Abstract
Objectives
The Pasteur effect in cellular energy metabolism is defined as the switch to anaerobic glycolysis due to alterations in ambient oxygen concentration or inhibition of the electron transport chain (ETC) and oxidative phosphorylation. This study aimed to determine the effect of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection on the energy metabolism of leukocytes among Coronavirus Disease 2019 (COVID-19) patients at various stages.
Methods
A total of 90 cases were analyzed in asymptomatic, mild, moderate, and severe COVID-19 patients. Levels of aerobic glycolysis intermediates and products of anaerobic glycolysis were measured in leukocytes with liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Results
A decrease in the rate of aerobic glycolysis was found as the disease progressed (p-values<0.001), while the anaerobic glycolysis rate was unchanged in all study groups because lactate levels had no difference between asymptomatic, mild, moderate, and severe groups (p=0.754). Moreover, the ratios of lactate to Tricarboxylic Acid (TCA) cycle parameters demonstrated a significant reduction in the rate of aerobic glycolysis of COVID-19 patients at different stages (p-values<0.001). Furthermore, Receiver Operating Characteristic (ROC) curve analysis for citrate, alpha-ketoglutarate, fumarate, lactate, lactate/citrate, lactate/alpha-ketoglutarate, and lactate/fumarate had significant cut-off values in Moderate and Severe patients (p<0.001).
Conclusions
Our results suggest that the Pasteur effect from aerobic to anaerobic glycolysis was shown to be induced as the severity of COVID-19 progresses.
Introduction
Metabolism involves the synthesis and breakdown of molecules in living organisms, both in normal and pathological conditions. The primary metabolic pathway in healthy cells is aerobic glycolysis. Dietary carbohydrates, lipids, and proteins are broken down into their monomers and converted into acetyl-CoA, a common intermediate, before being oxidized in the tricarboxylic acid (TCA) cycle. This is followed by the electron transport chain (ETC) and oxidative phosphorylation (OxP) for adenosine triphosphate (ATP) synthesis [1], 2].
Anaerobic glycolysis is a catabolic pathway that occurs in cells lacking mitochondria, such as erythrocytes and lens tissue, as well as in muscle cells during especially intense training. This pathway results in the formation of lactate through the lactate dehydrogenase (LDH) enzyme, providing less ATP per glucose molecule. It is important to note that this process occurs without the presence of an adequate oxygen supply. Therefore, anaerobic glycolysis leads to the consumption of 15–16 times more substrate (depending on the shuttle system) than aerobic glycolysis to produce the same amount of ATP [3], [4], [5], [6], [7], [8].
Cells exhibit altered metabolic pathways in various pathological conditions, resulting in different metabolic effects. The Pasteur effect is a metabolic phenomenon observed in cancer cells, certain bacterial and parasitic cells, and healthy skeletal muscle cells during intensive training. It is characterized by a shift towards anaerobic glycolysis depending on the oxygen concentration of the surrounding environment and/or due to inhibition of ETS and OxP [3], [4], [5], [6], [7], [8], [9].
Studies have demonstrated that the fundamental metabolic pathways involved in energy production remain consistent across both normal and pathological conditions. However, the consumption rates of these metabolic pathways can vary depending on the situation, such as in cases of hematological tumors, metabolic diseases, or infectious diseases [10], [11], [12], [13]. Leukocytes are one of the main formed components of blood and play a role in inducing immune response against infection [10], [11], [12], [13], [14].
Coronavirus 2019 (COVID-19) is a severe acute respiratory syndrome caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [15]. SARS-CoV-2 belongs to the Coronaviridae family, which includes large, enveloped, single-stranded RNA viruses. SARS-CoV-2 infection can cause a range of symptoms, including fever, cough, headache, pneumonia, dyspnea, chest pain, malaise, and multiple organ failure [15], [16], [17]. Patients with COVID-19 can be classified as asymptomatic, mild, moderate, or severe based on the severity of their illness [15], [16], [17]. Numerous scientific studies have reported variations in routine laboratory results, particularly in hematological and metabolic parameters, that allow checking the status of SARS-CoV-2 infection because the hematopoietic system and metabolic pathways are significantly affected by the evolution of COVID-19 [15].
In this study, we aimed to investigate the Pasteur effect in COVID-19 patients with different stages by assessing aerobic and anaerobic energy metabolism parameters, including citrate, α-ketoglutarate, fumarate, and lactate levels in leukocytes.
Materials and methods
This study was carried out at the Gulhane Training and Research Hospital (GTRH) with Ethical Approval of the University of Health Sciences’ Ethics Committee (November 30, 2020, numbered 2020-477) and by the permission of the Scientific Research Platform underlined by the Ministry of Health (2020-11-20T12_01_17) and the Medical Board of the GTRH (18.02.2021/2–24). The samples from COVID-19 patients were collected between December 2020 – April 2021, and the study was conducted between May – July 2023. Using the statistical power analysis program, G*Power V3.1.9, a total of 76 patients were divided into four groups, each consisting of at least 19 patients, with the acceptance of a 95 % confidence interval, 95 % power value, and 0.5 effect size. Written informed consent was obtained from all patients.
Levels of aerobic glycolysis intermediates, including citrate, alpha-ketoglutarate (AKG), and fumarate, as well as lactate, the end product of anaerobic glycolysis, were analyzed in leukocytes from COVID-19 patients. We also measured serum LDH levels in all patient groups.
Subjects
The asymptomatic group (As, n=22) consisted of patients who were examined with suspicion of COVID-19 due to filiation, tested positive by PCR, and were followed as outpatients without any clinical symptoms and/or radiological findings [18].
