Abstract
A bioanalytically validated potentiometric method was proposed for the assay of bisphenol A (BPA) using a MWCNT-modified graphite ion selective electrode. The sensitivity of measurements was significantly enhanced by using MWCNTs as an ion-to-electron transducer layer. A precise linear analytical curve was developed to determine BPA in a specified linear range of 10,000–0.01 μmol·L−1, with a detection limit of 0.000104 μmol·L−1. After confirming that the results obtained with the proposed potentiometric sensor are not affected by the presence of possible interferents, the method was successfully applied to determine BPA concentration in different saliva samples, giving good accuracy and precision.
Graphical abstract
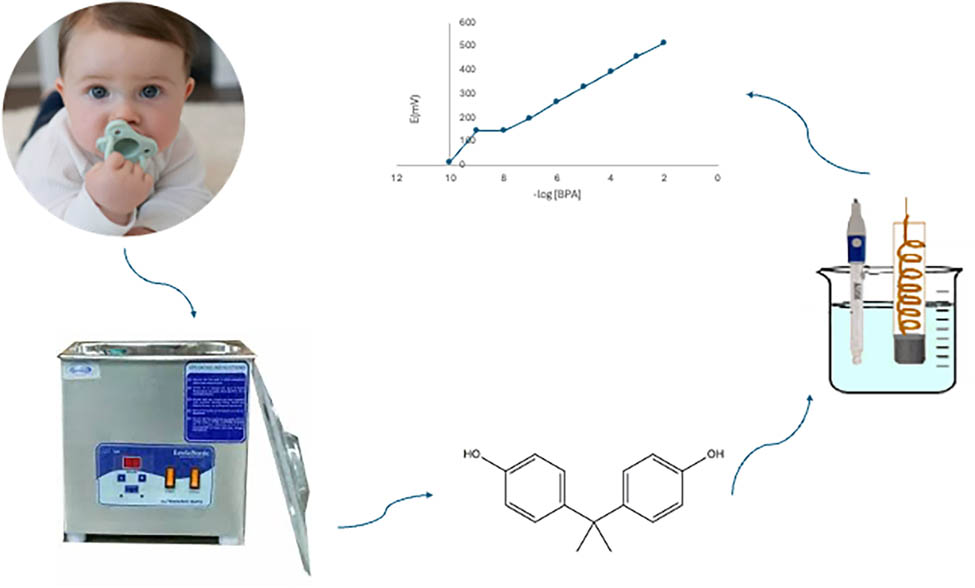
1 Introduction
Bisphenol A (BPA) is a monomer that was first progressed in the 1890s; it has moderate water solubility, a low vapor pressure [1], and a pK a of 10.6 [2]. BPA was introduced as an artificial estrogen and was reported to have the activity of estrone in activating the reproductive system in female rats in the 1930s.
BPA has been used in several commercial products, including thermal receipts, dental sealants, plastics, PVC, and food packaging. Humans are exposed to BPA through dermal exposure, their diet, and inhalation of household dust. BPA is a familiar endocrine disruptor; in laboratory studies, it has been discovered to link to estrogen receptors and to have estrogenic influence [3].
Babies have been renowned as a distinguished population to be considered in risk evaluation because their health menace can vary from that of adults. This can be ascribed to physiological variations, which can be declared as immature immune system functions and lower metabolism capacities. Consequently, toxic hazard elimination is not as efficient as in adults [4]. On the other hand, due to the frequent mouthing of objects by babies, the level of exposure varies, as infants face a greater likelihood of oral exposure compared to adults [5]. Baby pacifiers, bibs, and teethers are dramatically used by infants and can be deemed as a main cause of their oral exposure to several dangerous chemicals such as monomers.
Numerous commercial baby products designed for intraoral use, such as nipples, pacifiers, and baby bottles, have been merchandised and suggested as most suitable for fitting a baby’s mouth, consolidate appropriate tongue and jaw positioning, nasal breathing, and lip closure during feeding, and to facilitate a healthful oral growth. Children frequently use these items from infancy through early childhood, several times a day. BPA is used as a starting material in the industrialization of some of these products, which can be emitted into the oral environment during regular use.
Polycarbonate baby bottles have been identified as the primary cause of BPA exposure in children 6 years and below. Additionally, a survey disclosed that 21 bottle-fed babies had elevated levels of BPA in their blood serum [6].
Multiple methods have been reported in the literature for the determination of BPA in different matrices such as waste plastics [7], water [8,9], juice [10], baby bottles [11], food samples [12], saliva [13], and milk [14,15,16]. A very serious task is developing BPA screening methods and tools in children’s saliva to prevent and minimize its negative effects on the body. For many years, electrochemical ion-selective sensors have been extensively employed as in situ analyzers in fields such as medicine, environmental monitoring, and various industries [17]. Hence, in this study, a simple, sensitive, and rapid potentiometric sensor for monitoring BPA (Figure 1) in saliva samples of babies was proposed. To the best of our knowledge, this is the first potentiometric approach for measuring BPA in saliva samples of baby bibs, pacifiers, and teethers. It is a carbon-based ISE modified with MWCNTs for better sensitivity and more reproducible response. This study’s novelty is illustrated in attempting to establish a sensitive, fast, and reliable membrane electrode to selectively detect BPA, where MWCNTs are used for the first time in the potentiometric determination of BPA.
