals in the control group (n=55, 19–49 years) had regular menstrual cycles, and none of them were using hormonal preparations or any other medications. The PCOS group subjects (n=55, ages 18–25 years) were diagnosed according to Rotterdam criteria. All subjects in both groups were non-smoking women. Using the G-Power 3.1.9.2 software, α=0.05, power (1-β)=0.95, and a medium effect size (when taken as 0.5), the smallest sample size for each group was calculated as 55, for a total of 110 participants. Ethics approval was obtained from Ankara Bilkent City Hospital’s Ethic Committee for this study (E1-22-3153). At every stage of our study, we worked in accordance with the Declaration of Helsinki. Appropriate informed consent was obtained from each research participant.
Laboratory and clinical measurements
Serum samples were obtained after overnight fasting from individuals in the follicular phase of the menstrual cycle (1–7 days after spontaneous menstruation). Serum fasting glucose, FSH, luteinizing hormone (LH), prolactin, insulin, estradiol (E2), thyroid stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), progesterone, total testosterone, dehydroepiandrostenedione sulfate (DHEA-S), androstenedione and sex hormone-binding globulin (SHBG) levels were analyzed on the day of sampling. Remaining serum samples were aliquoted, frozen immediately, and stored at −80 °C until serum AMH measurement.
AMH measurement with Vidas® methodology is based on the combination of the single-step enzyme immunoassay sandwich method with fluorescence detection in the final stage. The final reading takes place at 450 nm, and the results obtained are proportional to the fluorescence density. AMH measurement with Atellica® methodology is an automated method using acridinium ester chemiluminescence technology. It contains two antibodies: the first antibody is a mouse monoclonal anti-AMH antibody and it is labeled with an acridinium ester, and the second antibody is a biotinylated antibody that binds to streptavidin-coated paramagnetic microparticles on the solid phase. There is a direct relationship between the relative light detected by the system and the amount of AMH in the sample. The limit of detection of Vidas® and Atellica® methods are as follows, respectively; 0.01 ng/mL and 0.02 ng/mL. The limit of quantitation of Vidas® and Atellica® methods are as follows, respectively; 0.02 ng/mL and 0.03 ng/mL.
Serum fasting glucose, FSH, LH, prolactin, insulin, E2, TSH, fT3, fT4, progesterone, total testosterone, DHEA-S, androstenedione, and SHBG levels were obtained from Ankara Bilkent City Hospital’s Laboratory Information System. Serum fasting glucose, FSH, LH, prolactin, insulin, E2, TSH, fT3, fT4, progesterone, total testosterone, and DHEA-S levels were measured using an Atellica® autoanalyzer Siemens Healthineers, Erlangen, Germany). Serum androstenedione and SHBG levels were measured by Immulite 2,000 (Siemens Healthineers, Erlangen, Germany) autoanalyzer.
Homeostasis model assessment-estimated insulin resistance index (HOMA-IR) was calculated according to the formula by using the formula described by Matthews et al.: [insulin (mU/L) × glucose (mmol/L)]/22.5 [19]. Our hospital uses internal and external quality controls, and during the study, all tests were in the acceptable quality control range.
Statistical analyses
Our study included age, the analysis results of AMH Vidas®, AMH Atellica®, FSH, LH, E2, prolactin, and androstenedione tests for both groups, while it included fasting glucose, insulin, HOMA-IR, androstenedione, total testosterone, DHEA-S, and SHBG tests only for the PCOS group.
In our study, age and the tests including AMH Vidas®, AMH Atellica®, TSH, fT3, fT4, fasting glucose, insulin, androstenedione, total testosterone, and DHEA-S distributions were statistically parametric, while FSH, LH, E2, prolactin, progesterone, HOMA-IR, and SHBG’s distributions’ were statistically non-parametric. For parametric parameters, the Student’s t-test, and for non-parametric parameters Mann-Whitney U test were performed. The mean, and the standard deviation values of the parametric tests and, the median and interquartile range values of the non-parametric tests are presented. To determine the best cut-off point to differentiate women with PCOS from control subjects, a receiver operating characteristic (ROC) curve analysis was done. For all these statistical analyses, IBM SPSS Statistics 20.0 (SPSS, Inc., IBM Corp., New York, NY, USA) was used, and a p-value <0.05 was assessed as statistically significant. Passing–Bablok regression analysis and Bland Altman plots were performed with MedCalc® (version 23.1.6 free trial) statistical software.
