Abstract
C33H22O6Sn, triclinic, P1̄ (no. 2), a = 9.5394(11) Å, b = 10.2394(12) Å, c = 15.3202(18) Å, α = 105.195(3)°, β = 91.321(2)°, γ = 105.703(3)°, V = 1383.1(3) Å3, Z = 2, Rgt(F) = 0.0452, wRref(F2) = 0.1115, T = 298(2) K.
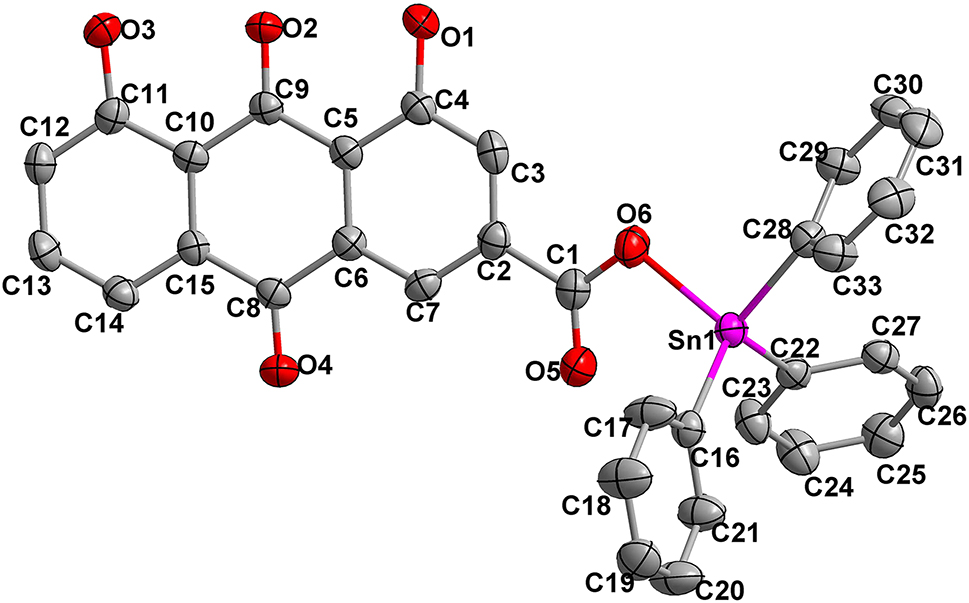
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.28 × 0.16 × 0.11 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.97 mm−1 |
| Diffractometer, scan mode: θmax, completeness: |
Bruker SMART-1000, φ and ω 25.0°, 98 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6729, 4782, 0.038 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), |
| N(param)refined: | 363 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.29797 (4) | 0.27801 (3) | 0.35704 (2) | 0.05113 (14) |
| O1 | −0.2602 (4) | −0.0751 (3) | −0.0159 (3) | 0.0672 (10) |
| H1 | −0.320996 | −0.140775 | −0.051958 | 0.101* |
| O2 | −0.3973 (4) | −0.3386 (3) | −0.0980 (2) | 0.0600 (9) |
| O3 | −0.5583 (4) | −0.5997 (4) | −0.1698 (2) | 0.0637 (9) |
| H3 | −0.536892 | −0.513529 | −0.159076 | 0.095* |
| O4 | −0.0454 (4) | −0.5134 (4) | 0.1040 (2) | 0.0642 (9) |
| O5 | 0.2225 (4) | −0.0137 (4) | 0.2664 (3) | 0.0764 (11) |
| O6 | 0.1304 (4) | 0.1587 (4) | 0.2506 (3) | 0.0697 (10) |
| C1 | 0.1316 (6) | 0.0283 (6) | 0.2298 (4) | 0.0592 (13) |
| C2 | 0.0138 (5) | −0.0763 (5) | 0.1559 (3) | 0.0544 (12) |
| C3 | −0.0712 (5) | −0.0289 (5) | 0.1024 (3) | 0.0546 (13) |
| H3A | −0.056226 | 0.067490 | 0.111385 | 0.066* |
| C4 | −0.1803 (5) | −0.1276 (5) | 0.0345 (3) | 0.0516 (12) |
| C5 | −0.2021 (5) | −0.2743 (5) | 0.0201 (3) | 0.0459 (11) |
| C6 | −0.1127 (5) | −0.3201 (5) | 0.0751 (3) | 0.0476 (11) |
| C7 | −0.0055 (5) | −0.2206 (5) | 0.1412 (3) | 0.0528 (12) |
| H7 | 0.053706 | −0.251122 | 0.175771 | 0.063* |
| C8 | −0.1317 (5) | −0.4754 (5) | 0.0615 (3) | 0.0475 (11) |
| C9 | −0.3197 (5) | −0.3804 (5) | −0.0484 (3) | 0.0466 (11) |
| C10 | −0.3459 (5) | −0.5308 (5) | −0.0568 (3) | 0.0453 (11) |
| C11 | −0.4637 (5) | −0.6348 (5) | −0.1179 (3) | 0.0515 (12) |
| C12 | −0.4864 (6) | −0.7788 (5) | −0.1255 (3) | 0.0588 (13) |
| H12 | −0.562099 | −0.845942 | −0.165924 | 0.071* |
| C13 | −0.3970 (6) | −0.8214 (5) | −0.0733 (4) | 0.0646 (14) |
| H13 | −0.413807 | −0.917225 | −0.078955 | 0.078* |
| C14 | −0.2816 (5) | −0.7233 (5) | −0.0120 (3) | 0.0572 (13) |
| H14 | −0.222682 | −0.754066 | 0.022566 | 0.069* |
| C15 | −0.2551 (5) | −0.5790 (5) | −0.0028 (3) | 0.0469 (11) |
| C16 | 0.2689 (5) | 0.2008 (5) | 0.4754 (3) | 0.0532 (12) |
| C17 | 0.1436 (7) | 0.1917 (8) | 0.5189 (5) | 0.096 (2) |
| H17 | 0.067122 | 0.216950 | 0.495724 | 0.115* |
