Abstract
A poly(vinylidene fluoride) (PVDF) nanocomposite incorporated with polydopamine-coated reduced graphene oxide (rGO@PDOPA) nanosheets has been developed with high dielectric permittivity and low loss for electricity storage applications. Structural analysis indicates that PDOPA was successfully anchored onto rGOs through non-covalent linkage under mild conditions. The presence of PDOPA layer between rGO and PVDF can not only prevent the agglomeration and direct contact of original rGOs but also enhance the interaction between PVDF and rGO and microcapacitor formation. Compared to rGO/PVDF composites, higher dielectric permittivity and lower loss factor were achieved simultaneously in rGO@PDOPA/PVDF nanocomposites at low and moderate frequencies. The PDOPA interface layers are effective in modifying the dielectric characteristics of the composites to increase the dielectric permittivity without the introduction of loss mechanisms. This study demonstrates that PDOPA is an ideal interface layer for the development of new percolative dielectric composites with high dielectric permittivity and low loss.
1 Introduction
Electricity storage can overcome the mismatch and optimize the energy flows between supply and demand, especially for electricity generated by variable and renewable resources, such as water, wind and solar [1]. Among the principal electricity storage technologies, dielectric capacitors possess the advantage of high power density with extremely high charge and discharge rates [2], [3]. To increase the energy-storing ability of a capacitor, dielectric materials with high permittivity and low dielectric loss are highly desirable for future energy storage and management [4], [5], [6], [7], [8], [9].
Graphene/ferroelectric polymer composites are one of the prospective candidates in this regard as they exhibit high permittivity, ease of processing and light weight, which leads to the compact storage devices exhibiting high energy density [10], [11], [12]. More importantly, graphene possesses a flake shape with atomic or molecular thickness, ultrahigh aspect ratio and extremely high electron conductivity [13], [14], [15], [16], [17]. Furthermore, the geometry of graphene is prone to facilitate the microcapacitor formation in composites [11], [12]. Nevertheless, readily formed percolation pathways caused by the high aspect ratio of graphene can lead to an unexpected high dielectric loss. Moreover, poor compatibility between the pristine graphene without any polar group and polar ferroelectric polymers, such as poly(vinylidene fluoride) (PVDF), impedes the formation of a homogeneous composite and thus induces an adverse excessive agglomeration of graphene and vacancies at interfaces. The recent studies [18], [19], [20], [21] show that a thin insulator layer inserted between graphene and ferroelectric polymer can significantly improve the dispersion of graphene nanosheets as well as dielectric properties.
Polydopamine (PDOPA), a kind of bio-inspired polymer, has attracted more and more attention in recent years [22], [23], [24], [25]. The dopamine could self-polymerize and then adhere firmly to the surfaces in mild aqueous environments. The PDOPA can be coated non-selectively from solution onto virtually any surface, including graphene or polymers. Therefore, it is of interest as interface layer between graphene and ferroelectric polymers. Strong interactions between PDOPA and graphene or polymers are in favor of increased interfacial bonding. Besides, PDOPA insulator layer with adjustable thickness can easily anchor onto graphene surfaces under mild reaction conditions.
In the present study, we report the preparation and dielectric properties of hybrid percolative dielectric composites consisting of ferroelectric PVDF host and PDOPA-coated reduced graphene oxide (rGO@PDOPA) nanosheets as the fillers. The rGO@PDOPA nanosheets can be conveniently prepared in mild aqueous environments. Technically, PDOPA can be used in any percolative composite system as interface layers. PDOPA can form strong interactions between PVDF and graphene, which thus stabilizes the dispersion of graphene in PVDF host. The presence of PDOPA insulating layer also prevents the direct contact of graphene nanosheets and thus reduces the dielectric loss. The composites, denoted as rGO@PDOPA/PVDF, exhibit significantly improved dielectric permittivity and suppress loss compared to the rGO/PVDF composites. This makes the hybrid percolative composites suitable to develop novel, flexible and easy to process capacitors with relatively high dielectric permittivity and low loss for energy storage applications.