The mild group (Mi, n=24) contained patients with mild influenza-like symptoms (fever, cough, headache, sore throat, malaise, muscle pain, but no dyspnea), no radiological findings or minimal lung infiltration, and oxygen saturation measured by pulse oximetry (SpO2)≥93 % on room air at sea level and patients who received inpatient treatment without supplemental oxygen therapy [18].
The moderate group (Mo, n=22) contains patients with lower respiratory disease during clinical examination (respiratory rate≥24 breaths/min) or radiological diagnosis showing moderate lung infiltration and SpO2≤93 on room air at sea level and patients who received inpatient treatment with supplemental oxygen therapy [18].
The severe group (Se, n=22) included patients who developed acute respiratory distress syndrome (ARDS), had SpO2<93 on room air at sea level, had a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2)<300 mmHg, received inpatient treatment with high-flow nasal cannula oxygen therapy or who were connected to mechanical ventilation for treatment [18].
Sample preparation for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
Venous blood samples (10 mL) were collected from patients and stored in ethylenediaminetetraacetate (EDTA)-coated tubes for peripheral blood mononuclear cells (PBMCs) isolation. PBMCs were isolated using Ficoll-hypaque (Capricorn Scientific GmbH, Ebsdorfergrund, Germany) density gradient centrifugation according to the manufacturer’s instructions. The fractionated cells were stored at −20 °C until the experiment.
LC-MS/MS analysis was performed using the JASEM Organic Acid LC-MS/MS Analysis Kit (JASEM, Istanbul, Turkey). 100 µL of the samples were transferred to a centrifuge tube. Then, 50 µL of internal standard and 350 µL of extraction solution (Reagent-1) were added. The mixture was vortexed for 10 s and centrifuged at 1,800×g for 10 min. The resulting supernatant was collected into a new vial, and 10 µL of the sample was loaded onto an LC-MS/MS system.
Analytical procedure
LC-MS/MS analysis was performed using the Agilent 6,470 Triple Quad LC-MS/MS system (Table 1) coupled with the 1,290 Binary Pump system and 1,290 Autosampler system from Agilent Technologies, Inc., USA. The sensitivity limits were tested by measuring different concentrations of citrate, α-ketoglutarate, fumarate, and lactate through constant dilution of the stock solution. Tables 1 and 2 present the LC-MS/MS parameters with multiple reaction monitoring (MRM) transitions. The limit of quantification (LOQ) values for citrate, α-ketoglutarate, fumarate, and lactate were calculated and found to be 0.39, 0.61, 0.06, and 3.54 mg/L, respectively. The recovery assay values for these four analytes ranged between 75.9 and 118 %.
Parameters and conditions of each recommended setpoint for the Agilent 6470 LC-MS/MS Mass Spectrometer System.
| Parameters | Conditions |
|---|---|
| Ion source | ESI (Agilent Jet Stream) |
| Polarity | Positive/Negative |
| Gas temperature | 150 °C |
| Gas flow | 10 L/min |
| Nebulizer pressure | 40 psi |
| Sheath gas temperature | 400 °C |
| Sheath gas flow | 10 L/min |
| Capillary voltage | 2500 V (positive)/2500 V (negative) |
| Nozzle voltage | 0 V |
| Resolution MS1 and MS2 | Unit resolution |
The precursor ions, product ions, dwell time, fragmentor voltage (FV), collision energy (CE), collision cell acceleration voltage (CAV), and polarity of each measured analyte.
| Analyte | Precursor ion, m/z | Product ion, m/z | Dwell time, ms | FV, V | CE, eV | CAV, V | Polarity |
|---|---|---|---|---|---|---|---|
| Citrate | 191 | 86.9 | 10 | 90 | 14 | 7 | Negative |
| α-ketoglutarate | 145 | 100.9 | 10 | 70 | 2 | 7 | Negative |
| Fumarate | 114.9 | 71 | 5 | 90 | 2 | 7 | Negative |
| Lactate | 89 | 45 | 10 | 110 | 7 | 7 | Negative |
| Pyruvate-ISTD | 88 | 44.1 | 10 | 100 | 4 | 7 | Negative |
| Glutarate-ISTD | 134.9 | 115.8 | 10 | 60 | 8 | 7 | Negative |
| Fumarate-ISTD | 3,000 | 89.1 | 10 | 90 | 2 | 7 | Negative |
| Lactate-ISTD | 92 | 45.1 | 100 | 85 | 10 | 7 | Negative |
-
ISTD, internal standard.
Statistical analysis
Statistical analyses were performed with SPSS 21.0 (IBM, Inc., USA). The normality of variables was assessed using the Shapiro-Wilks test. The aerobic/anaerobic glycolysis parameters of study groups were analyzed using the Kruskal-Wallis test. Mann-Whitney U test was applied for pairwise comparison of the parameters following a Kruskal-Wallis test. Receiver Operating Characteristic (ROC) curve analyses were performed for aerobic and anaerobic glycolysis markers according to the clinical severity of two groups of COVID-19 patients (one group with As and Mi disease and the other with Mo and Se disease). p-Values<0.05 were accepted as significant.
Results
This study analyzed a total of 90 cases, comprising 22 asymptomatic (As), 24 mild (Mi), 22 moderate (Mo), and 22 severe (Se) COVID-19 patients. The levels of aerobic glycolysis intermediates, including citrate, α-ketoglutarate (AKG), and fumarate, as well as anaerobic glycolysis end product lactate levels, were reported for all study groups as medians and interquartile ranges (25–75 %), along with statistical significance information, in Table 3.