| C18 | 0.1283 (8) | 0.1451 (9) | 0.5975 (5) | 0.109 (3) |
| H18 | 0.041457 | 0.138087 | 0.624719 | 0.130* |
| C19 | 0.2387 (7) | 0.1105 (7) | 0.6340 (4) | 0.0856 (19) |
| H19 | 0.228699 | 0.081017 | 0.686577 | 0.103* |
| C20 | 0.3650 (8) | 0.1191 (8) | 0.5930 (5) | 0.096 (2) |
| H20 | 0.441213 | 0.095114 | 0.617667 | 0.115* |
| C21 | 0.3811 (6) | 0.1644 (7) | 0.5130 (4) | 0.0790 (17) |
| H21 | 0.467616 | 0.169688 | 0.485624 | 0.095* |
| C22 | 0.5089 (5) | 0.2908 (5) | 0.3070 (3) | 0.0517 (12) |
| C23 | 0.5587 (6) | 0.1718 (6) | 0.2731 (4) | 0.0706 (15) |
| H23 | 0.497147 | 0.082055 | 0.268376 | 0.085* |
| C24 | 0.6997 (7) | 0.1871 (7) | 0.2464 (5) | 0.0884 (19) |
| H24 | 0.731389 | 0.107392 | 0.224410 | 0.106* |
| C25 | 0.7928 (7) | 0.3191 (7) | 0.2522 (4) | 0.0786 (17) |
| H25 | 0.886832 | 0.328223 | 0.234707 | 0.094* |
| C26 | 0.7454 (6) | 0.4375 (6) | 0.2842 (4) | 0.0676 (15) |
| H26 | 0.807367 | 0.526465 | 0.287382 | 0.081* |
| C27 | 0.6050 (6) | 0.4243 (5) | 0.3117 (3) | 0.0578 (13) |
| H27 | 0.574545 | 0.504740 | 0.333487 | 0.069* |
| C28 | 0.2494 (5) | 0.4759 (5) | 0.3768 (3) | 0.0519 (12) |
| C29 | 0.2515 (6) | 0.5409 (6) | 0.3061 (4) | 0.0651 (14) |
| H29 | 0.265497 | 0.492744 | 0.247970 | 0.078* |
| C30 | 0.2335 (6) | 0.6741 (6) | 0.3208 (5) | 0.0788 (18) |
| H30 | 0.236051 | 0.714688 | 0.273037 | 0.095* |
| C31 | 0.2116 (7) | 0.7473 (6) | 0.4070 (5) | 0.086 (2) |
| H31 | 0.201433 | 0.837709 | 0.417363 | 0.103* |
| C32 | 0.2051 (7) | 0.6852 (7) | 0.4774 (5) | 0.093 (2) |
| H32 | 0.188321 | 0.732985 | 0.534917 | 0.112* |
| C33 | 0.2237 (7) | 0.5502 (6) | 0.4620 (4) | 0.0725 (16) |
| H33 | 0.218859 | 0.509296 | 0.509717 | 0.087* |
1 Source of material
1,8-Dihydroxy-3-carboxyanthraquinone (0.284 g, 1 mmol) and sodium ethoxide (0.068 g, 1 mmol) were dissolved in 30 mL of methanol in a Schlenk flask and stirred for 30 min. Subsequently, triphenyltin chloride (0.385 g, 1 mmol) was introduced, and the reaction mixture was stirred at room temperature for 12 h. After filtration, the solvent in the filtrate was gradually evaporated under vacuum until a solid product formed. This solid was recrystallized from methanol, resulting in the formation of orange crystals with a yield of 68 %.
2 Experimental details
The structure was solved by Direct Methods with the SHELXS program. Hydrogen atoms were positioned geometrically (C–H = 0.93–0.98 Å). Their U iso values were set to 1.2 U eq or 1.5 U eq of the parent atoms.
3 Comment
