Abstract
C26H26N4O5, triclinic, P
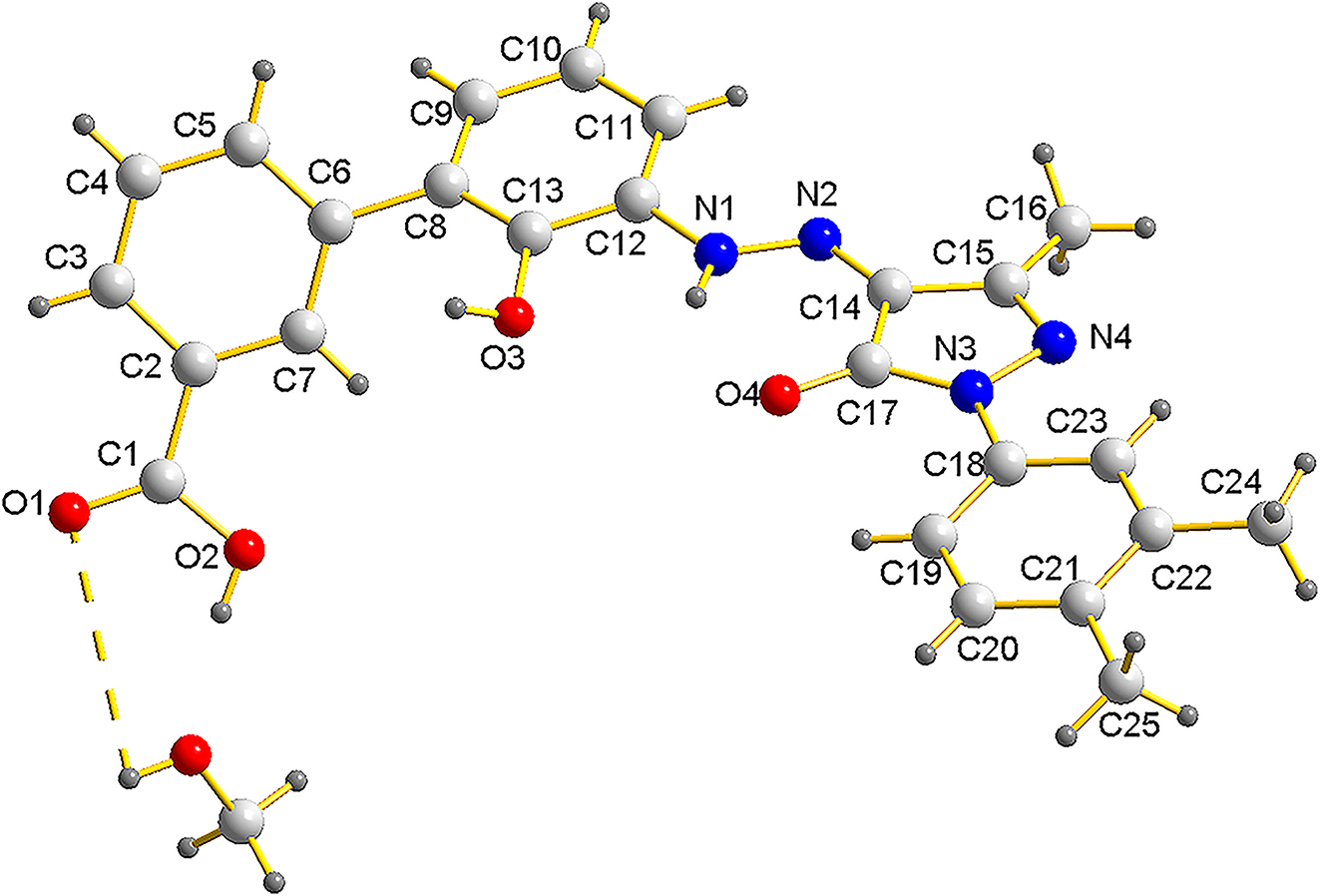
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.22 × 0.18 × 0.14 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.77 mm−1 |
| Diffractometer, scan mode: | Bruker D8 VENTURE, φ and ω |
| θmax, completeness: | 68.4°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 16,092, 4,309, 0.049 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3,634 |
| N(param)refined: | 323 |
| Programs: | Bruker 1 , SHELX 2 , 3 , Diamond 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.0628 (2) | 0.05022 (12) | 0.34557 (11) | 0.0532 (4) |
| C2 | −0.0894 (2) | 0.10910 (12) | 0.40441 (10) | 0.0492 (3) |
| C3 | −0.2746 (2) | 0.08385 (13) | 0.41119 (12) | 0.0588 (4) |
| H3A | −0.300764 | 0.032705 | 0.377887 | 0.071* |
| C4 | −0.4182 (2) | 0.13485 (14) | 0.46728 (13) | 0.0615 (4) |
| H4 | −0.541692 | 0.118607 | 0.471199 | 0.074* |
| C5 | −0.3805 (2) | 0.20991 (13) | 0.51778 (12) | 0.0555 (4) |
| H5 | −0.478693 | 0.242776 | 0.556440 | 0.067* |
| C6 | −0.19585 (19) | 0.23731 (12) | 0.51157 (10) | 0.0471 (3) |
| C7 | −0.05201 (18) | 0.18627 (12) | 0.45365 (10) | 0.0471 (3) |
| H7 | 0.071176 | 0.204086 | 0.447816 | 0.057* |
| C8 | −0.16012 (18) | 0.32311 (12) | 0.56156 (10) | 0.0467 (3) |
| C9 | −0.2761 (2) | 0.42146 (13) | 0.55180 (12) | 0.0537 (3) |
| H9 | −0.378371 | 0.429541 | 0.517684 | 0.064* |
| C10 | −0.2429 (2) | 0.50653 (13) | 0.59127 (12) | 0.0574 (4) |
| H10 | −0.322353 | 0.571059 | 0.583379 | 0.069* |
| C11 | −0.0925 (2) | 0.49695 (12) | 0.64254 (12) | 0.0537 (3) |
| H11 | −0.069373 | 0.554704 | 0.668816 | 0.064* |
| C12 | 0.02352 (19) | 0.39959 (12) | 0.65419 (10) | 0.0471 (3) |
| C13 | −0.00921 (18) | 0.31256 (11) | 0.61493 (10) | 0.0447 (3) |
| C14 | 0.3813 (2) | 0.44718 (13) | 0.77778 (11) | 0.0530 (3) |
| C15 | 0.4638 (3) | 0.52909 (14) | 0.80971 (13) | 0.0617 (4) |
| C16 | 0.4120 (4) | 0.65026 (17) | 0.78853 (19) | 0.0897 (7) |
| H16A | 0.476097 | 0.683314 | 0.827434 | 0.134* |