Median and interquartile range (25–75 %) levels of leukocyte aerobic glycolysis intermediates, citrate, alpha-ketoglutarate (AKG), and fumarate as well as anaerobic glycolysis end product, lactate, for all study groups.
| Groups | Asymptomatic, As | Mild, Mi | Moderate, Mo | Severe, Se | Total | p-Values | |
|---|---|---|---|---|---|---|---|
| Parameters, µg/L/PBMC | |||||||
| Citratea | Sigb | 18.00 (12.71–30.16) | 2.58 (1.86–6.59) | 0.82 (0.53–1.46) | 1.34 (0.84–2.47) | 2.27 (1.03–11.87) | <0.001 |
| AKGa | Sigb | 4.31 (3.84–6.58) | 2.83 (1.90–5.21) | 1.39 (0.82–2.83) | 1.63 (0.84–2.20) | 2.36 (1.33–4.26) | <0.001 |
| Fumaratea | Sigc | 1.08 (0.61–1.96) | 0.43 (0.26–1.05) | 0.25 (0.17–0.60) | 0.30 (0.18–0.46) | 0.45 (0.21–0.97) | <0.001 |
| Lactatea | 3.44 (2.06–7.61) | 3.16 (1.74–8.67) | 3.29 (2.32–7.59) | 4.18 (2.85–9.87) | 3.51 (2.21–7.83) | 0.724 | |
| Serum LDH | Sigd | 188 (153–220) | 235 (197–345) | 362 (269–449) | 438 (323–698) | 278 (213–412) | <0.001 |
| Lactate/Citrate | Sigb | 0.20 (0.11–0.45) | 1.06 (0.76–1.80) | 4.24 (1.31–5.95) | 3.19 (2.26–4.09) | 1.77 (0.64–3.46) | <0.001 |
| Lactate/AKG | Sige | 0.76 (0.50–1.65) | 1.30 (0.52–2.79) | 2.99 (0.87–4.99) | 3.30 (1.37–7.51) | 1.54 (0.80–3.50) | <0.001 |
| Lactate/Fumarate | Sigf | 3.53 (2.64–4.89) | 8.27 (4.74–10.56) | 11.60 (5.44–21.82) | 19.94 (7.36–24.93) | 8.38 (4.46–18.72) | <0.001 |
| AKG/Fumarate | 3.92 (3.30–6.57) | 5.90 (3.42–13.06) | 5.79 (1.83–8.93) | 5.45 (2.27–12.34) | 4.87 (3.10–8.04) | 0.586 | |
-
Sig: Denotes statistical significance with p-values < 0.05 reached from the Mann-Whitney U test following a Kruskal-Wallis test; a Citrate, α-ketoglutarate (AKG); fumarate, and lactate values were corrected according to peripheral blood mononuclear cell (PBMC) counts (106 leucocytes/mm3); b As – Mi, As – Mo, As – Se; Mi – Mo, Mi – Se; c As – Mi, As – Mo, As – Se; d As – Mi, As – Mo, As – Se; Mi – Mo, Mi – Se; Mo – Se; e As – Mo, As – Se; Mi – Mo, Mi – Se; f As – Mi, As – Mo, As – Se; Mi – Se.
A statistically significant decrease was observed in all aerobic glycolysis parameters, including citrate, AKG, and fumarate levels, with prognosis severity of COVID-19 patients (Table 3, Supplemental Figure 1A–C, p-values<0.001). As seen in Table 3 and Supplemental Figure 1A, citrate levels were reduced by 7–22 times while both AKG and fumarate levels were dropped by two–four folds between As and Mi, Mo, and Se groups, individually (p-values<0.001). These results indicated a significant reduction in the rate of aerobic glycolysis with increasing disease severity. However, the statistical analysis of an anaerobic glycolysis parameter, lactate levels, reported no significant difference among all study groups (Table 3, Supplemental Figure 1D, p-value=0.724>0.05). Serum LDH activities rose considerably as the severity of the disease increased (Table 3), reflecting enhanced anaerobic catabolism of glucose in the body.
A significant reduction was also found in the citrate and AKG levels of Mo and Se groups when compared with those in the Mi group (Table 3, Supplemental Figure 1A and B, p-values<0.01). We further calculated the ratios of lactate to the parameters, including citrate, AKG, and fumarate, as well as the ratio of AKG to fumarate, separately to better evaluate the catabolic reprogramming in leukocyte metabolism. Lactate/citrate and lactate/fumarate ratios were significantly increased in Mi, Mo, and Se groups as compared to those in As group (Figure 1A–C, p-values<0.001) while lactate/AKG ratios were significantly higher in Mo and Se but, not in Mi, than As group (Figure 1B, p-values<0.001). Our results, in general, indicated that lactate/citrate, lactate/AKG, and lactate/fumarate ratios were found to be significantly higher as the severity of the disease increased (Figure 1A–C, p-values<0.001). Although the comparisons of parameter measurements proposed similar conclusions (Table 3, Supplemental Figure 1), the calculated ratios (Table 3, Figure 1) recurrently revealed a remarkable decrease in the rate of aerobic glycolysis within the leukocyte metabolism of COVID-19-patients with different stages. However, we found no variation in AKG/fumarate ratios amongst the study groups (Figure 1D, p-value=0.586>0.05).