Results
The PCOS group’s age (21.3 ± 1.89 years) was less than the control group (31.7 ± 7.83 years) (p<0.001). Serum prolactin concentrations were statistically higher in the control group. (p=0.002) In contrast, serum E2 levels, serum AMH levels measured by Vidas®, and Atellica® were statistically higher in the PCOS group (p=0.001, <0.001, p=0.001, respectively). Statistically, no difference was observed in serum FSH, LH, TSH, fT3, fT4, and progesterone levels between the groups. Hormonal and metabolic parameters of the groups are presented in Table 1.
Hormonal and metabolic parameters in PCOS and control groups.
| PCOS group | Control group | p-Value | |
|---|---|---|---|
| (n=55) | (n=55) | ||
| Fasting glucose, mg/dL | 83.5 ± 4.9 | ||
| FSH, U/L | 6.06 [5.5–6.9] | 6.68 [5.3–6.9] | 0.125 |
| LH, U/L | 5.77 [3.2–5.9] | 5.71 [3.6–5.71] | 0.619 |
| E2, ng/L | 84.6 [48–85] | 56.3 [41–58] | 0.01a |
| TSH, mU/L | 2.0 ± 1.07 | 2.17 ± 0.88 | 0.294 |
| fT3, ng/L | 3.6 ± 0.27 | 3.52 ± 0.19 | 0.151 |
| fT4, ng/dL | 1.2 ± 0.12 | 1.15 ± 0.12 | 0.063 |
| Prolactin, µg/L | 10.6 [8.1–11.2] | 13 [9.2–13.47] | 0.002a |
| Androstenedione, nmol/L | 11.3 ± 4.90 | ||
| Progesterone, µg/L | 1.6 [0.8–1.6] | 2.14 [0.46–2.4] | 0.371 |
| Total testosterone, ug/L | 0.3 ± 0.07 | ||
| Insulin, mU/L | 11.6 ± 5.10 | ||
| HOMA-IR | 2.86 [1.8–2.86] | ||
| DHEA-S, µg/dL | 284.4 ± 72.0 | ||
| SHBG, nmol/L | 39.9 [34–39.9] | ||
| AMH Vidas®, µg/L | 4.48 ± 2.36 | 2.78 ± 2.20 | <0.001b |
| AMH Atellica®, µg/L | 4.88 ± 2.65 | 3.17 ± 2.61 | 0.001b |
| Age, years | 21.3 ± 1.9 | 31.7 ± 7.8 | <0.001b |
-
aMann- Whitney U test; bStudent’s t-test. A p-value <0.05 was assessed as statistically significant. Data are presented with mean and standard deviations for normally distributed parameters (AMH, Vidas®, AMH, Atellica®, TSH, fT3, fT4, fasting glucose, insulin, androstenedione, total testosterone, DHEA-S, and age) and median and interquartile ranges for non-normally distributed parameters (FSH, LH, E2, prolactin, progesterone; HOMA-IR, and SHBG). FSH, folicule-stimulating hormone; LH, luteinizing hormone; E2, estradiol; TSH, thyroid stimulating hormone; fT3, free triiodothyronine; fT4, free thyroxine; HOMA-IR, homeostatic model assessment of insulin resistance; DHEA-S, dehydroepiandrosterone sulfate; SHBG, sex hormone-binding globulin; AMH, anti-müllerian hormone.
The positive prediction values for Vidas® and Atellica® methods were 0.66 and 0.65, respectively. The negative prediction values for Vidas® and Atellica® methods were 0.34 and 0.35, respectively. The positive and negative likelihood ratios of the Vidas® and Atellica® methods were 1, 1.03 and 1, 0.95, respectively.
Serum AMH concentrations in PCOS and control groups
Serum AMH concentrations of PCOS group were significantly higher than the control group measured by Vidas® (4.48 ± 2.36 μg/L vs. 2.78 ± 2.20 μg/L, p<0.001) and by Atellica® (4.88 ± 2.65 μg/L vs. 3.17 ± 2.61 μg/L, p=0.001).