| H16B | 0.278525 | 0.663358 | 0.806902 | 0.134* |
| H16C | 0.447953 | 0.682353 | 0.717575 | 0.134* |
| C17 | 0.4940 (2) | 0.34317 (13) | 0.80869 (11) | 0.0514 (3) |
| C18 | 0.7731 (2) | 0.30618 (15) | 0.90186 (12) | 0.0599 (4) |
| C19 | 0.8359 (3) | 0.20495 (18) | 0.88273 (14) | 0.0720 (5) |
| H19 | 0.779069 | 0.175850 | 0.840744 | 0.086* |
| C20 | 0.9854 (3) | 0.1470 (2) | 0.92714 (16) | 0.0860 (6) |
| H20 | 1.027418 | 0.077700 | 0.915137 | 0.103* |
| C21 | 1.0758 (3) | 0.1888 (2) | 0.98951 (14) | 0.0822 (6) |
| C22 | 1.0108 (3) | 0.2902 (2) | 1.00826 (17) | 0.0864 (6) |
| C23 | 0.8605 (3) | 0.34794 (18) | 0.96455 (17) | 0.0819 (6) |
| H23 | 0.816693 | 0.416652 | 0.977437 | 0.098* |
| C24 | 1.1018 (5) | 0.3387 (3) | 1.0762 (3) | 0.1404 (14) |
| H24A | 1.101275 | 0.287380 | 1.141021 | 0.211* |
| H24B | 1.032754 | 0.406767 | 1.085157 | 0.211* |
| H24C | 1.229210 | 0.352845 | 1.045675 | 0.211* |
| C25 | 1.2407 (4) | 0.1226 (3) | 1.0352 (2) | 0.1106 (9) |
| H25A | 1.341939 | 0.169446 | 1.024480 | 0.166* |
| H25B | 1.281988 | 0.062100 | 1.003519 | 0.166* |
| H25C | 1.202998 | 0.094405 | 1.106702 | 0.166* |
| C26 | 0.6304 (3) | 0.0380 (3) | 0.17458 (19) | 0.1040 (8) |
| H26A | 0.618332 | 0.035096 | 0.107121 | 0.156* |
| H26B | 0.755745 | 0.011378 | 0.187136 | 0.156* |
| H26C | 0.606080 | 0.113060 | 0.181320 | 0.156* |
| N1 | 0.17966 (17) | 0.38416 (10) | 0.70430 (9) | 0.0511 (3) |
| H1 | 0.238477 | 0.319766 | 0.718465 | 0.061* |
| N2 | 0.23656 (18) | 0.46707 (10) | 0.72969 (9) | 0.0519 (3) |
| N3 | 0.6224 (2) | 0.37131 (11) | 0.85751 (10) | 0.0585 (3) |
| N4 | 0.6026 (2) | 0.48493 (12) | 0.85682 (11) | 0.0668 (4) |
| O1 | 0.03772 (19) | −0.02442 (10) | 0.30888 (9) | 0.0695 (3) |
| O2 | 0.23019 (16) | 0.08517 (12) | 0.33573 (11) | 0.0712 (4) |
| H2 | 0.307229 | 0.049123 | 0.302925 | 0.107* |
| O3 | 0.11747 (13) | 0.22214 (8) | 0.62915 (9) | 0.0544 (3) |
| H3 | 0.063056 | 0.165804 | 0.638128 | 0.082* |
| O4 | 0.47475 (16) | 0.25184 (9) | 0.79389 (9) | 0.0604 (3) |