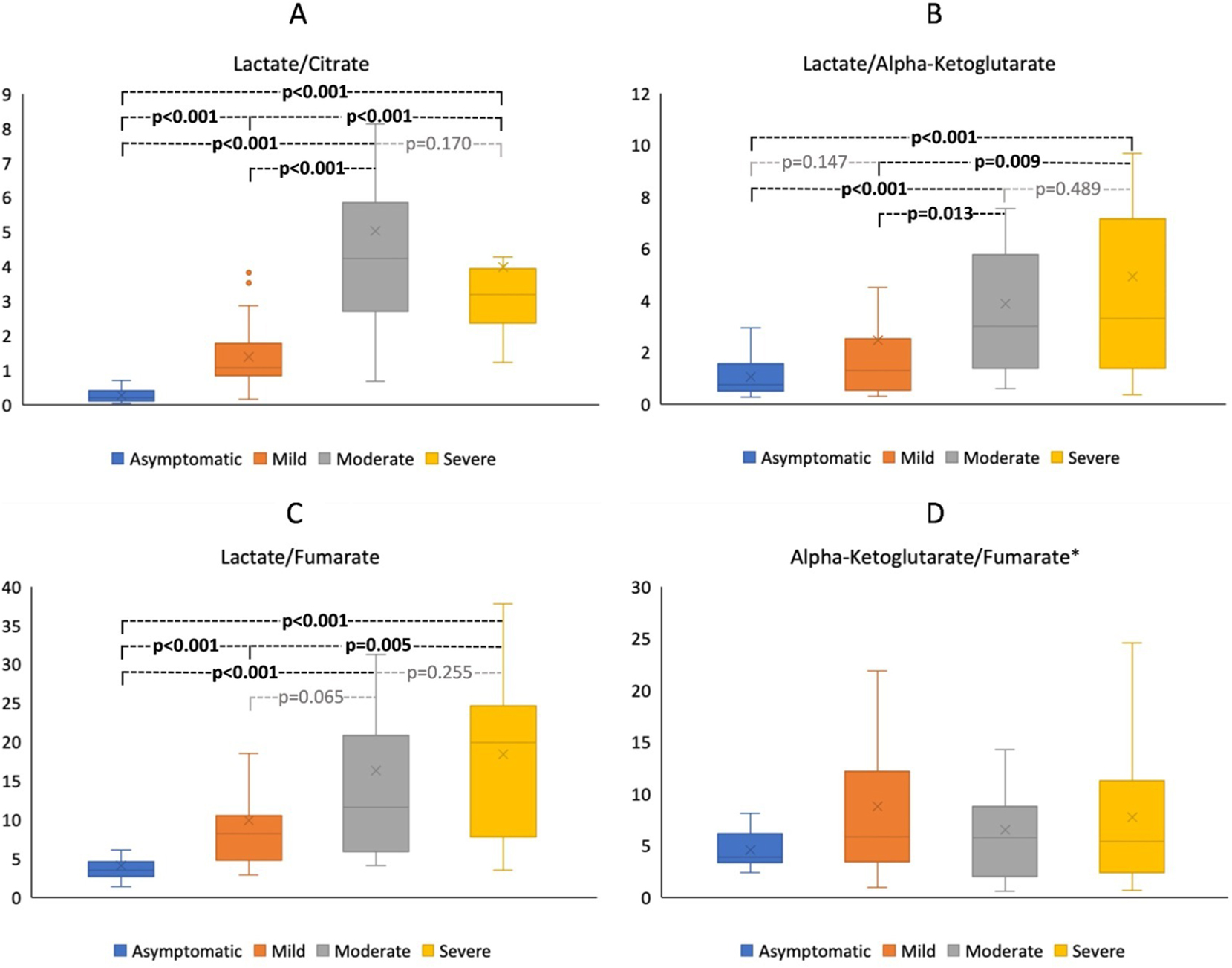
The comparison of lactate/citrate (A) and lactate/alpha-ketoglutarate (B), lactate/fumarate (C), and alpha-ketoglutarate/fumarate (D) ratios in leukocytes of patients with asymptomatic, mild, moderate and severe COVID-19 infection. p-Values were reached from the Mann-Whitney U test following a Kruskal-Wallis test. p-Values<0.05 were considered as significant. *No statistical significance was observed between groups for alpha-ketoglutarate/fumarate ratios.
ROC curve analyses were performed to evaluate the area under the curve (AUC) and cut-off values of aerobic and anaerobic glycolysis parameters in determining the severity of COVID-19. The cut-off points that predicted the severity of COVID-19 patients (between As+Mi and Mo+Se) in the ROC curve using the Youden’s index for citrate and for lactate/citrate ratio were <1.85 μg/L/PBMC (sensitivity 77.3 %, specificity 89.1 %, AUC: 0.891, CI 95 %: 0.824–0.957) and >1.85 μg/L/PBMC (sensitivity 86.4 %, specificity 91.3 %, AUC: 0.941, CI 95 %: 0.894–0.988), respectively. Moreover, the ROC analysis for AKG, fumarate, ratio of lactate/AKG, and lactate/fumarate also estimated significant threshold values between As+Mi and Mo+Se COVID-19 patients, and the data were presented in Figure 2 and Table 4.

ROC curves were constructed to illustrate the AUC for aerobic and anaerobic glycolysis markers associated with the severity of COVID-19. The prognostic value of AKG, fumarate, lactate, and citrate levels (A), and the ratios of lactate to the mentioned parameters in determining the degree of severity in patients with COVID-19 and the proportion of AKG to fumarate to demonstrate the integrity of the TCA cycle (B).
Receiver Operating Characteristic (ROC) analysis data for alpha-ketoglutarate (AKG), fumarate, and citrate.
| Parameters | Cut off, µg/L/PBMC | Sensitivity, % | Specificity, % | AUC | CI 95 % | p-Values |
|---|---|---|---|---|---|---|
| AKG | <3.60 | 93.2 | 63.0 | 0.833 | 0.750–0.916 | <0.001 |
| Fumarate | <0.52 | 75.0 | 67.4 | 0.764 | 0.665–0.863 | <0.001 |
| Citrate | <1.85 | 77.3 | 89.1 | 0.891 | 0.824–0.957 | <0.001 |
| Lactate/Citrate | >1.85 | 86.4 | 91.3 | 0.941 | 0.894–0.988 | <0.001 |
| Lactate/AKG | >0.84 | 95.5 | 50.0 | 0.786 | 0.691–0.880 | <0.001 |
| Lactate/Fumarate | >10.58 | 61.4 | 89.1 | 0.809 | 0.721–0.896 | <0.001 |
-
Lactate and AKG/Fumarate values were not presented due to p-values = 0.50 and 0.96, respectively; AUC, area under the curve; CI, confidence interval.