According to the ROC curve analysis; we got 3.23 μg/L as the best cut-off AMH level with a sensitivity of 65.5 % and, a specificity of 34.5 % by using the Vidas® method and we got 3.66 μg/L as the best cut off AMH level with sensitivity 65.4 % and specificity 36.4 % by using Atellica® method. The area under the curve (AUC) and 95 % confidence interval (CI) of the Vidas® and the Atellica® method were 0.702 (0.605–0.799) and 0.689 (0.59–0.789), respectively (Figure 1). Passing Bablock regression analysis was presented in Figure 2. Bland Altman plot presents the scatter of difference values against the mean of the measurements of Atellica® and Vidas® methods. Bias distribution was given in Bland-Altman plots (Figure 3).

Receiver operating characteristic-curve shows the best cut-off values of serum AMH levels between the PCOS and the control groups according to the Vidas® (3.23 μg/L as the best cut-off AMH level with a sensitivity of 65.5 % and a specificity of 34.5 %) and the Atellica® (3.23 μg/L as the best cut-off AMH level with a sensitivity of 65.5 % and a specificity of 34.5 %).
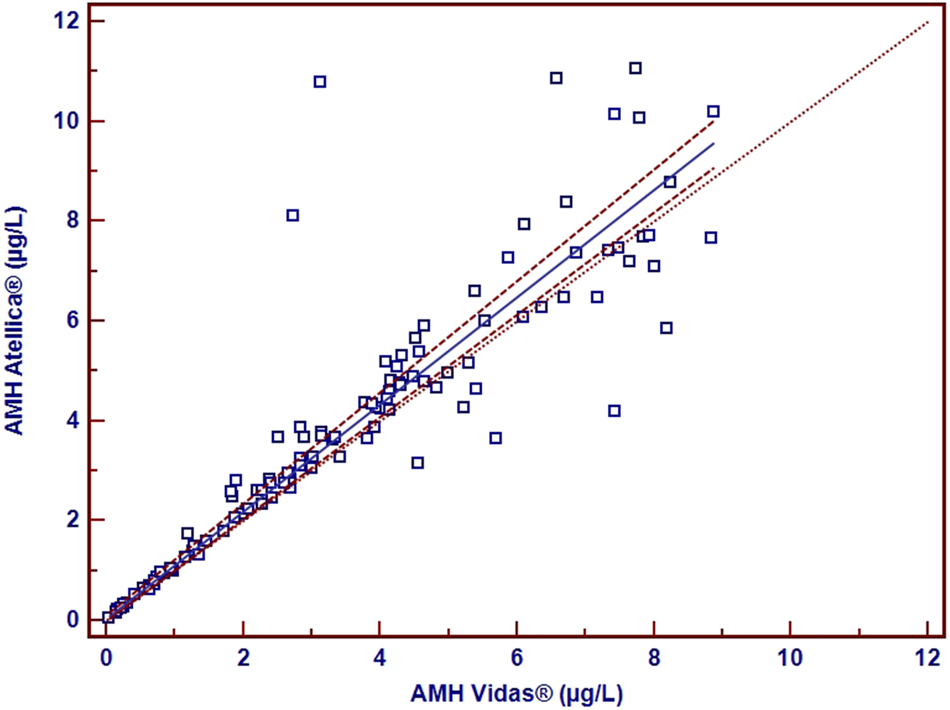
Passing-Bablok regression analysis (y=0.0413 + 1.073x, n=110) (The 95 % CI for the intercept: −0.049, 0.799) (The 95 % CI for the slope: 0.908–1.102) (The area between dashed lines: the confidence interval) (r: 0.891).

Bland-Altman plot graph.
Comparison of serum AMH concentrations
PCOS women’s mean serum AMH level was 4.88 ± 2.6 μg/L with Atellica® and 4.48 ± 2.36 μg/L with Vidas®. The correlation coefficient value was 0.899.
Serum AMH concentrations and the hormonal parameters
AMH measurement results performed by the Atellica® device showed a positive correlation between fasting glucose (r=0.385, p=0.004) and LH (r=0.285, p=0.035), and a negative correlation between age (r= −0.289, p=0.032) and progesterone (r= −0.389, p=0.003) in the PCOS group. AMH measurement results performed by the Vidas® device showed a significantly negative correlation existed between age (r= −0.365, p=0.006) and progesterone (r= −0.32, p=0.017).