2 Materials and methods
2.1 Materials
Poly(vinylidene fluoride) (PVDF) with a melt flow index of 10 g/min was purchased from Shanghai 3F New Materials (China) and used as received. Graphite flakes were purchased from Beijing Nanopowder Science & Technology (China). Dopamine hydrochloride was obtained from Sinopharm Chemical Reagents (China). Other chemical reagents were of analytical grade purchased from Sinopharm Chemical Reagents (China) and used as received.
2.2 Preparation of rGO@PDOPA nanosheets
Firstly, graphene oxide (GO) was synthesized through a modified Hummers method from graphite flakes [16]. Afterwards, 100 mg GO was dissolved in 10 mm Tris-HCl (pH 8.5, 200 ml), followed by a sonication of 2 h at room temperature. Then, 400 mg of dopamine hydrochloride (2 mg/ml) was added into the above suspension and stirred for 30 min. The solution’s color changed to dark brown due to pH-induced oxidation. The PDOPA-coated GO (GO@PDOPA) nanosheets were centrifuged and rinsed with distilled water three times. Afterwards, an aqueous solution of hydrazine (0.4 g in 2 ml deionized water) was slowly added to the GO@PDOPA dispersion (1 mg ml-1, 100 ml), keeping stirring and refluxing for 6 h at 85°C. After multiple washes with distilled water and then drying in vacuum for 24 h at 60°C, rGO@PDOPA nanosheets could be obtained.
2.3 Preparation of rGO@PDOPA/PVDF composites
To fabricate the rGO@PDOPA/PVDF composites, rGO@PDOPA nanosheets were first dispersed in dimethylformamide with the aid of sonication at ambient temperature. Three hundred milligrams of PVDF powder was added to the above well-mixed solution under stirring, and the mixture was stirred vigorously overnight to give a homogeneous dispersion, which was then drop-casted on glass plates. The cast dispersions were first dried at 60°C for 6 h and subsequently peeled off the glass plates for further drying in vacuum at 80°C for 48 h. The solution-cast films were then hot pressed at 180°C and 10 MPa for 10 min to ensure the formation of uniform films around 0.15 mm in thickness and then cooled down to room temperature while maintaining the pressure.
2.4 Characterization
Infrared measurements were made on a Fourier transform infrared spectrometer (FTIR, Thermo Nicolet Nexus, USA) using KBr pellets. Thermogravimetric analysis (TGA) measurements were performed at a rate of 10°C min-1 to 800°C with a simultaneous thermal analyzer (Netzsch, STA 499C, Germany) under N2 flow. UV-Vis spectra in the wavelength range of 200–800 nm were recorded using a Shimadzu UV-2550 spectrophotometer (Japan). Atomic force microscopy (AFM) was performed with a commercial AFM (DI Nanoscope IV, USA) equipped with a tapping probe. Transmission electron microscopy (TEM, Joel JEM-2001F, Japan) images were obtained by placing a few drops of the dispersion on a copper grid and evaporating them at room temperature prior to observation. The cryogenic fracture surface morphologies of the rGO@PDOPA/PVDF composite films were examined by scanning electron microscopy (SEM) on an ULTRA PLUS-43-13 scanning electron microscope (Germany). The dielectric properties of the rGO@PDOPA/PVDF composite films were determined using a Hioki 3532-50 LCR meter (Japan) at ambient temperature. The composite films were coated with a silver film on the top and bottom surfaces of the films.
3 Results and discussion
3.1 Structural analysis of rGO@PDOPA hybrid nanosheets
Chemical characterizations of GO@PDOPA and rGO@PDOPA were carried out using FTIR spectroscopy, and the results are shown in Figure 1. The spectrum of PDOPA presents intense absorption peaks around 3426 cm-1 from catechol -OH groups and 1434 cm-1 from -NH2 groups. The peaks at 1646 cm-1 and ~1560 cm-1 can be assigned to an amide carbonyl-stretching mode and N-H shearing vibration of the amide group, respectively. For GO@PDOPA, the peak at 1717 cm-1 represents the stretching vibration of functional groups of C=O. For rGO@PDOPA, the peak from the carbonyl stretch at 1717 cm-1 disappears, while the peak at 1434 cm-1 attributed to -NH2 group of PDOPA still remains after hydrazine reduction. The FTIR analysis validates the successful wrapping of PDOPA onto GO surface and suggests that the reducing agent hydrazine has no significant influence on the surface coating layer of PDOPA.