Discussion
Different metabolic adaptations have been identified in human bodies with various cancer types, pathologies, and metabolic diseases or in healthy cells (for instance, muscle tissues during vigorous activity) as a result of changing environmental conditions. The Pasteur effect is such a metabolic adaptation characterized by a switch from aerobic glycolysis to anaerobic glycolysis in response to low oxygen levels of the environment, interference with the ETS and OP, or both [3], [4], [5], [6], [7], [8], [9]. Consequently, net ATP gain from one mol of glucose decreases while the excessive substrate utilization and lactate concentration in the cells increase [3], [4], [5], [6], [7], [8], [9], [10, 14]. Although there have been several studies on the host metabolism landscape of COVID-19 patients, metabolic regulation that occurs during different stages of infection has not been thoroughly investigated [15], [19], [20], [21], [22], [23], [24].
In this study, we measured citrate, AKG, fumarate, and lactate levels as indicators of aerobic and anaerobic glycolysis to determine the Pasteur effect in leukocytes of COVID-19 patients at different disease phases. The measured citrate, AKG, and fumarate levels, as well as the ratios of lactate to citrate, to AKG, and to fumarate, showed that the rate of aerobic glycolysis decreased with the progression of COVID-19 infection, whereas anaerobic glycolysis level did not significantly alter in all study groups (Table 3, Figure 1, and Supplemental Figure 1). In addition, serum LDH levels as a metabolic and prognostic biomarker of immune surveillance [25] increased significantly with disease severity and favored anaerobic glycolysis and respiratory failure during COVID-19 infection in our study (Table 3). The comparison of LDH levels in serum and leukocytes does not have to show a correlation [26]. Thus, the increase in serum LDH levels was not reflected in PBMS lactate levels in our study. Although patients in the Mo and Se groups were hospitalized and received medication and supplemental oxygen therapy, the fact that aerobic glycolysis reduced prominently with increasing disease severity can be considered evidence of hypoxemia in COVID-19 patients [24]. Thus, the metabolic switch from aerobic to anaerobic glycolysis reveals the induction of the Pasteur effect in progressive COVID-19 cases.
The induction of the Pasteur effect in COVID-19 patients might be due to the high energy demands of invading SARS-CoV-2 viruses for viral replication and immune response regulation [27]. Although anaerobic glycolysis yields less ATP than aerobic glycolysis for each molecule of glucose, it enables approximately 100 times faster synthesis of ATP than OP [28], 29]. Thus, the metabolism of COVID-19 patients shifted towards anaerobic glycolysis to supply energy for rapid viral proliferation, especially during the hyperinflammatory phase of the disease [28], 29].
Defective mitochondrial quality and function were also observed in diverse tissue cells of patients with COVID-19 [30], 31]. Although possible mitochondrial dysfunction due to COVID-19 infection could be responsible for the depression of aerobic glycolysis, our data revealed no changes in AKG/fumarate ratios across study groups (Figure 1D). The constant rate of AKG/fumarate suggested that the TCA cycle was not fully affected in terms of its ATP production function. This finding has also confirmed that there is no problem with the mitochondrial metabolism of pyruvate into acetyl CoA, which is directed towards the TCA cycle, but there was a shift to lactate production, indicating anaerobic glycolysis [32]. In our study, the shift to anaerobic glycolysis was supported by the increase in lactate to AKG, citrate, and fumarate ratios with disease severity.
Reduced levels of aerobic glycolysis intermediates in COVID-19 patients suggest the need for dietary support, particularly with citrate, AKG, and fumarate. Our assumption was confirmed by Agarwal et al. 2022, who showed that dietary supplementation of SARS-CoV2-infected mice with AKG significantly alleviated pro-inflammatory and pro-thrombotic responses in their leukocytes and platelets [33]. Furthermore, AKG rescued the animals from COVID-19 infection and restored normal SpO2 levels in the blood circulation [33]. One another study performed by Pérez-Hernández et al. indicated that the treatment of human monocytes with fumarate considerably enhanced the capacity of mitochondria for energy production [34]. Therefore, support for COVID-19 patients with supplements rich in TCA cycle intermediates such as citrate, AKG, and fumarate may not only speed up the healing time from the disease but also compensate for the decreased rate of aerobic glycolysis as reported in our study.
A recent study by Ceballos et al. 2022 characterized plasma metabolic and cytokine profiles at COVID-19 onset [35]. Lactate, aspartate, and l-tryptophan metabolites were differentially expressed between plasma samples of COVID-positive and COVID-negative patients as well as between severe and moderate groups, while citrate, citrulline, isocitrate, l-glycine, and cysteine glutathione were dysregulated between plasma samples of symptomatic mild and asymptomatic as well as severe and moderate groups [35]. In consistency with our results in leukocytes (Table 3), there was a substantial downregulation of citrate levels in samples of the Mi, Mo, and Se group as compared to the As group [35]. Additionally, a significant reduction was also detected in the AKG levels of the Mo and Se groups in comparison with those in the As and Mi groups (Table 3). These findings strongly supported that the rate of aerobic glycolysis significantly decreased as the severity of the disease increased [35]. Although no significant difference was found in leukocyte AKG levels between Mo and Se groups (Table 3), it was suggested as a downregulated intermediate in the pairwise comparison of Se and Mo patients’ plasma samples [35].