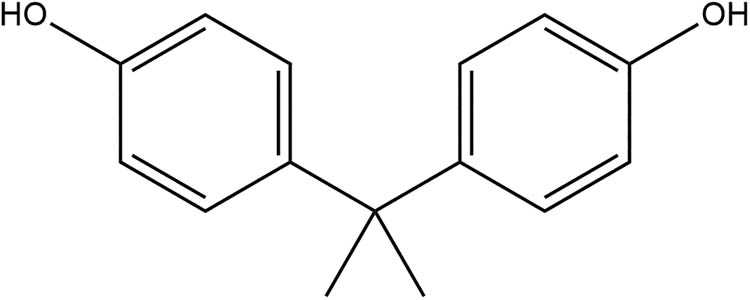
Bisphenol A chemical structure (driven by chemibio draw ultra).
2 Experimental
2.1 Materials and reagents
BPA (purity ≥ 97.00%) was purchased from Sigma-Aldrich (Cairo, Egypt); purity was confirmed by using a previously reported method [7] and found to be 99.66 ± 1.05%. All chemicals and reagents used were of analytical grade, and water was bi-distilled. Polyvinyl chloride (PVC) was bought from Fluka AG (Buchs, Switzerland). Tetrahydrofuran (THF) was purchased from Millipore Sigma (Darmstadt, Germany). Dioctyl phthalate (DOP) was bought from Acros Organics (Morris Plains, NJ, USA). Multi-walled carbon nanotubes (MWCNTs) were purchased from XFnano Materials Tech Co., Ltd. (Nanjing, China). Methanol was bought from El Nasr Company (Cairo, Egypt). Acetic acid, boric acid, phosphoric acid, and potassium chloride (KCl) were purchased from Rebain International (Rotterdam, Netherlands). Human saliva samples were collected from healthy volunteers and used within 24 h. Saliva samples were collected by spitting sampling method, and sampling of whole saliva is the most common and less invasive procedure [18]. The samples were stored at −20°C when not in use.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.2 Standard solutions
2.2.1 Standard BPA stock solution
A stock solution of BPA (1.0 × 10−2 mol·L−1) was prepared by weighing 0.228 g of BPA into a 100.0 mL volumetric flask, solubilized in 0.5 mL methanol before completing to the mark using distilled water.
2.2.2 Standard BPA working solutions
Solutions of various concentrations (ranging from 1.0 × 10−3 to 1.0 × 10−9 mol·L−1) were prepared by diluting BPA stock solution using Britton–Robinson buffer (BRB) [19] adjusted to pH 7.
2.3 Procedures
2.3.1 Ion-selective membrane (ISM) preparation
The ISM was prepared in a 5 cm Petri dish by blending 0.01 g of MWCNTs, 0.10 g of PVC, and 0.4 mL of DOP, dissolved in 6.0 mL of THF, and then sonication was applied for 5 min till complete homogeneity.
2.3.2 Electrode assembly
The potentiometric sensor was made from a 2.5 cm graphite rod (about 3 mm in diameter). One end of the rod was utilized for connection, whereas the other end, approximately 1 cm in length, was coated with a layer >0.01 cm of the ion-sensing mixture. The electrode was coated by dipping in the electroactive membrane mixture several times until the proper thickness was obtained. It was then allowed to completely dry overnight at room temperature. The uncoated graphite rod’s end was plugged into a polytetraethylene tube, which was stuffed with metallic mercury. A copper wire, approximately 1 mm in diameter, was submerged in mercury. For preconditioning, the sensor was dipped in a BPA solution with a concentration of 1.0 × 10−2 mol·L−1 at 25°C for 2 h before its initial use. It was stored in 1.0 × 10−2 mol·L−1 KCl when not in use.
2.3.3 Potentiometric measurements
Potentiometric measurements were conducted using a Jenway pH meter 3310 Orion, equipped with a double-junction AgǀAgCl reference electrode. A schematic diagram of the electrode assembly is demonstrated in Scheme 1. For pH adjustment, a Bandelin Sonorox Rx 510S magnetic stirrer (Budapest, Hungary) and a Jenway pH glass electrode (UK) were employed. The calibration curve was constructed by immersion of the developed electrode, in conjunction with the reference electrode, in BPA solutions of concentrations ranging from 1.0 × 10−2 to 1.0 × 10−9 mol·L−1. The electrode was left immersed in solutions till a constant reading was obtained. The spotted emf from the proposed sensor was plotted in relation to −log [BPA] concentrations. A regression equation was calculated for the suggested sensor. IUPAC recommendations were followed for the performance validation of the sensor [20].