Discussion
In the present study, a regression analysis was performed to compare AMH levels among Atellica® and the Vidas® devices, and a fairly strong positive relationship was observed (r=0.891) (Figure 2). The scatter of the difference values against the mean of the measurements of Atellica® and Vidas® methods was presented with the Bland Altman plot (Figure 3). Higher serum AMH concentrations were observed in PCOS women, and serum AMH cut-off values were presented with both the Atellica® and the Vidas® devices.
Additionally, we performed correlation analyses for variables with both Atellica® and Vidas® methods. We report that, serum AMH levels of women with PCOS, according to the Atellica® method, were positively correlated with both serum fasting glucose and LH levels and negatively correlated with serum progesterone levels and age. We also report that serum AMH levels of women with PCOS, according to the Vidas® method, were negatively correlated with serum progesterone levels and age. A statistically significant difference was observed in serum E2 and serum prolactin levels between the groups, but there was no statistically significant difference in serum FSH, TSH, fT3, and fT4 levels between the groups.
In this study, the mean age of the groups was not similar, but all the participants were of reproductive age. In the study conducted by La Marka et al., it was shown that AMH values decrease with increasing age and fall to undetectable levels after spontaneous menopause [20]. De Vet et al. reported that serum AMH levels in young normo-ovulatory women decreased with time [21]. In our study, there was a negative correlation between age and serum AMH levels, consistent with previous studies [20], 21].
In this study, we obtained results using the Atellica® and the Vidas® methods, and both represent higher mean serum AMH levels in women with PCOS compared to controls, in agreement with former studies. As mentioned in previous studies; this situation shows that serum AMH concentrations may be useful [22], [23], [24]. However, AMH was not included in the 2003 Rotterdam consensus for the diagnosis of PCOS.
In this study, we state two different best cut-off level values for serum AMH levels, but still, AMH alone is not sufficient diagnostic value to distinguish PCOS patients from controls. The Vidas® method presents 3.23 μg/L (sensitivity 65.5 % and specificity 34.5 %) and the Atellica® method presents 3.66 μg/L (sensitivity 65.4 % and specificity 36.4 %) and our results are nearly similar to the study by Sahmay et al. [24].
However, in some studies, cut-off values of serum AMH concentrations are higher to distinguish women with PCOS from controls [12], 25], 26]. In this academic work, mean serum AMH concentrations show a strong correlation between the Vidas® and the Atellica® methods (r=0.899).
In the study conducted by Halder et al., the AUC for total PCOS cases was 0.93 (95 % CI: 0.88–0.96) [27]. But in the present study, AUC of the Vidas® method was 0.702 (95 % CI: 0.605–0.799) and the AUC of the Atellica® method was 0.689 (95 % CI: 0.59–0.789).
AMH has important roles in gonadal functions and sexual differentiation. AMH has a potent effect on GnRH neuron firing and can increase GnRH-dependent LH pulse and secretion. LH pulsatility increment role is crucial for the PCOS’s pathophysiological process [28]. In this study, there was no statistically significant difference between the groups, but a positive correlation was observed between serum AMH and LH levels of women with PCOS, consistent with previous studies [29], 30].
PCOS is a common endocrine ailment, including progesterone secretion disorder [31]. Women with PCOS have low progesterone levels; because of oligo/anovulation [32]. In this academic work, serum AMH levels of women with PCOS were negatively correlated with serum progesterone levels, in line with previous studies [31], 32].
Zarei et al. stated higher E2 levels in women with PCOS than in controls. Our study was in line with the study by Zarei et al., having higher serum E2 levels in PCOS group and showing a significant difference between the groups [33]. In this study, serum prolactin levels showed statistical differences and were lower in women with PCOS according to the literature [34].
Women with PCOS are known to have elevated insulin and HOMA-IR levels and are expected to have an increased risk of type-2 diabetes mellitus [17]. Approximately, 40 % of PCOS patients have prediabetes [35]. Li et al. stated higher serum fasting glucose levels in women with PCOS compared with controls [36].