Moreover, fumarate was upregulated in COVID-19-negative patients compared to COVID-19-positive ones, while it was not significant when comparing patients with COVID-19 at different stages [35]. However, we have shown a significant decrease in leukocyte fumarate levels of the Mi, Mo, and Se groups as compared to the As group (Table 3). Surprisingly, lactate levels were significantly lower in plasma samples of the Se group than in the Mo group [34], for which we could not find any significant difference in isolated PBMC samples of these two groups (Table 3). Our results indicated that the rate of aerobic glycolysis declined in the later stages of the disease despite the medication and supplemental oxygen therapy. Although plasma levels of some common metabolites such as citrate, AKG, fumarate, and lactate were checked in previous studies, the Pasteur effect in leukocyte energy metabolism of COVID-19 patients was first declared by this study.
ROC, sensitivity, specificity, and cut-off values were determined for the levels and ratios of aerobic and anaerobic glycolysis markers. The predicted threshold values for the citrate level (<1.85 μg/L/PBMC, p<0.001) and ratio of lactate to citrate (>1.85, p<0.001) appeared to show metabolic reprogramming in COVID-19 patients who progressed from Mi to Se state (Figure 2 and Table 4). Particularly, lactate to citrate ratio of >1.85 might be applied to clinical practice to have the best adequate discriminatory power for the prognosis severity of COVID-19. However, several studies have suggested certain hematologic parameters (such as alanine aminotransferase (ALT/SGPT), neutrophil-to-leukocyte ratio and high-sensitive-C-reactive protein [36] and serum metabolome (including d-fructose, succinate and 2-hydroxybutyrate) [37] as a predictor of COVID-19 severity, none of those have mentioned the lactate/citrate ratio as a biochemical indicator of metabolic alterations in predicting the progression of Mo to Se degrees of severity in COVID-19 patients [36], [37], [38]. Therefore, the present study is expected to contribute to existing literature in this regard.
In conclusion, COVID-19 might not only suggest impaired immune function and cytotoxicity but also explain the Pasteur effect of SARS-CoV-2 infection on the energy metabolism of leukocytes among different COVID-19 severity groups. However, further studies are needed to elucidate the molecular mechanisms underpinning adaptations in carbohydrate, lipid, and protein metabolisms during COVID-19. Ultimately, the knowledge gained of SARS-CoV-2-induced metabolic reprogramming would enable the development of novel therapeutic strategies against the COVID-19 pandemic.
-
Research ethics: University of Health Sciences’ Ethics Committee (November 30, 2020, numbered 2020-477).
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data can be obtained on request from the corresponding author.
References
1. Krohn, KA, Link, JM, Mason, RP. Molecular imaging of hypoxia. J Nucl Med 2008;49:129S–48S. https://doi.org/10.2967/jnumed.107.045914.Search in Google Scholar PubMed
2. Baker, JM, Nederveen, JP, Parise, G. Aerobic exercise in humans mobilizes HSCs in an intensity-dependent manner. J Appl Physiol 2017;122:182–90. https://doi.org/10.1152/japplphysiol.00696.2016.Search in Google Scholar PubMed PubMed Central
3. Tielens, AG. Energy generation in parasitic helminths. Parasitol Today 1994;10:346–52. https://doi.org/10.1016/0169-4758(94)90245-3.Search in Google Scholar PubMed
4. Tielens, AG, van de Pas, FA, van den Heuvel, JM, van den Bergh, SG. The aerobic energy metabolism of Schistosoma mansoni miracidia. Mol Biochem Parasitol 1991;46:181–4. https://doi.org/10.1016/0166-6851(91)90211-n.Search in Google Scholar PubMed
5. Summers, JE, Ratcliffe, RG, Jackson, MB. Anoxia tolerance in the aquatic monocot Potamogeton pectinatus absence of oxygen stimulates elongation in association with an unusually large pasteur effect. J Exp Bot 2000;51:1413–22. https://doi.org/10.1093/jexbot/51.349.1413.Search in Google Scholar
6. Boyunaga, H, Schmitz, MG, Brouwers, JF, Van Hellemond, JJ, Tielens, AG. Fasciola hepatica miracidia are dependent on respiration and endogenous glycogen degradation for their energy generation. Parasitology 2001;122:169–73. https://doi.org/10.1017/s0031182001007211.Search in Google Scholar PubMed
7. Epstein, T, Xu, L, Gillies, RJ, Gatenby, RA. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metabol 2014;2:7. https://doi.org/10.1186/2049-3002-2-7.Search in Google Scholar PubMed PubMed Central
8. Harvey, RA, Ferrier, DR. Lippincott’s illustrated reviews: biochemistry, 5th ed. Philadelphia: Wolters Kluwer Health; 2011:520 p.Search in Google Scholar
9. Pasteur, L. Expériences et vues nouvelles sur la nature des fermentations. Comptes Rendus 1861;52:1260–4.Search in Google Scholar
10. Gibellini, L, De Biasi, S, Paolini, A, Borella, R, Boraldi, F, Mattioli, M, et al.. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol Med 2020;12:e13001. https://doi.org/10.15252/emmm.202013001.Search in Google Scholar PubMed PubMed Central
11. Beck, WS, Valentine, WN. The aerobic carbohydrate metabolism of leukocytes in health and leukemia. I. Glycolysis and respiration. Cancer Res 1952;12:818–22.Search in Google Scholar
12. Webster, KA. Evolution of the coordinate regulation of glycolytic enzyme genes by hypoxia. J Exp Biol 2003;206:2911–22. https://doi.org/10.1242/jeb.00516.Search in Google Scholar PubMed