FT-IR spectra of GO@PDOPA and rGO@PDOPA.
UV-Vis spectroscopy was utilized to monitor the reduction process of the hybrid nanosheets. As can be seen in Figure 2, the absorption peak of the GO@PDOPA dispersion at 225 nm red shifted to 270 nm after it was converted to rGO@PDOPA, while no visible peak could be detected within this region in the UV-Vis curve of PDOPA, suggesting the restoration of electronic conjugation in the basal plane of graphene upon reduction.

UV-vis spectra of PDOPA, GO@PDOPA and rGO@PDOPA.
Figure 3A and B show typical topographic images and height profiles of GO and GO@PDOPA nanosheets. A thickness of 1.5–1.7 nm was identified in a typical hybrid planar flake of GO@PDOPA, which is thicker than that of the GO nanoflake (i.e. ~0.9 nm). This denotes a ~0.35 nm thickness of the PDOPA coating layer on each side of the GO sheet in the hybrid nanosheet. Figure 3C illustrates the TEM image of an individual GO@PDOPA nanosheet with rough surface morphology due to PDOPA coating on rGO surfaces.

AFM images and the corresponding height profiles of (A) GO and (B) GO@PDOPA nanosheets. (C) TEM image of GO@PDOPA nanosheets.
The hybrid nanosheets were analyzed by thermogravimetry. TGA shows that no well-defined decomposition temperature can be found for PDOPA, suggesting that PDOPA has a heterogeneous structure and the presence of different chemical groups. As showed in Figure 4, rGO started to lose weight at around 280°C, while pure PDOPA started to decompose early from 60°C. The rGO@PDOPA also started to lose weight from 60°C in a slow rate. The TGA results reveal that rGO undergoes 19.91% weight loss as the temperature increases to 800°C due to the existence of oxygen groups. By comparison, the weight loss for rGO@PDOPA is larger than that for rGO, which is ascribed to the decomposition of PDOPA. The char yield at 800°C of rGO@PDOPA is about 60.67%, higher than the 47.16% of PDOPA. The composition of rGO@PDOPA is calculated as 58.97 wt% PDOPA and 41.03 wt% rGO according to the TGA results.

TGA traces of rGO, PDOPA and rGO@PDOPA.
3.2 Dielectric properties of rGO@PDOPA/PVDF composite films
Figure 5A shows the dielectric permittivity of the rGO@PDOPA/PVDF nanocomposite films as a function of frequency with different rGO loadings. The rGO@PDOPA volume ratio data in the composites shown in this paper exclude the volume of PDOPA. As can be seen, no abrupt increases in permittivity are observed below 1.32 vol% of rGO content, suggesting that conductive pathways were not formed in the films. When the rGO content is beyond 1.5 vol%, the electrical conductivity of the composites is apparently higher than those below 1.5 vol% over the whole frequency range (Figure 5B), indicating the threshold percolation of the composites below 1.5 vol%, as previously estimated about 1.32 vol%. It was also observed that the frequency dependence of the electrical conductivity for the composites was relatively weak over the whole frequency range, indicating a relatively good insulating property. The permittivity improved sharply in the low-frequency range as the rGO content approached the percolation threshold, followed by a gradual decrease in the high-frequency range. As summarized in Figure 5A and D, the permittivity increased dramatically and up to more than 279 at 100 Hz when the rGO content reached 1.32 vol%, which is ~30 times larger than that of pure PVDF (about 9).