In our study, the control group’s serum fasting glucose levels, insulin levels, and HOMA-IR levels could not be obtained, but a positive correlation was observed between serum AMH and fasting glucose levels in women with PCOS. Zhao et al. stated a weak correlation between serum AMH and plasma fasting glucose levels in the classical PCOS phenotype [3]. Our result was in line with their study but not with some other studies [37], 38]. Further studies including more participants of similar ages with different serum AMH measurement methods may be useful to investigate whether serum AMH levels can determine the PCOS patients from healthy controls and to evaluate its relationship with hormonal parameters of PCOS patients.
A limitation of the this study is the inclusion of individuals of the same ethnicity and, given the study limitations, there were not many participants in any group. Another limitation of the present study is that serum fasting glucose, total testosterone, insulin, Homa-IR, DHEA-S, SHBG, and androstenedione levels were only available for women with PCOS. There was a discrepancy in age between the PCOS and control groups.
We stated the cut-off points for each method and performed correlation analyses with both the Vidas® and the Atellica® methods. Future investigations on this topic may find great value in all of these data points. To our knowledge, this is the first study to compare serum AMH levels using the Atelllica® and Vidas® methods and evaluate serum AMH levels in PCOS and control groups with both devices.
Conclusions
In conclusion, serum AMH levels analyzed with the Atellica® showed good correlation with serum AMH levels analyzed with the Vidas®. Our study showed that serum AMH levels measured with both the Atellica® and the Vidas® present significant differences between PCOS patients and the controls but still AMH alone was not diagnostic enough to distinguish PCOS patients from controls.
Furthermore, AMH were positively correlated with fasting glucose and LH level and negatively correlated with age and serum progesterone level. Further research with a larger sample of similar ages would be beneficial.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Dunaif, A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774–800. https://doi.org/10.1210/er.18.6.774.Search in Google Scholar
2. Sirmans, SM, Pate, KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013;6:1–13. https://doi.org/10.2147/clep.s37559.Search in Google Scholar
3. Zhao, H, Zhou, D, Liu, C, Zhang, L. The relationship between insulin resistance and obesity and serum anti-müllerian hormone level in Chinese women with polycystic ovary syndrome: a retrospective, single-center cohort study. Int J Womens Health 2023;15:151–66. https://doi.org/10.2147/ijwh.s393594.Search in Google Scholar PubMed PubMed Central
4. Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 2018;182:27–36. https://doi.org/10.1016/j.jsbmb.2018.04.008.Search in Google Scholar PubMed
5. Teede, H, Deeks, A, Moran, L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. https://doi.org/10.1186/1741-7015-8-41.Search in Google Scholar PubMed PubMed Central
6. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004.Search in Google Scholar PubMed
7. Shrikhande, L, Shrikhande, B, Shrikhande, A. AMH and its clinical implications. J Obstet Gynaecol India 2020;70:337–41. https://doi.org/10.1007/s13224-020-01362-0.Search in Google Scholar PubMed PubMed Central
8. Dewailly, D, Barbotin, AL, Dumont, A, Catteau-Jonard, S, Robin, G. Role of anti-müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front Endocrinol 2020;11:641. https://doi.org/10.3389/fendo.2020.00641.Search in Google Scholar PubMed PubMed Central
9. Visser, JA, de Jong, FH, Laven, JS, Themmen, AP. Anti-müllerian hormone: a new marker for ovarian function. Reproduction 2006;131:1–9. https://doi.org/10.1530/rep.1.00529.Search in Google Scholar PubMed