13. Hume, DA, Weidemann, MJ. Role and regulation of glucose metabolism in proliferating cells. J Natl Cancer Inst 1979;62:3–8.Search in Google Scholar
14. Kenar, L, Boyunaga, H, Serdar, M, Karayilanoglu, T, Erbil, MK. Effect of nitrogen mustard, a vesicant agent, on lymphocyte energy metabolism. Clin Chem Lab Med 2006;44:1253–7. https://doi.org/10.1515/cclm.2006.220.Search in Google Scholar
15. Layla, KN, Yeasmin, S, Khan, SA, Shaila, KN, Azad, AB, Ahmad, R, et al.. White blood cell profile among different clinical stages of COVID-19 patients. EJMED 2021;3:73–6. https://doi.org/10.24018/ejmed.2021.3.5.1051.Search in Google Scholar
16. Zhu, N, Zhang, D, Wang, W, Li, X, Yang, B, Song, J, et al.. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. https://doi.org/10.1056/nejmoa2001017.Search in Google Scholar PubMed PubMed Central
17. Rabaan, AA, Smajlović, S, Tombuloglu, H, Ćordić, S, Hajdarević, A, Kudić, N, et al.. SARS-CoV-2 infection and multi-organ system damage: a review. Bosn J Basic Med Sci 2022. https://doi.org/10.17305/bjbms.2022.7762. [Internet] [cited 2024 Jun 28]; Available from: https://www.bjbms.org/ojs/index.php/bjbms/article/view/7762.Search in Google Scholar PubMed PubMed Central
18. Ortatatli, M, Fatsa, T, Mulazimoglu, DD, Oren, S, Artuk, C, Hosbul, T, et al.. Role of vitamin D, ACE2, and the proteases as TMPRSS2 and furin on SARS-CoV-2 pathogenesis and COVID-19 severity. Arch Med Res 2023;54:223–30. https://doi.org/10.1016/j.arcmed.2023.02.002.Search in Google Scholar PubMed PubMed Central
19. Cheng, SC, Scicluna, BP, Arts, RJW, Gresnigt, MS, Lachmandas, E, Giamarellos-Bourboulis, EJ, et al.. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 2016;17:406–13. https://doi.org/10.1038/ni.3398.Search in Google Scholar PubMed
20. Japiassú, AM, Santiago, APSA, d’Avila J da, CP, Garcia-Souza, LF, Galina, A, Castro Faria-Neto, HC, et al.. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit Care Med 2011;39:1056–63. https://doi.org/10.1097/ccm.0b013e31820eda5c.Search in Google Scholar PubMed
21. Kramer, PA, Ravi, S, Chacko, B, Johnson, MS, Darley-Usmar, VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol 2014;2:206–10. https://doi.org/10.1016/j.redox.2013.12.026.Search in Google Scholar PubMed PubMed Central
22. Merad, M, Martin, JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol 2020;20:355–62. https://doi.org/10.1038/s41577-020-0331-4.Search in Google Scholar PubMed PubMed Central
23. Tay, MZ, Poh, CM, Rénia, L, MacAry, PA, Ng, LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74. https://doi.org/10.1038/s41577-020-0311-8.Search in Google Scholar PubMed PubMed Central
24. Swenson, KE, Hardin, CC. Pathophysiology of hypoxemia in COVID-19 lung disease. Clin Chest Med 2023;44:239–48. https://doi.org/10.1016/j.ccm.2022.11.007.Search in Google Scholar PubMed PubMed Central
25. Gupta, GS. The lactate and the lactate dehydrogenase in inflammatory diseases and major risk factors in COVID-19 patients. Inflammation 2022;45:2091–123. https://doi.org/10.1007/s10753-022-01680-7.Search in Google Scholar PubMed PubMed Central
26. Ghosh, K, Malik, K, Das, KC. Serum and leukocyte lactate dehydrogenase activity in leukaemias. Haematologia 1988;21:227–32.Search in Google Scholar
27. Shen, T, Wang, T. Metabolic reprogramming in COVID-19. Int J Mol Sci 2021;22:11475. https://doi.org/10.3390/ijms222111475.Search in Google Scholar PubMed PubMed Central
28. Melkonian, EA, Schury, MP. Biochemistry, anaerobic glycolysis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. [Internet] [cited 2024 Jun 28]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK546695/.Search in Google Scholar
29. Peek, CB, Levine, DC, Cedernaes, J, Taguchi, A, Kobayashi, Y, Tsai, SJ, et al.. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab 2017;25:86–92. https://doi.org/10.1016/j.cmet.2016.09.010.Search in Google Scholar PubMed PubMed Central
30. Ajaz, S, McPhail, MJ, Singh, KK, Mujib, S, Trovato, FM, Napoli, S, et al.. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol 2021;320:C57–65. https://doi.org/10.1152/ajpcell.00426.2020.Search in Google Scholar PubMed PubMed Central
31. Swain, O, Romano, SK, Miryala, R, Tsai, J, Parikh, V, Umanah, GKE. SARS-CoV-2 neuronal ınvasion and complications: potential mechanisms and therapeutic approaches. J Neurosci 2021;41:5338–49. https://doi.org/10.1523/jneurosci.3188-20.2021.Search in Google Scholar PubMed PubMed Central
32. Martínez-Reyes, I, Chandel, NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 2020;11:102. https://doi.org/10.1038/s41467-019-13668-3.Search in Google Scholar PubMed PubMed Central
33. Agarwal, S, Kaur, S, Asuru, TR, Joshi, G, Shrimali, NM, Singh, A, et al.. Dietary alpha-ketoglutarate inhibits SARS CoV-2 infection and rescues inflamed lungs to restore O2 saturation by inhibiting pAkt. Clin Transl Med 2022;12:e1041. https://doi.org/10.1002/ctm2.1041.Search in Google Scholar PubMed PubMed Central
34. Pérez-Hernández, CA, Moreno-Altamirano, MMB, López-Villegas, EO, Butkeviciute, E, Ali, M, Kronsteiner, B, et al.. Mitochondrial ultrastructure and activity are differentially regulated by glycolysis-krebs cycle-and microbiota-derived metabolites in monocytes. Biology (Basel) 2022;11:1132. https://doi.org/10.3390/biology11081132.Search in Google Scholar PubMed PubMed Central
35. Ceballos, FC, Virseda-Berdices, A, Resino, S, Ryan, P, Martínez-González, O, Peréz-García, F, et al.. Metabolic profiling at COVID-19 onset shows disease severity and sex-specific dysregulation. Front Immunol 2022;13:925558. https://doi.org/10.3389/fimmu.2022.925558.Search in Google Scholar PubMed PubMed Central