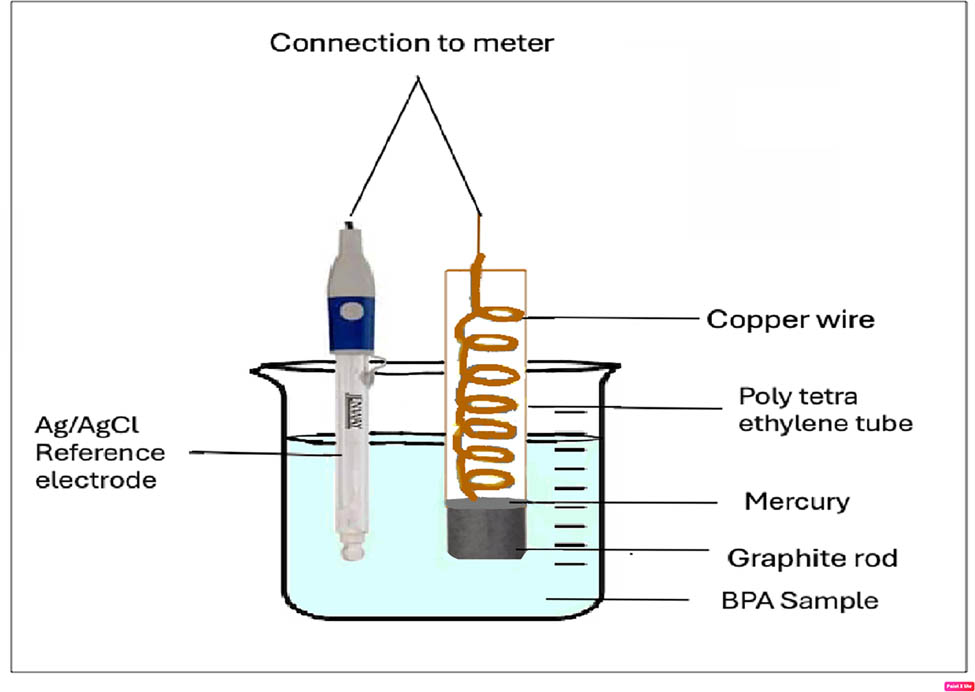
Schematic diagram of electrode assembly.
2.3.4 Sensor selectivity
The selectivity of BPA sensing electrode was investigated by observing the potential of 1.0 × 10−4 mol·L−1 BPA with the same concentration of interfering ions such as phthalates (organic leaching chemicals termed as endocrine-disrupting compounds), inorganic leaching compounds (PbCl2 and ZnO), and saliva components (sodium chloride, magnesium bicarbonate, calcium bicarbonate, sodium phosphate, urea, and ammonium oxide). Selectivity coefficient values (K pot A,B) were obtained using the separate solution method (SSM) [21] in which the potentials were obtained separately for 1.0 × 10−4 mol·L−1 BPA and then for 1.0 × 10−4 mol·L−1 interferent solution. The selectivity coefficients were obtained using the following equation:
where K pot AB is the selectivity coefficient, E A and E B are the potentials of the drug and interferent solutions, respectively, S is the slope of the calibration plot, a A is the activity of the drug, and Z A and Z B are charges of the drug and interfering ions, respectively.
2.3.5 pH effect on sensor performance
Over a pH range of 2.0–11.0 regulated by using Britton–Robinson buffer, the influence of pH on the readings of the investigated sensor was investigated for 1.0 × 10−4 and 1.0 × 10−5 mol·L−1 BPA solutions. Potential values were attained and registered at each pH level.
2.3.6 Application to saliva in contact with baby bibs, pacifiers, and teethers
Simulation of the real status of babies chewing bibs, teethers, or pacifiers was done by leaving each one separately in contact with human saliva for 3 h at 37°C in a stirring water bath. Into a set of volumetric flasks (10.0 mL), 1 mL of each of the resultant saliva was added and then accurately spiked with different volumes of the prepared stock standard solution and filled to the mark with BRB (pH 7) to obtain 0.01–8.0 μg·mL−1 BPA. All biological samples were stored at −20°C until use. The measurements were completed in agreement with the “Potentiometric measurements” section. The corresponding regression equation was used to obtain the recovery percentages.
3 Results and discussion
Potentiometric methods offer several benefits compared to other sample analysis techniques. These include a wide linear range, quick response time, low cost and energy consumption, ease of preparation, and excellent sensitivity and selectivity for a variety of samples. Additionally, potentiometric methods do not require pre-treatment or the use of organic solvents, making them more environmentally friendly. In contrast, other analytical methods are often unsuitable for large-scale monitoring due to their complexity, high cost, energy, and time demands, and need for sample pre-treatment. As a result, potentiometric assays are a more advantageous choice for sample analysis [22]. A big challenge that has always been facing analysts is building a solid contact ion selective electrode (SC-ISE) with harmonious and repeatable potentials. Thus, the main goal of this work is the synthesis of a portable, economical, solid-contact sensor with high sensitivity, reproducibility, and harmonious readings for selective determination of BPA.