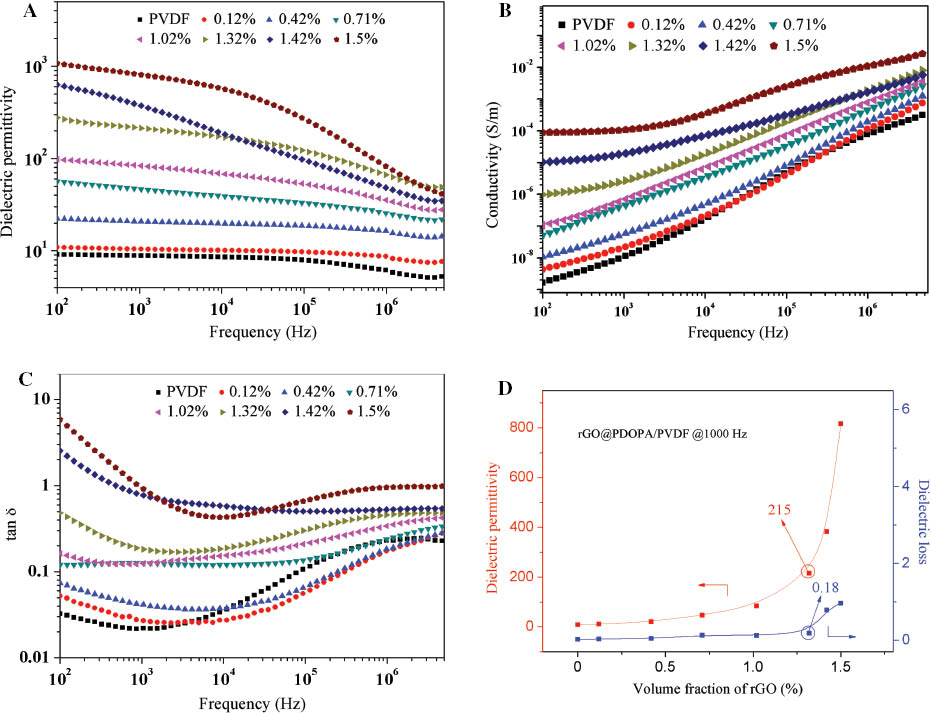
Dependence of (A) dielectric permittivity, (B) electrical conductivity and (C) dielectric loss on the frequency for rGO@PDOPA/PVDF composites. (D) Dielectric permittivity and dielectric loss of rGO@PDOPA/PVDF as a function of volume fraction of rGO at 1000 Hz.
The percolation threshold of rGO@PDOPA/PVDF nanocomposites in the present study is much higher than those of PVDF nanocomposites filled with pristine rGOs, according to the results of previous studies on rGO/PVDF composites [11], [21]. This reveals that the PDOPA layers anchored on rGO surfaces can prevent the direct contact of the conductive rGOs and thus make the percolative network of rGOs harder to form. Compared with the rGO/PVDF composites, the permittivity of rGO@PDOPA/PVDF composites at low and modest frequencies (e.g. <105 Hz) is higher near the percolation threshold owing to increased interfacial polarization. The enhancement of the interfacial polarization is mainly attributed to increased interfacial area and improved interfacial bonding between rGOs and PVDF.
As far as the dielectric loss is concerned, the loss tangents for the rGO@PDOPA/PVDF composites displayed a similar dielectric relaxation to that of the pure PVDF below the threshold (Figure 5C). An abrupt increase in loss tangent was observed near the percolation threshold as one important feature of the percolative composites (Figure 5D), while the loss factor maintained a value under 0.5 over the whole frequency range. Near the threshold, the loss tangent of the rGO@PDOPA/PVDF composite is 0.18 at 1000 Hz, which is about 5.5 times lower than that of the rGO/PVDF composite, which is acceptable for applications such as high-charge storage capacitors.
As the rGO content increased beyond the percolation threshold, the dielectric permittivity of the rGO@PDOPA/PVDF composites continued to rise, to an exceptionally high value of about 1100. At this point, the loss tangents of the rGO@PDOPA/PVDF composites maintained below 1 at moderate and high frequencies. The observed depressed loss had been rationalized by the presence of the insulating PDOPA layers, which separated the conductive rGOs and thus reduced conduction loss.