36. Angky, S, Vincentius, DS, Margaret, GH, Margareth Ayuni, TA, Vicky, SA. Hematologic parameters as predictor of COVID-19 severity. Jurnal Widya Medika 2021;7:100–15.Search in Google Scholar
37. Shi, D, Yan, R, Lv, L, Jiang, H, Lu, Y, Sheng, J, et al.. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism 2021;118:154739. https://doi.org/10.1016/j.metabol.2021.154739.Search in Google Scholar PubMed PubMed Central
38. Quraishi, E, Jibuaku, C, Lisik, D, Wennergren, G, Lötvall, J, Nyberg, F, et al.. Comparison of clinician diagnosis of COVID-19 with real-time polymerase chain reaction in an adult-representative population in Sweden. Respir Res 2023;24:10. https://doi.org/10.1186/s12931-023-02315-7.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/tjb-2024-0301).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Unveiling the hidden clinical and economic impact of preanalytical errors
- Research Articles
- To explore the role of hsa_circ_0053004/hsa-miR-646/CBX2 in diabetic retinopathy based on bioinformatics analysis and experimental verification
- Study on the LINC00578/miR-495-3p/RNF8 axis regulating breast cancer progression
- Comparison of two different anti-mullerian hormone measurement methods and evaluation of anti-mullerian hormone in polycystic ovary syndrome
- The evaluation of the relationship between anti angiotensin type I antibodies in hypertensive patients undergoing kidney transplantation
- Evaluation of neopterin, oxidative stress, and immune system in silicosis
- Assessment of lipocalin-1, resistin, cathepsin-D, neurokinin A, agmatine, NGF, and BDNF serum levels in children with Autism Spectrum Disorder
- Regulatory nexus in inflammation, tissue repair and immune modulation in Crimean-Congo hemorrhagic fever: PTX3, FGF2 and TNFAIP6
- Pasteur effect in leukocyte energy metabolism of patients with mild, moderate, and severe COVID-19
- Thiol-disulfide homeostasis and ischemia-modified albumin in patients with sepsis
- Myotonic dystrophy type 1 and oxidative imbalance: evaluation of ischemia-modified albumin and oxidant stress
- Antioxidant and alpha-glucosidase inhibitory activities of flavonoids isolated from fermented leaves of Camellia chrysantha (Hu) Tuyama
- Examination of the apelin signaling pathway in acetaminophen-induced hepatotoxicity in rats
- Integrating network pharmacology, in silico molecular docking and experimental validation to explain the anticancer, apoptotic, and anti-metastatic effects of cosmosiin natural product against human lung carcinoma
- Validation of Protein A chromatography: orthogonal method with size exclusion chromatography validation for mAb titer analysis
- The evaluation of the efficiency of Atellica UAS800 in detecting pathogens (rod, cocci) causing urinary tract infection
- Case Report
- Exploring inherited vitamin B responsive disorders in the Moroccan population: cutting-edge diagnosis via GC-MS profiling
- Letter to the Editor
- Letter to the Editor: “Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase”
Articles in the same Issue
- Frontmatter
- Review Article
- Unveiling the hidden clinical and economic impact of preanalytical errors
- Research Articles
- To explore the role of hsa_circ_0053004/hsa-miR-646/CBX2 in diabetic retinopathy based on bioinformatics analysis and experimental verification
- Study on the LINC00578/miR-495-3p/RNF8 axis regulating breast cancer progression
- Comparison of two different anti-mullerian hormone measurement methods and evaluation of anti-mullerian hormone in polycystic ovary syndrome
- The evaluation of the relationship between anti angiotensin type I antibodies in hypertensive patients undergoing kidney transplantation
- Evaluation of neopterin, oxidative stress, and immune system in silicosis
- Assessment of lipocalin-1, resistin, cathepsin-D, neurokinin A, agmatine, NGF, and BDNF serum levels in children with Autism Spectrum Disorder
- Regulatory nexus in inflammation, tissue repair and immune modulation in Crimean-Congo hemorrhagic fever: PTX3, FGF2 and TNFAIP6
- Pasteur effect in leukocyte energy metabolism of patients with mild, moderate, and severe COVID-19
- Thiol-disulfide homeostasis and ischemia-modified albumin in patients with sepsis
- Myotonic dystrophy type 1 and oxidative imbalance: evaluation of ischemia-modified albumin and oxidant stress
- Antioxidant and alpha-glucosidase inhibitory activities of flavonoids isolated from fermented leaves of Camellia chrysantha (Hu) Tuyama
- Examination of the apelin signaling pathway in acetaminophen-induced hepatotoxicity in rats
- Integrating network pharmacology, in silico molecular docking and experimental validation to explain the anticancer, apoptotic, and anti-metastatic effects of cosmosiin natural product against human lung carcinoma
- Validation of Protein A chromatography: orthogonal method with size exclusion chromatography validation for mAb titer analysis
- The evaluation of the efficiency of Atellica UAS800 in detecting pathogens (rod, cocci) causing urinary tract infection
- Case Report
- Exploring inherited vitamin B responsive disorders in the Moroccan population: cutting-edge diagnosis via GC-MS profiling
- Letter to the Editor
- Letter to the Editor: “Gene mining, recombinant expression and enzymatic characterization of N-acetylglucosamine deacetylase”