Organotin compounds have drawn growing interest owing to their diverse structures and widespread applications. 5 For instance, they participate in the synthesis of many complex organic molecules, including drugs, pesticides, and functional compounds. 6 They also serve as additives or catalysts for polymer modification and functionalization. 7 Furthermore, some organotin carboxylates exhibit biological activities such as antimicrobial, anticancer, and antiviral properties. 8 With increasing understanding of the structure-property relationships of organotin carboxylates, researchers are actively designing and developing novel compounds to expand their application scope.
In this study, we synthesized a new organotin carboxylate compound C33H22O6Sn by reacting 1, 8-dihydroxy-3-carboxyanthraquinone with triphenyltin chloride. The title complex is a monomer with one deprotonated ligand and one triphenyltin unit. The tin atom is linked to three phenyl groups and one oxygen atom, leading to a distorted tetrahedral geometry. The bond length Sn1–O6 [2.118(3) Å] is smaller than the sum of covalent radii of Sn and O [2.13 Å], which proves the existence of strong covalent bond between Sn and O. 9 The angle O6–Sn1–C28 96.92(16)° deviates significantly from the ideal tetrahedral coordination angle due to the interaction of O5 with the Sn atom. All these values are similar to the reported analogue compounds. 10 , 11 , 12 , 13 , 14 , 15 , 16
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by Shandong Provincial Natural Science Foundation under Grant ZR2020MB019.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Tiekink, E. R. T. Structural Chemistry of Organotin Carboxylates: a Review of the Crystallographic Literature. Appl. Organomet. Chem. 1991, 5, 1–23; https://doi.org/10.1002/aoc.590050102.Search in Google Scholar
6. Hadjikakou, S. K.; Hadjiliadis, N. Antiproliferative and Anti-tumor Activity of Organotin Compounds. Coord. Chem. Rev. 2009, 253, 235–249; https://doi.org/10.1016/j.ccr.2007.12.026.Search in Google Scholar
7. Gorelik, D. J.; Turner, J. A.; Virk, T. S.; Foucher, D. A.; Taylor, M. S. Site- and Stereoselective C–H Akylations of Carbohydrates Enabled by Cooperative Photoredox, Hydrogen Atom Transfer, and Organotin Catalysis. Org. Lett. 2021, 23, 2180–5185.10.1021/acs.orglett.1c01718Search in Google Scholar PubMed
8. Du, X.-M.; Zhang, R.-F.; Li, Q.-L.; Cheng, S.; Li, Y.-X.; Ru, J.; Ma, C.-L. Organotin(IV) Complexes Derived from 1,4-naphthalenedicarboxylic Acid: Synthesis, Structure, In Vitro Cytostatic Activity. J. Organomet. Chem. 2021, 935, 121654; https://doi.org/10.1016/j.jorganchem.2020.121654.Search in Google Scholar
9. Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451; https://doi.org/10.1021/j100785a001.Search in Google Scholar
10. Kovala-Demertzi, D.; Dokoro, V.; Primikiri, A.; Vargas, R.; Silvestru, C.; Russo, U.; Demertzis, M. A. Organotin Meclofenamic Complexes: Synthesis, Crystal Structures and Antiproliferative Activity of the First Complexes of Meclofenamic Acid – Novel Anti-tuberculosis Agents. J. Inorg. Biochem. 2009, 103, 738–744; https://doi.org/10.1016/j.jinorgbio.2009.01.014.Search in Google Scholar PubMed
11. Chandrasekhar, V.; Baskar, V.; Boomishankar, R.; Gopal, K.; Zacchini, S.; Bickley, J. F.; Steiner, A. Solventless Reactions for the Synthesis of Organotin Clusters and Cages. Organometallics 2003, 22, 3710–3716; https://doi.org/10.1021/om030338c.Search in Google Scholar
12. James, B. D.; Kivlighon, L. M.; Skelton, B. W.; White, A. H. Triphenyltin(IV) Compounds with Biologically Active Anionic Groups: Crystal and Molecular Structures of the P-Ethoxybenzoic Acid, Acetylsalicylic Acid, Phthalic Acid and Salicylaldehyde Derivatives. Appl. Organomet. Chem. 1998, 12, 13–23; https://doi.org/10.1002/(sici)1099-0739(199801)12:1<13::aid-aoc648>3.0.co;2-4.10.1002/(SICI)1099-0739(199801)12:1<13::AID-AOC648>3.0.CO;2-4Search in Google Scholar
13. Ma, C.-L.; Han, Y.-W.; Zhang, R.-F.; Wang, D.-Q. Self-assembled Triorganotin(IV) Moieties with 1,3,5-benzenetricarboxylic Acid: Syntheses and Crystal Structures of Monomeric, Helical, and Network Triorganotin(IV) Complexes. Eur. J. Inorg. Chem. 2005, 2005, 3024–3033; https://doi.org/10.1002/ejic.200500152.Search in Google Scholar
14. Wang, S.; Li, Q.-L.; Zhang, R.-F.; Du, J.-Y.; Li, Y.-X.; Ma, C.-L. Novel Organotin(IV) Complexes Derived from 4-carboxybenzenesulfonamide: Synthesis, Structure and In Vitro Cytostatic Activity Evaluation. Polyhedron 2019, 158, 15–24; https://doi.org/10.1016/j.poly.2018.10.048.Search in Google Scholar
15. Chandrasekhar, V.; Thilagar, P.; Steiner, A.; Bickley, J. F. Inorganic-cored Photoactive Assemblies: Synthesis, Structure, and Photochemical Investigations on Stannoxane-Supported Multifluorene Compounds. Chem. Eur. J. 2006, 12, 8847–8861; https://doi.org/10.1002/chem.200600556.Search in Google Scholar
16. Tian, W.; Wang, J.; Zhong, W.; Huang, H.; Ji, M.; Yang, T.; Lin, S.; Chen, S.; Yang, P. Synthesis, Crystal Structure and Antitumor Activity of Trialkyltin (IV) 9,10-Anthraquinone-2-Carboxylate. J. Organomet. Chem. 2024, 1006, 122997; https://doi.org/10.1016/j.jorganchem.2023.122997.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