3.1 Performance of the investigated sensors
The calibration plot slope for the developed MWCNT-SCE exhibited a cationic response and was found to be 62.48 mV/concentration decade. Polyvinyl chloride (PVC) served as the polymer matrix in creating the ion-sensing mixture, and DOP was used as a plasticizer, along with THF. Additionally, MWCNTs were added and proven to be utilized as a transducer layer to enhance sensitivity and increase stability and reproducibility [23].
In this study, the performance of a sensor based on MWCNTs was assessed. As per IUPAC guidelines, the performance characteristics of the proposed sensor were evaluated [24], as shown in Table 1. The suggested sensor covered a wide dynamic linear concentration range of 1.0 × 10−2–1.0 × 10−8 M, as illustrated in Figure 2. The point of intersection of the linear extrapolated portions of the two curves is used for determining the limit of detection (LOD) and was found to be 1.04 × 10−10 M [24]. Fast response time is a major factor for the rapid analysis of a large number of samples and, therefore, participates in the real application of the developed sensor [25]. A short response time of 10 s was observed for the developed sensor. The short response time is because of the use of MWCNTs characterized by causing a high double-layer capacitance. As opposed to conducting polymers, the transduction mechanism is found in redox reactions and ion-exchange mechanisms, and MWCNTs are sturdier against any redox side reactions that might occur in the sensor [26].
Performance characteristics of BPA by the suggested sensor
| Parameter | Sensor |
|---|---|
| Slope (mV/decade)a | 62.48 |
| Intercept (mV)a | 642.95 |
| Correlation coefficient (r) | 0.9995 |
| Response time | 10 s |
| Working pH range | 6–8 |
| Concentration range (mol·L−1) | 1.0 × 10−2–1.0 × 10−8 |
| Stability (days) | 30 |
| Accuracya (mean ± SD) | 100.62 ± 1.171 |
| Precision (SD) | |
| Inter-day precisionb | 1.061 |
| LOD (mol·L−1)c | 1.04 × 10−10 |
aAverage of three determinations. bInter-day precision was evaluated with a sample size of 9, involving the average of three concentrations repeated over three consecutive days. cLimit of detection, is determined by the intersection of the extrapolated arms of the Nernstian and non-responsive calibration plot parts.
![Figure 2
Profile of the proposed sensor’s potential (mV) vs −log BPA [M] concentrations.](/document/doi/10.1515/revac-2025-0085/asset/graphic/j_revac-2025-0085_fig_002.jpg)
Profile of the proposed sensor’s potential (mV) vs −log BPA [M] concentrations.
3.2 pH effect on sensor performance
To optimize experimental conditions, the impact of pH on the efficacy of the proposed sensor was examined. No significant variation in the sensor’s measurements was observed within the pH range of 6.0–8.0. Above pH 8, E decreases dramatically, and below pH 6, BPA begins to precipitate; therefore, E is not stable anymore, as illustrated in Figure 3. This could be attributed to the fact that BPA, at pK a = 10.6 [2], in aqueous solutions, and at pH values <10.6, exists in its molecular form, while at pH values >10.6, it is present as ionized forms (HBPA− or as BPA2−). Inspired by previous findings in the literature [27], the application of the nonclassical response in BPA detection was explored. In order to guarantee that BPA exists mostly in its neutral form, BRB with a pH of 7 was used as the background solution to achieve sensitive detection of neutral BPA.