10. Visser, JA, Themmen, AP. Anti-Müllerian hormone and folliculogenesis. Mol Cell Endocrinol 2005;234:81–6. https://doi.org/10.1016/j.mce.2004.09.008.Search in Google Scholar PubMed
11. Durlinger, AL, Gruijters, MJ, Kramer, P, Karels, B, Kumar, TR, Matzuk, MM, et al.. Anti-müllerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 2001;142:4891–9. https://doi.org/10.1210/endo.142.11.8486.Search in Google Scholar PubMed
12. Jacob, SL, Field, HP, Calder, N, Picton, HM, Balen, AH, Barth, JH. Anti-Müllerian hormone reflects the severity of polycystic ovary syndrome. Clin Endocrinol 2017;86:395–400. https://doi.org/10.1111/cen.13269.Search in Google Scholar PubMed
13. Pigny, P, Merlen, E, Robert, Y, Cortet-Rudelli, C, Decanter, C, Jonard, S, et al.. Elevated serum level of anti-müllerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab 2003;88:5957–62. https://doi.org/10.1210/jc.2003-030727.Search in Google Scholar PubMed
14. Laven, JS, Mulders, AG, Visser, JA, Themmen, AP, De Jong, FH, Fauser, BC. Anti-müllerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 2004;89:318–23. https://doi.org/10.1210/jc.2003-030932.Search in Google Scholar PubMed
15. Eilertsen, TB, Vanky, E, Carlsen, SM. Anti-müllerian hormone in the diagnosis of polycystic ovary syndrome: can morphologic description be replaced? Hum Reprod 2012;27:2494–502. https://doi.org/10.1093/humrep/des213.Search in Google Scholar PubMed
16. Casadei, L, Madrigale, A, Puca, F, Manicuti, C, Emidi, E, Piccione, E, et al.. The role of serum anti-müllerian hormone (AMH) in the hormonal diagnosis of polycystic ovary syndrome. Gynecol Endocrinol 2013;29:545–50. https://doi.org/10.3109/09513590.2013.777415.Search in Google Scholar PubMed
17. Sova, H, Unkila-Kallio, L, Tiitinen, A, Hippeläinen, M, Perheentupa, A, Tinkanen, H, et al.. Hormone profiling, including anti-müllerian hormone (AMH), for the diagnosis of polycystic ovary syndrome (PCOS) and characterization of PCOS phenotypes. Gynecol Endocrinol 2019;35:595–600. https://doi.org/10.1080/09513590.2018.1559807.Search in Google Scholar PubMed
18. Punchoo, R, Bhoora, S. Variation in the measurement of anti-müllerian hormone - what are the laboratory issues? Front Endocrinol 2021;12:719029. https://doi.org/10.3389/fendo.2021.719029.Search in Google Scholar PubMed PubMed Central
19. Matthews, DR, Hosker, JP, Rudenski, AS, Naylor, BA, Treacher, DF, Turner, RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9. https://doi.org/10.1007/bf00280883.Search in Google Scholar
20. La Marca, A, De Leo, V, Giulini, S, Orvieto, R, Malmusi, S, Giannella, L, et al.. Anti-müllerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J Soc Gynecol Invest 2005;12:545–8. https://doi.org/10.1016/j.jsgi.2005.06.001.Search in Google Scholar PubMed
21. de Vet, A, Laven, JS, de Jong, FH, Themmen, AP, Fauser, BC. Anti-müllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 2002;77:357–62. https://doi.org/10.1016/s0015-0282(01)02993-4.Search in Google Scholar PubMed
22. Rudnicka, E, Kunicki, M, Calik-Ksepka, A, Suchta, K, Duszewska, A, Smolarczyk, K, et al.. Anti-müllerian hormone in pathogenesis, diagnostic and treatment of PCOS. Int J Mol Sci 2021;22:12507. https://doi.org/10.3390/ijms222212507.Search in Google Scholar PubMed PubMed Central
23. Stracquadanio, M, Ciotta, L, Palumbo, MA. Relationship between serum anti-mullerian hormone and intrafollicular AMH levels in PCOS women. Gynecol Endocrinol 2018;34:223–8. https://doi.org/10.1080/09513590.2017.1381838.Search in Google Scholar PubMed
24. Sahmay, S, Atakul, N, Aydogan, B, Aydin, Y, Imamoglu, M, Seyisoglu, H. Elevated serum levels of anti-müllerian hormone can be introduced as a new diagnostic marker for polycystic ovary syndrome. Acta Obstet Gynecol Scand 2013;92:1369–74. https://doi.org/10.1111/aogs.12247.Search in Google Scholar PubMed
25. Matsuzaki, T, Munkhzaya, M, Iwasa, T, Tungalagsuvd, A, Yano, K, Mayila, Y, et al.. Relationship between serum anti-müllerian hormone and clinical parameters in polycystic ovary syndrome. Endocr J 2017;64:531–41. https://doi.org/10.1507/endocrj.ej16-0501.Search in Google Scholar PubMed