The morphology of the rGO@PDOPA/PVDF composite containing an rGO volume fraction of 1.32%, as shown in Figure 6, indicates that the rGO@PDOPA nanoplates homogeneously dispersed in the PVDF matrix without serious aggregation. This can be ascribed to strong interactions between the functional groups of the PDOPA and the -CF2- groups of the PVDF [26]. Therefore, the wrapping of PDOPA onto rGO surfaces led to homogeneous dispersion of rGOs and enhanced interfacial polarization effect induced by increased phase interfaces between rGOs and PVDF. Furthermore, it should be noted that the rGO@PDOPA nanosheets tended to lie down inside the PVDF matrix due to the 2D structure of the nanosheets [11], [12]. As a result, the faces of the rGO@PDOPAs are almost parallel to the nanocomposite-film plate. That is, the rGO@PDOPA nanoplates are prone to be parallel to each other, isolated by a polymer layer as a medium between them. This promoted the formation of a larger number of microcapacitors in the nanocomposites [18], [21]. It suggests that the enhancement of the dielectric permittivity can be mainly ascribed to enhanced interfacial polarization and the formation of microcapacitor networks. Therefore, we believe that the non-covalent coating of PDOPA insulating layers on rGO surfaces and the existence of many microcapacitors in the PVDF matrix are beneficial in achieving a high dielectric permittivity, increased percolation threshold and reduced dielectric loss.
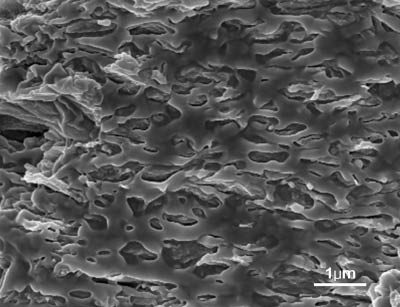
SEM image of fracture surface of rGO@PDOPA/PVDF hybrid film containing 1.32 vol% rGO nanosheets.
4 Conclusions
This contribution reports the preparation and characterization of PVDF-based nanocomposites filled with rGO@PDOPA nanosheets. The rGOs coated by PDOPA were observed to be well-dispersed, nearly parallel to each other and isolated by a polymer layer within the PVDF matrix. The values of percolation threshold for rGO@PDOPA/PVDF were estimated to be 1.32 vol%, which is much higher than that of PVDF nanocomposites filled with rGOs. Near the percolation threshold, the dielectric permittivity increased approximately 30-fold for pristine PVDF at 100 Hz while preserving relatively low dielectric loss. Compared with the rGO/PVDF composites, higher dielectric permittivity together with lower loss factor were achieved in rGO@PDOPA/PVDF nanocomposites at low and moderate frequencies. These can be well explained by enhanced interfacial polarization, microcapacitor model and suppressed conduction loss. The PDOPA interface layers were effective to modify the dielectric characteristics of the composites and could thus lead to greater energy storage capacity than the PVDF matrix and its composites filled with pristine graphene. The novel strategy based on PDOPA interface layers shows significant improvement over those currently available techniques using chemical functionalization and inorganic coating methods. We believe that such hybrid nanocomposite films are attractive for practical applications in energy storage devices.
Acknowledgments
We gratefully acknowledge the support by the National Natural Science Foundation of China (No. 51173139).