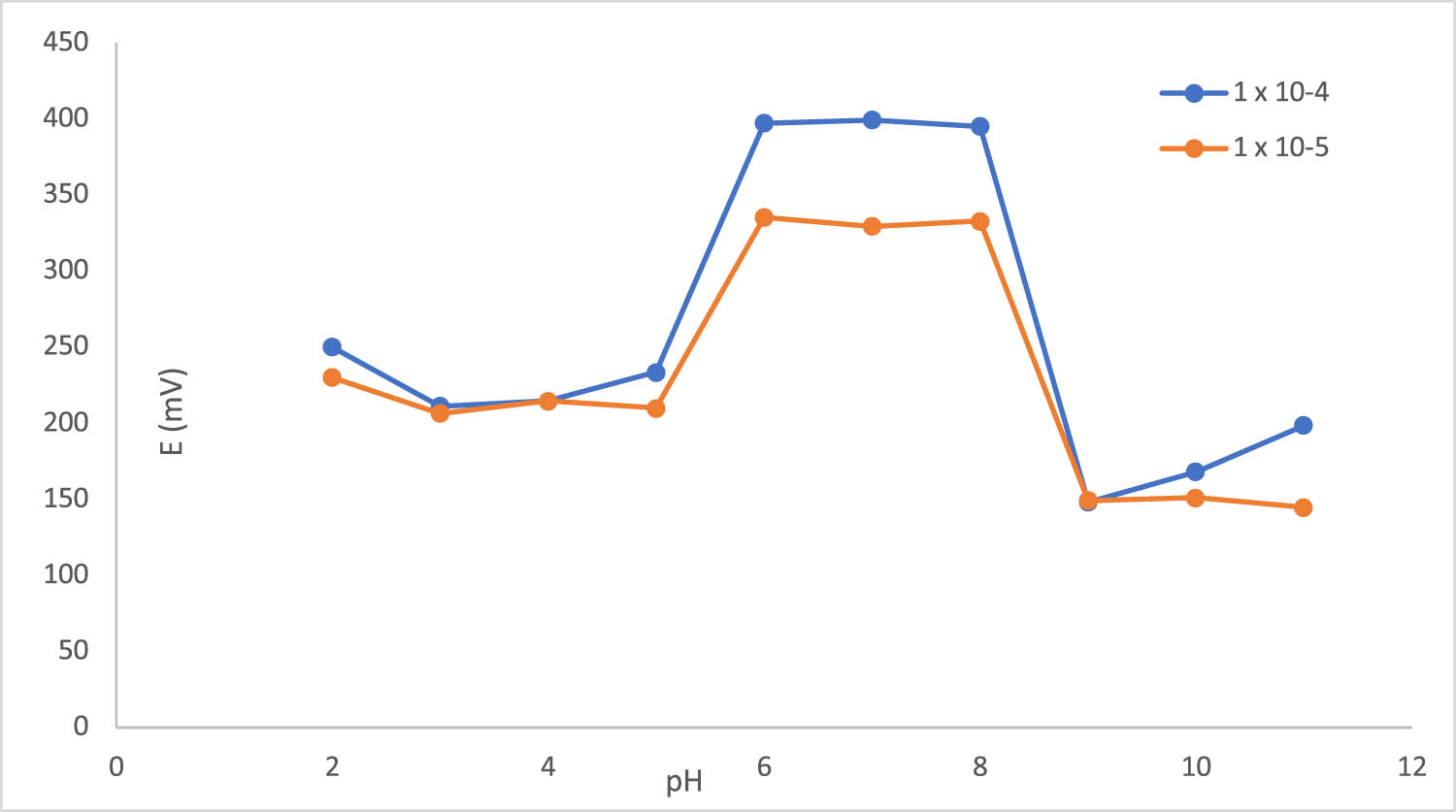
Effect of pH on the sensor response (2–11).
3.3 Determination of the sensor’s selectivity coefficients
Selectivity of the ion-selective electrode is one of its most fateful characteristics. It is frequently utilized to determine the potential accuracy of measuring the sample of interest. Evaluation of selectivity coefficients was performed utilizing a separate solution technique [21]. Selectivity coefficients were assessed for organic leaching chemicals, termed endocrine-disrupting compounds, such as phthalates, inorganic leaching compounds (PbCl2 and ZnO), and saliva components (sodium chloride, magnesium bicarbonate, calcium bicarbonate, sodium phosphate, urea, and ammonium oxide). Table 2 lists the selectivity coefficient values. The results indicate that MWCNT-SCE exhibited perfect selectivity toward BPA as opposed to other examined interferents. The sensor showed the highest selectivity for BPA in the presence of calcium bicarbonate, while the least selectivity was recorded in the presence of lead chloride. As K pot BPA, Interferent value reduces, it indicates that the electrode is more selective to the studied analyte (BPA) [28].
Potentiometric selectivity coefficients of the suggested sensors (K potBPA, interferents)
| Interfering species (I) | (K pot BPA, interferent) |
|---|---|
| Dibutyl phthalate | 1.5 × 10−3 |
| Diethyl phthalate | 2.2 × 10−4 |
| PbCl2 | 7.8 × 10−2 |
| ZnO | 3.5 × 10−2 |
| NaCl | 2.0 × 10−4 |
| Mg (HCO3)2 | 3.8 × 10−5 |
| Ca (HCO3)2 | 3.2 × 10−5 |
| Sodium phosphate | 4.2 × 10−4 |
| Urea | 6.0 × 10−5 |
| Ammonium oxide | 6.2 × 10−4 |
3.4 Analysis of BPA in spiked saliva samples
The primary goal of this study was to devise a sensitive and quick method for detecting BPA in saliva that comes in contact with baby bibs, pacifiers, and teethers. Thus, the ability of the introduced SC-ISE to detect BPA in biological fluids was evaluated by direct testing of spiked saliva samples without any prior extraction steps. The proposed sensor was felicitous in obtaining accurate and precise recoveries, as shown in Table 3.
Analysis of BPA in spiked saliva samples
| Plastic bibs | Teethers | Pacifiers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Spiked (μg·mL−1) | Found (μg·mL−1) | R%a | Spiked (μg·mL−1) | Found (μg·mL−1) | R%a | Spiked (μg·mL−1) | Found (μg·mL−1) | R%a | |
| Blank | 0 | ND | ND | 0 | ND | ND | 0 | ND | ND |
| Sample 1 | 0.5 | 0.51 | 102 | 0.5 | 0.5 | 100 | 0.5 | 0.53 | 106 |
| Sample 2 | 1 | 1.01 | 101 | 1 | 1.05 | 105 | 1 | 1 | 100 |
| Sample 3 | 3 | 3.1 | 103.33 | 3 | 2.8 | 93.33 | 3 | 3.02 | 100.66 |
| Mean ± SD | 102.11 ± 1.1717 | 99.44 ± 5.853 | 102.22 ± 3.289 | ||||||
aAverage of three determinations.