26. Sathyapalan, T, Al-Qaissi, A, Kilpatrick, ES, Dargham, SR, Atkin, SL. Anti-müllerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin Endocrinol 2018;88:258–62. https://doi.org/10.1111/cen.13517.Search in Google Scholar PubMed
27. Halder, A, Kumar, H, Sharma, M, Jain, M, Kalsi, AK, Pandey, S. Serum anti-müllerian hormone: a potential biomarker for polycystic ovary syndrome. Indian J Med Res 2023;158:397–406. https://doi.org/10.4103/ijmr.ijmr_4608_20.Search in Google Scholar PubMed PubMed Central
28. Cimino, I, Casoni, F, Liu, X, Messina, A, Parkash, J, Jamin, SP, et al.. Novel role for anti-müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun 2016;7:10055. https://doi.org/10.1038/ncomms10055.Search in Google Scholar PubMed PubMed Central
29. Esparza, LA, Schafer, D, Ho, BS, Thackray, VG, Kauffman, AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology 2020;161:bqaa018. https://doi.org/10.1210/endocr/bqaa018.Search in Google Scholar PubMed PubMed Central
30. Coutinho, EA, Kauffman, AS. The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med Sci 2019;7:84. https://doi.org/10.3390/medsci7080084.Search in Google Scholar PubMed PubMed Central
31. Yang, Y, Liu, B, Wu, G, Yang, J. Exploration of the value of progesterone and progesterone/estradiol ratio on the hCG trigger day in predicting pregnancy outcomes of PCOS patients undergoing IVF/ICSI: a retrospective cohort study. Reprod Biol Endocrinol 2021;19:184. https://doi.org/10.1186/s12958-021-00862-6.Search in Google Scholar PubMed PubMed Central
32. Luan, YY, Zhang, L, Peng, YQ, Li, YY, Liu, RX, Yin, CH. Immune regulation in polycystic ovary syndrome. Clin Chim Acta 2022;531:265–72. https://doi.org/10.1016/j.cca.2022.04.234.Search in Google Scholar PubMed
33. Zarei, E, Binabaj, MM, Zadeh, FM, Bakhshandeh Nosrat, S, Veghari, G, Mansourian, A. Kisspeptin levels in relation to sex hormone profile among PCOS patients. Ir J Med Sci 2022;191:1711–6. https://doi.org/10.1007/s11845-021-02733-w.Search in Google Scholar PubMed
34. Yang, H, Di, J, Pan, J, Yu, R, Teng, Y, Cai, Z, et al.. The association between prolactin and metabolic parameters in PCOS women: a retrospective analysis. Front Endocrinol 2020;11:263. https://doi.org/10.3389/fendo.2020.00263.Search in Google Scholar PubMed PubMed Central
35. Tao, T, Zhang, Y, Zhu, YC, Fu, JR, Wang, YY, Cai, J, et al.. Exenatide, metformin, or both for prediabetes in PCOS: a randomized, open-label, parallel-group controlled study. J Clin Endocrinol Metab 2021;106:e1420–32. https://doi.org/10.1210/clinem/dgaa692.Search in Google Scholar PubMed PubMed Central
36. Li, XJ, Wang, H, Lu, DY, Yu, TT, Ullah, K, Shi, XY, et al.. Anti-müllerian hormone accelerates pathological process of insulin resistance in polycystic ovary syndrome patients. Horm Metab Res 2021;53:504–11. https://doi.org/10.1055/a-1499-7718.Search in Google Scholar PubMed
37. Cui, L, Qin, Y, Gao, X, Lu, J, Geng, L, Ding, L, et al.. Anti-müllerian hormone: correlation with age and androgenic and metabolic factors in women from birth to postmenopause. Fertil Steril 2016;105:481–5. e1. https://doi.org/10.1016/j.fertnstert.2015.10.017.Search in Google Scholar PubMed
38. Ou, M, Xu, P, Lin, H, Ma, K, Liu, M. AMH is a good predictor of metabolic risk in women with PCOS: a cross-sectional study. Internet J Endocrinol 2021;2021:9511772. https://doi.org/10.1155/2021/9511772.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Unveiling the hidden clinical and economic impact of preanalytical errors
- Research Articles
- To explore the role of hsa_circ_0053004/hsa-miR-646/CBX2 in diabetic retinopathy based on bioinformatics analysis and experimental verification
- Study on the LINC00578/miR-495-3p/RNF8 axis regulating breast cancer progression
- Comparison of two different anti-mullerian hormone measurement methods and evaluation of anti-mullerian hormone in polycystic ovary syndrome