References
[1] Hadjipaschalis I, Poullikkas A, Efthimiou V. Renew Sust. Energ. Rev. 2009, 13, 1513–1522.10.1016/j.rser.2008.09.028Search in Google Scholar
[2] Chu B, Zhou X, Ren K, Neese B, Lin M, Wang Q, Bauer F, Zhang QM. Science 2006, 313, 334–336.10.1126/science.1127798Search in Google Scholar PubMed
[3] Wang Q, Zhu L. J. Polym. Sci., Part B: Polym. Phys. 2011, 49, 1421–1429.10.1002/polb.22337Search in Google Scholar
[4] Wen F, Xu Z, Tan S, Xia W, Wei X, Zhang Z. ACS Appl. Mater. Interfaces 2013, 5, 9411–9420.10.1021/am401784pSearch in Google Scholar PubMed
[5] Yao J, Xiong C, Dong L, Chen C, Lei Y, Chen L, Li R, Zhu Q, Liu X. J. Mater. Chem. 2009, 19, 2817–2821.10.1039/b819910hSearch in Google Scholar
[6] Li H, Jiang M, Dong L, Xie H, Xiong C. J. Macromol. Sci. B: Phys. 2013, 52, 1058–1066.10.1080/00222348.2012.754707Search in Google Scholar
[7] Kumar RP, Thomas S. Sci. Eng. Compos. Mater. 2011, 8, 311–326.10.1515/SECM.1999.8.6.311Search in Google Scholar
[8] Dong L, Xiong C, Quan H, Zhao G. Scripta Mater. 2006, 55, 835–837.10.1016/j.scriptamat.2006.07.001Search in Google Scholar
[9] Sirisathitkul C, Jantaratana P, Muensit N. Sci. Eng. Compos. Mater. 2012, 19, 255–258.10.1515/secm-2012-0018Search in Google Scholar
[10] Romasanta LJ, Hernández M, López-Manchado MA, Verdejo R. Nanoscale Res. Lett. 2011, 6, 508.10.1186/1556-276X-6-508Search in Google Scholar PubMed PubMed Central
[11] Fan P, Wang L, Yang J, Chen F, Zhong M. Nanotechnology 2012, 23, 365702.10.1088/0957-4484/23/36/365702Search in Google Scholar PubMed
[12] Almadhoun MN, Hedhili MN, Odeh IN, Xavier P, Bhansali US, Alshareef HN. Chem. Mater. 2014, 26, 2856–2861.10.1021/cm5004565Search in Google Scholar
[13] Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS. Nature 2006, 442, 282–286.10.1038/nature04969Search in Google Scholar PubMed
[14] Miller JR, Outlaw RA, Holloway BC. Science 2010, 329, 1637–1639.10.1126/science.1194372Search in Google Scholar PubMed
[15] Ansari S, Giannelis EP. J. Polym. Sci., Part B: Polym. Phys. 2009, 47, 888–897.10.1002/polb.21695Search in Google Scholar
[16] Li H, Jiang M, Li Q, Li D, Chen Z, Hu W, Huang J, Xu X, Dong L, Xie H, Xiong C. Energ. Convers. Manage. 2013, 75, 482–487.10.1016/j.enconman.2013.07.005Search in Google Scholar
[17] Wen F, Xu Z, Xia W, Wei X, Zhang Z. J. Adv. Dielect. 2013, 3, 1350010 1–6.10.1142/S2010135X13500100Search in Google Scholar
[18] Han K, Li Q, Chen Z, Gadinski MR, Dong L, Xiong C, Wang Q. J. Mater. Chem. C 2013, 1, 7034–7042.10.1039/c3tc31556hSearch in Google Scholar
[19] Özçelik VO, Ciraci S. J. Phys. Chem. C 2013, 117, 15327–15334.10.1021/jp403706eSearch in Google Scholar
[20] Shen Y, Guan Y, Hu Y, Lei Y, Song Y, Lin Y, Nan CW. Appl. Phys. Lett. 2013, 103, 072906.10.1063/1.4818763Search in Google Scholar
[21] Wang D, Bao Y, Zha JW, Zhao J, Dang ZM, Hu GH. ACS Appl. Mater. Interfaces 2012, 4, 6273–6279.10.1021/am3018652Search in Google Scholar PubMed
[22] Long Y, Wu J, Wang H, Zhang X, Zhao N, Xu J. J. Mater. Chem. 2011, 21, 4875–4881.10.1039/c0jm03838eSearch in Google Scholar
[23] Hu H, Yu B, Ye Q, Gu Y, Zhou F. Carbon 2010, 48, 2347–2353.10.1016/j.carbon.2010.03.014Search in Google Scholar
[24] Lee H, Dellatore SM, Miller WM, Messersmith PB. Science 2007, 318, 426–430.10.1126/science.1147241Search in Google Scholar PubMed PubMed Central
[25] Zhang L, Wu J, Wang Y, Long Y, Zhao N, Xu J. J. Am. Chem. Soc. 2012, 134, 9879–9881.10.1021/ja303037jSearch in Google Scholar PubMed
[26] Thakur VK, Lin M, Tan EJ, Lee PS. J. Mater. Chem. 2012, 22, 5951–5959.10.1039/c2jm15665bSearch in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- Wave propagation in functionally graded piezoelectric-piezomagnetic rectangular bars