| O5 | 0.5027 (2) | −0.02735 (13) | 0.24382 (14) | 0.0929 (5) |
| H5A | 0.490608 | −0.081728 | 0.223226 | 0.139* |
1 Source of materials
All reagents for the synthesis were commercially procured and used without further treatment. A solution of 3′-amino-2′-hydroxy-1,1′-biphenyl-3- carboxylic acid (1.0 g, 4.36 mmol) in methanol (20 mL) was stirred at 0–5 °C, 4 M hydrochloric acid (2.9 g, 10.73 mmol) was added. A solution of sodium nitrite (0.31 g, 4.36 mmol) in water (2 mL) was added over 20 min. The reaction was stirred at 0–5 °C for 1 h. Triethylamine (0.8 g, 7.85 mmol) was added to bring the pH to 8–9 and 2-(3,4-dimethylphenyl)-1,2-dihydro-5-methyl-3H-pyrazol-3-one (0.79 g, 3.93 mmol) was added in one portion. The mixture was stirred for 3 h at 15–25 °C maintaining the pH 8–9. After the reaction was over, 4 M hydrochloric acid was added dropwise to adjust the pH to 1.5–2. The temperature was kept for 1 h, the precipitate was filtered, washed with water and dried at 45 °C to yield (Z)-3′-(2-(1-(3,4- dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylic acid (1.70 g, 88 % yield) as a yellow solid. 0.5 g of (Z)-3′-(2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-5-pyrazol-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylic acid was dissolved in 20 mL of methanol. After filtration, the solution was left to evaporate slowly at room temperature with the cap being left only slightly open. After a period of 7 days, yellow crystals of the title compound were obtained.
2 Experimental details
Hydrogen atom were placed in their geometrically idealized positions and constrained to ride on their parent atoms with C–H = 0.96 Å (methyl) or 0.93 Å (methylene), N–H = 0.86 Å, O–H = 0.82 Å. For H atoms in methyl and hydroxyl groups, the uncertainty displacement parameter (Uiso(H)) was constrained to 1.5 times the equivalent isotropic displacement parameter (Ueq), and for all other H atoms, it was constrained to 1.2 times Ueq of the parent atoms.