3.5 Statistical analysis
The variance ratio F test was used to examine the validity of the proposed approach. A statistical comparison of the suggested sensor and the reported method [7] for BPA is presented in Table 4. The results show that there is no statistically significant difference between the reported method and the proposed sensor; the calculated F values were less than the theoretical one at p = 0.05.
Statistical comparison between the proposed sensor and the reported reference method for the determination of BPA
| Parameter | Reported method** | Proposed sensor |
|---|---|---|
| Mean | 99.66 | 100.62 |
| SD | 1.05 | 1.171 |
| Variance | 1.103 | 1.371 |
| n | 5 | 5 |
| F (6.39)* | — | 1.243 |
*Tabulated F value at p = 0.5. **Reported method [7]: Voltammetric determination of bisphenol A using a carbon paste electrode based on the enhancement of cetyltrimethylammonium bromide (CTAB).
3.6 Comparison of the proposed sensor to other reported potentiometric methods for determination of BPA
The proposed sensor was compared with other reported potentiometric sensors for the determination of BPA, as summarized in Table 5. The developed electrode has a wider linear range and lower LOD and is considered the first potentiometric sensor for measuring BPA in saliva samples in baby bibs, pacifiers, and teethers.
Comparison of the proposed sensor to other reported potentiometric methods for determination of BPA in different matrices
| Technique | Matrix | Linear range (mol·L−1) | LOD (μmol·L−1) | Reference |
|---|---|---|---|---|
| Potentiometric immunosensor | Real spiked water sample | 2.2 × 10−7 to 6.85 × 10−6 | 0.14 | [29] |
| MIP-based potentiometric sensor | Real plastic samples | 1.0 × 10−7 to 1.0 × 10−6 | 0.02 | [30] |
| MIP paper-based potentiometric sensor | Real plastic samples | 5.0 × 10−7 to 1.3 × 10−5 | 0.15 | [27] |
| Multifunctional MIP receptor-based potentiometric sensor | Spiked lake and river water samples | 5.0 × 10−7 to 2.0 × 10−5 | 0.23 | [31] |
| MWCNT-modified graphite ion-selective electrode | Real spiked saliva samples in contact with baby bibs, teethers, and pacifiers | 1.0 × 10−8 to 1.0 × 10−2 | 0.000104 | This work |
4 Conclusion
In this research, the developed sensor was utilized for directly analyzing BPA in saliva samples in contact with baby bibs, pacifiers, and teethers. It experienced several advantages over previously developed instrumental methods, including being economic, portable, environmentally friendly, of real-time analysis, energy saving, and easy to miniaturize. Our proposed sensor exhibited accurate, selective, and precise potentiometric readings in the presence of various potential interferents. Thus, it could be applied for routine analysis without tedious extraction procedures. The addition of carbon nanotubes as ion to electron transducer led to a short response time and low signal drift.
-
Funding information: Authors state no funding involved.
-
Author contributions: Eman Ali Al-Harbi: conceptualization, methodology, validation, investigation, and visualization. Amira Mabrouk El-Kosasy: conceptualization, methodology, validation, investigation, and visualization. Yossra Ahmed Trabik: validation, investigation, writing – original draft, and visualization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Groshart CP, Okkeman PC, Pijnenburg AM. Chemical study on bisphenol A. Netherlands: Rijkswaterstaat, RIKZ; 2001 Jul 1.Search in Google Scholar
[2] Rekos K, Kampouraki ZC, Sarafidis C, Samanidou V, Deliyanni E. Graphene oxide based magnetic nanocomposites with polymers as effective bisphenol–A nano adsorbents. Materials. 2019 Jun;12(12):1987.10.3390/ma12121987Search in Google Scholar PubMed PubMed Central
[3] Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol. 2013 Dec;42:132–55.10.1016/j.reprotox.2013.08.008Search in Google Scholar PubMed
[4] United States. Environmental Protection Agency. Risk Assessment Forum. Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. United States: US Environmental Protection Agency, Risk Assessment Forum.Search in Google Scholar
[5] Yoon H, Yoo SK, Seo J, Kim T, Kim P, Kim PJ, et al. Development of general exposure factors for risk assessment in Korean children. Int J Environ Res Public Health. 2020 Mar;17(6):1988.10.3390/ijerph17061988Search in Google Scholar PubMed PubMed Central
[6] Sharma R, Kotyk MW, Wiltshire WA. An investigation into bisphenol A leaching from materials used intraorally. J Am Dental Assoc. 2016 Jul;147(7):545–50.10.1016/j.adaj.2016.01.013Search in Google Scholar PubMed
[7] Huang W. Voltammetric determination of bisphenol A using a carbon paste electrode based on the enhancement effect of cetyltrimethylammonium bromide (CTAB). Bull Korean Chem Soc. 2005;26(10):1560–4.10.5012/bkcs.2005.26.10.1560Search in Google Scholar