- Graphene/poly(vinylidene fluoride) dielectric composites with polydopamine as interface layers
- A novel biaxial double-negative metamaterial for electromagnetic rectangular cloaking operation
- Formation of homogenous copper film on MWCNTs by an efficient electroless deposition process
- Nano-SiCp/Al2014 composites with high strength and good ductility
- Microstrip line-fed monopole antenna on an epoxy-resin-reinforced woven-glass material for super wideband applications
- Influence of casting speed on fabricating Al-1%Mn and Al-10%Si alloy clad slab
- Thermal insulating epoxy composite coatings containing sepiolite/hollow glass microspheres as binary fillers: morphology, simulation and application
- Analysis of influence of fibre type and orientation on dynamic properties of polymer laminates for evaluation of their damping and self-heating
- Dynamic stability of nanocomposite viscoelastic cylindrical shells coating with a piezomagnetic layer conveying pulsating fluid flow
- Buckling and layer failure of composite laminated cylinders subjected to hydrostatic pressure
- One-step preparation and characterization of core-shell SiO2/Ag composite spheres by pulse plating
- The failure mechanism of carbon fiber-reinforced composites under longitudinal compression considering the interface
- A thermal-plastic model of friction stir welding in aluminum alloy
- A model for longitudinal tensile strength prediction of low braiding angle three-dimensional and four-directional composites
- Nonlinear stability of shear deformable eccentrically stiffened functionally graded plates on elastic foundations with temperature-dependent properties
- Design and multibody dynamics analyses of the novel force-bearing structures for variable configuration spacecraft
Articles in the same Issue
- Frontmatter
- Original articles
- Wave propagation in functionally graded piezoelectric-piezomagnetic rectangular bars
- Graphene/poly(vinylidene fluoride) dielectric composites with polydopamine as interface layers
- A novel biaxial double-negative metamaterial for electromagnetic rectangular cloaking operation
- Formation of homogenous copper film on MWCNTs by an efficient electroless deposition process
- Nano-SiCp/Al2014 composites with high strength and good ductility
- Microstrip line-fed monopole antenna on an epoxy-resin-reinforced woven-glass material for super wideband applications
- Influence of casting speed on fabricating Al-1%Mn and Al-10%Si alloy clad slab
- Thermal insulating epoxy composite coatings containing sepiolite/hollow glass microspheres as binary fillers: morphology, simulation and application
- Analysis of influence of fibre type and orientation on dynamic properties of polymer laminates for evaluation of their damping and self-heating
- Dynamic stability of nanocomposite viscoelastic cylindrical shells coating with a piezomagnetic layer conveying pulsating fluid flow
- Buckling and layer failure of composite laminated cylinders subjected to hydrostatic pressure
- One-step preparation and characterization of core-shell SiO2/Ag composite spheres by pulse plating
- The failure mechanism of carbon fiber-reinforced composites under longitudinal compression considering the interface
- A thermal-plastic model of friction stir welding in aluminum alloy
- A model for longitudinal tensile strength prediction of low braiding angle three-dimensional and four-directional composites
- Nonlinear stability of shear deformable eccentrically stiffened functionally graded plates on elastic foundations with temperature-dependent properties
- Design and multibody dynamics analyses of the novel force-bearing structures for variable configuration spacecraft