- The evaluation of the relationship between anti angiotensin type I antibodies in hypertensive patients undergoing kidney transplantation
- Evaluation of neopterin, oxidative stress, and immune system in silicosis
- Assessment of lipocalin-1, resistin, cathepsin-D, neurokinin A, agmatine, NGF, and BDNF serum levels in children with Autism Spectrum Disorder
- Regulatory nexus in inflammation, tissue repair and immune modulation in Crimean-Congo hemorrhagic fever: PTX3, FGF2 and TNFAIP6
- Pasteur effect in leukocyte energy metabolism of patients with mild, moderate, and severe COVID-19
- Thiol-disulfide homeostasis and ischemia-modified albumin in patients with sepsis
- Myotonic dystrophy type 1 and oxidative imbalance: evaluation of ischemia-modified albumin and oxidant stress
- Antioxidant and alpha-glucosidase inhibitory activities of flavonoids isolated from fermented leaves of Camellia chrysantha (Hu) Tuyama
- Examination of the apelin signaling pathway in acetaminophen-induced hepatotoxicity in rats
- Integrating network pharmacology, in silico molecular docking and experimental validation to explain the anticancer, apoptotic, and anti-metastatic effects of cosmosiin natural product against human lung carcinoma
- Validation of Protein A chromatography: orthogonal method with size exclusion chromatography validation for mAb titer analysis
- The evaluation of the efficiency of Atellica UAS800 in detecting pathogens (rod, cocci) causing urinary tract infection
- Case Report
- Exploring inherited vitamin B responsive disorders in the Moroccan population: cutting-edge diagnosis via GC-MS profiling
- Letter to the Editor
- Letter to the Editor: “Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase”
Articles in the same Issue
- Frontmatter
- Review Article
- Unveiling the hidden clinical and economic impact of preanalytical errors
- Research Articles
- To explore the role of hsa_circ_0053004/hsa-miR-646/CBX2 in diabetic retinopathy based on bioinformatics analysis and experimental verification
- Study on the LINC00578/miR-495-3p/RNF8 axis regulating breast cancer progression
- Comparison of two different anti-mullerian hormone measurement methods and evaluation of anti-mullerian hormone in polycystic ovary syndrome
- The evaluation of the relationship between anti angiotensin type I antibodies in hypertensive patients undergoing kidney transplantation
- Evaluation of neopterin, oxidative stress, and immune system in silicosis
- Assessment of lipocalin-1, resistin, cathepsin-D, neurokinin A, agmatine, NGF, and BDNF serum levels in children with Autism Spectrum Disorder
- Regulatory nexus in inflammation, tissue repair and immune modulation in Crimean-Congo hemorrhagic fever: PTX3, FGF2 and TNFAIP6
- Pasteur effect in leukocyte energy metabolism of patients with mild, moderate, and severe COVID-19
- Thiol-disulfide homeostasis and ischemia-modified albumin in patients with sepsis
- Myotonic dystrophy type 1 and oxidative imbalance: evaluation of ischemia-modified albumin and oxidant stress
- Antioxidant and alpha-glucosidase inhibitory activities of flavonoids isolated from fermented leaves of Camellia chrysantha (Hu) Tuyama
- Examination of the apelin signaling pathway in acetaminophen-induced hepatotoxicity in rats
- Integrating network pharmacology, in silico molecular docking and experimental validation to explain the anticancer, apoptotic, and anti-metastatic effects of cosmosiin natural product against human lung carcinoma
- Validation of Protein A chromatography: orthogonal method with size exclusion chromatography validation for mAb titer analysis
- The evaluation of the efficiency of Atellica UAS800 in detecting pathogens (rod, cocci) causing urinary tract infection
- Case Report
- Exploring inherited vitamin B responsive disorders in the Moroccan population: cutting-edge diagnosis via GC-MS profiling
- Letter to the Editor
- Letter to the Editor: “Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase”