[8] Zhuang Y, Zhou M, Gu J, Li X. Spectrophotometric and high performance liquid chromatographic methods for sensitive determination of bisphenol A. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014 Mar;122:153–7.10.1016/j.saa.2013.11.015Search in Google Scholar PubMed
[9] Zhang X, Jin Y, Wang Y, Liang P, Zou M, Li S, et al. Measurement of trace bisphenol A in drinking water with combination of immunochromatographic detection technology and SERS method. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2022 Feb;267:120519.10.1016/j.saa.2021.120519Search in Google Scholar PubMed
[10] Beitollahi H, Mahmoudi Moghaddam H, Tajik S. Voltammetric determination of bisphenol A in water and juice using a lanthanum (III)-doped cobalt (II, III) nano cube modified carbon screen-printed electrode. Anal Lett. 2019 Jun;52(9):1432–44.10.1080/00032719.2018.1545132Search in Google Scholar
[11] Niu X, Yang W, Wang G, Ren J, Guo H, Gao J. A novel electrochemical sensor of bisphenol A based on stacked graphene nanofibers/gold nanoparticles composite modified glassy carbon electrode. Electrochim Acta. 2013 May;98:167–75.10.1016/j.electacta.2013.03.064Search in Google Scholar
[12] Najafi M, Khalilzadeh MA, Karimi-Maleh H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/ionic liquid paste electrode in food samples. Food Chem. 2014 Sep;158:125–31.10.1016/j.foodchem.2014.02.082Search in Google Scholar PubMed
[13] Russo G, Barbato F, Mita DG, Grumetto L. Simultaneous determination of fifteen multiclass organic pollutants in human saliva and serum by liquid chromatography–tandem ultraviolet/fluorescence detection: A validated method. Biomed Chromatogr. 2019 Mar;33(3):e4427.10.1002/bmc.4427Search in Google Scholar PubMed
[14] Xu Z, Wu Q, Duan Y, Yang M, Ou M, Xu X. Development of a novel spectrophotometric method based on diazotization-coupling reaction for determination of bisphenol A. J Braz Chem Soc. 2017 Aug;28(8):1475–82.10.21577/0103-5053.20160328Search in Google Scholar
[15] Yin H, Cui L, Chen Q, Shi W, Ai S, Zhu L, et al. Amperometric determination of bisphenol A in milk using PAMAM–Fe3O4 modified glassy carbon electrode. Food Chem. 2011 Apr;125(3):1097–103.10.1016/j.foodchem.2010.09.098Search in Google Scholar
[16] Karthika P, Shanmuganathan S, Viswanathan S, Delerue-Matos C. Molecularly imprinted polymer-based electrochemical sensor for the determination of endocrine disruptor bisphenol-A in bovine milk. Food Chem. 2021 Nov;363:130287.10.1016/j.foodchem.2021.130287Search in Google Scholar PubMed
[17] Golcs Á, Vermes B, Siwek DC, Huszthy P, Tóth T. Innovation in potentiometry: 3D-printed polylactic acid-based ion-selective bulk electrode membranes. J Appl Electrochem. 2022 Sep;52(9):1369–82.10.1007/s10800-022-01706-wSearch in Google Scholar
[18] Bellagambi FG, Lomonaco T, Salvo P, Vivaldi F, Hangouët M, Ghimenti S, et al. Saliva sampling: Methods and devices. An overview. TrAC Trends Anal Chem. 2020 Mar;124:115781.10.1016/j.trac.2019.115781Search in Google Scholar
[19] Lu D, Sullivan C, Brack EM, Drew CP, Kurup P. Simultaneous voltammetric detection of cadmium (II), arsenic (III), and selenium (IV) using gold nano star–modified screen-printed carbon electrodes and modified Britton-Robinson buffer. Anal Bioanal Chem. 2020 Jul;412:4113–25. Britton HT, Robinson RA. CXCVIII. —Universal buffer solutions and the dissociation constant of veronal. Journal of the Chemical Society (Resumed). 1931;1456–62.10.1007/s00216-020-02642-4Search in Google Scholar PubMed
[20] Buck RP, Lindner E. Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations 1994). Pure Appl Chem. 1994 Jan;66(12):2527–36.Search in Google Scholar
[21] Umezawa Y, Bühlmann P, Umezawa K, Tohda K, Amemiya S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report). Pure Appl Chem. 2000 Jan;72(10):1851–2082.10.1351/pac200072101851Search in Google Scholar
[22] Trabik YA, Ismail RA, Ayad MF, Hussein LA, Mahmoud AM. Microfabricated potentiometric sensor based on a carbon nanotube transducer layer for selective Bosentan determination. Rev Anal Chem. 2024 Feb;43(1):20230071.10.1515/revac-2023-0071Search in Google Scholar
[23] Trabik YA, Ayad MF, Mahmoud AM, Abdullatif HA, Michael AM. Eco-friendly electrochemical assay of oxytetracycline and flunixin in their veterinary injections and spiked milk samples. BMC Chem. 2024 Sep;18(1):179.10.1186/s13065-024-01282-4Search in Google Scholar PubMed PubMed Central
[24] Buck RP, Lindner E. IUPAC recommendations for nomenclature of ion-selective electrodes. Pure Appl Chem. 1994;66(2527):10–351.10.1351/pac199466122527Search in Google Scholar
[25] El-Kosasy AM, abd el Aziz L, Trabik YA. Comparative study of beta cyclodextrin and calix-8-arene as ionophores in potentiometric ion-selective electrodes for sitagliptin phosphate. J Appl Pharm Sci. 2012 Aug;2(8):51–6.10.7324/JAPS.2012.2806Search in Google Scholar
[26] Crespo GA, Macho S, Rius FX. Ion-selective electrodes using carbon nanotubes as ion-to-electron transducers. Anal Chem. 2008 Feb;80(4):1316–22.10.1021/ac071156lSearch in Google Scholar PubMed
[27] Kamel AH, Jiang X, Li P, Liang R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal Methods. 2018;10(31):3890–5.10.1039/C8AY01229FSearch in Google Scholar
[28] Umezawa Y, Umezawa K, Sato H. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KA, B pot values (Technical Report). Pure Appl Chem. 1995 Jan;67(3):507–18.10.1351/pac199567030507Search in Google Scholar
[29] Piao MH, Noh HB, Rahman MA, Won MS, Shim YB. Label‐free detection of bisphenol A using a potentiometric immunosensor. Electroanal: An Int J Devoted Fundam Pract Asp Electroanal. 2008 Jan;20(1):30–7.10.1002/elan.200704022Search in Google Scholar
[30] Kou LJ, Liang RN, Wang XW, Chen Y, Qin W. Potentiometric sensor for determination of neutral bisphenol A using a molecularly imprinted polymer as a receptor. Anal Bioanal Chem. 2013 May;405:4931–6.10.1007/s00216-013-6877-2Search in Google Scholar PubMed
[31] Wang C, Qi L, Liang R, Qin W. Multifunctional molecularly imprinted receptor-based polymeric membrane potentiometric sensor for sensitive detection of bisphenol A. Anal Chem. 2022 May;94(22):7795–803.10.1021/acs.analchem.1c05444Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Impressive stability-indicating RP-HPLC method for concurrent quantification of salbutamol, guaifenesin, and sodium benzoate in cough syrup: Application of six sigma and green metrics
- Bioanalytically validated potentiometric method for determination of bisphenol A: Application to baby bibs, pacifiers, and Teethers’ saliva samples
- Environmental impact of RP-HPLC strategy for detection of selected antibiotics residues in wastewater: Evaluating of quality tools
- Trace-level impurity quantification in lead-cooled fast reactors using ICP-MS: Methodology and challenges
- Picogram-level detection of three ACE inhibitors via LC–MS/MS: Comparing BMP and UOSA54 derivatization methods
- Eco-friendly RP-HPLC method for concurrent estimation of a promising combination of methocarbamol and etoricoxib in rat plasma
- Development of a point-of-care testing sensor using polypyrrole/TiO2 molecular imprinting technology for cinchocaine determination
- Green and sustainable RP-UPLC and UV strategies for determination of metformin and dapagliflozin: Evaluation of environmental impact and whiteness
- Review Articles
- Determination of montelukast and non-sedating antihistamine combination in pharmaceutical dosage forms: A review
- Extraction approaches for the isolation of some POPs from lipid-based environmental and food matrices: A review
- A review of semiconductor photocatalyst characterization techniques
- Analytical determination techniques for lithium – A review
- Fourier transform infrared spectroscopy study of polymer/filler/ionic liquid composites
Articles in the same Issue
- Research Articles
- Impressive stability-indicating RP-HPLC method for concurrent quantification of salbutamol, guaifenesin, and sodium benzoate in cough syrup: Application of six sigma and green metrics
- Bioanalytically validated potentiometric method for determination of bisphenol A: Application to baby bibs, pacifiers, and Teethers’ saliva samples
- Environmental impact of RP-HPLC strategy for detection of selected antibiotics residues in wastewater: Evaluating of quality tools
- Trace-level impurity quantification in lead-cooled fast reactors using ICP-MS: Methodology and challenges
- Picogram-level detection of three ACE inhibitors via LC–MS/MS: Comparing BMP and UOSA54 derivatization methods
- Eco-friendly RP-HPLC method for concurrent estimation of a promising combination of methocarbamol and etoricoxib in rat plasma
- Development of a point-of-care testing sensor using polypyrrole/TiO2 molecular imprinting technology for cinchocaine determination
- Green and sustainable RP-UPLC and UV strategies for determination of metformin and dapagliflozin: Evaluation of environmental impact and whiteness
- Review Articles
- Determination of montelukast and non-sedating antihistamine combination in pharmaceutical dosage forms: A review
- Extraction approaches for the isolation of some POPs from lipid-based environmental and food matrices: A review
- A review of semiconductor photocatalyst characterization techniques
- Analytical determination techniques for lithium – A review
- Fourier transform infrared spectroscopy study of polymer/filler/ionic liquid composites