3 Comment
Eltrombopag, chemically known as (Z)-3′-(2-(1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylic acid is a small molecule agonist of the c-mpl (TpoR) receptor, which is the physiological target of the hormone thrombopoietin. 5 Eltrombopag has a catalytic effect on the differentiation and proliferation of bone marrow progenitor cells, so it has been developed to treat immune thrombocytopenia (ITP). 6 , 7 , 8 , 9 , 10 The structural integrity and stability of Eltrombopag are crucial for its pharmaceutical applications. The study of its crystal structure not only provides insights into its physicochemical properties but also aids in the optimization of its formulation for improved bioavailability and therapeutic outcomes. 11 The crystalline structure elucidates that the two phenyl rings are not coplanar. 12 , 13 The dihedral angle between the best planes of the benzene ring (C2/C3/C4/C5/C6/C7) and benzene ring (C8/C9/C10/C11/C12/C13) is 44.61°. The crystalline structure elucidates that hydrogen bonds are important in the crystal structure. The hydroxyl group of methanol forms an O–H–O hydrogen bond to a carbonyl group. The complete set of X-ray diffraction data for the title compound was deposited to the Cambridge Crystallographic Data Centre (CCDC entry no. 2363039).
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Major Special Projects of Science and Technology Innovation of Tai’an (2021ZDZX029), Key Research and Development Program of Shandong Province (2021CXGC010514), Science and Technology Innovation Development Project of Tai’an (2023GX076), Double Ten Project of Tai’an (2023JSGG12).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B. A.; Thiessen, P. A.; Yu, B.; Zaslavsky, L.; Zhang, J.; Bolton, E. E. Pub Chem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. https://doi.org/10.1093/nar/gkaa971.Search in Google Scholar PubMed PubMed Central
6. Townsley, D. M.; Scheinberg, P.; Winkler, T.; Desmond, R.; Bogdan, D.; Rios, O.; Weinstein, B.; Valdez, J.; Lotter, J.; Feng, X. M.; Desierto, M.; Leuva, H.; Bevans, M.; Wu, C.; Larochelle, A.; Calvo, K. R.; Dunbar, C. E.; Young, N. S. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. New Engl. J. Med. 2017, 376, 1540–1550. https://doi.org/10.1056/nejmoa1613878.Search in Google Scholar PubMed PubMed Central
7. Fattizzo, B.; Levati, G.; Cassin, R.; Barcellini, W. Eltrombopag in Immune Thrombocytopenia, Aplastic Anemia, and Myelodysplastic Syndrome: From Megakaryopoiesis to Immunomodulation. Drugs 2019, 79, 1305–1319. https://doi.org/10.1007/s40265-019-01159-0.Search in Google Scholar PubMed
8. Olmsted, K. T.; Jenny, D.; Lambert, M. P. Eltrombopag for Use in Children with Immune Thrombocytopenia. Blood Adv. 2018, 2, 454–461. https://doi.org/10.1182/bloodadvances.2017010660.Search in Google Scholar PubMed PubMed Central
9. Desmond, R.; Townsley, D. M.; Dunbar, C.; Young, N. Eltrombopag in Aplastic Anemia. Semin. Hematol. 2015, 52, 31–37. https://doi.org/10.1053/j.seminhematol.2014.10.002.Search in Google Scholar PubMed PubMed Central
10. Li, R. X.; Wang, N. L.; Chai, X. X.; Yang, L. H.; Liu, K. K.; He, H. L.; Lin, S. Y.; Yang, Y.; Jia, J. S.; Zhang, D. H.; Gong, Y. M.; Shi, J. N.; He, G. S.; Li, J. Y. Prolonged Use of Eltrombopag in Patients with Severe Aplastic Anemia in the Real World. Clin. Exp. Med. 2023, 23, 2619–2627. https://doi.org/10.1007/s10238-023-00989-3.Search in Google Scholar PubMed
11. Huang, S. Y.; Yin, M. L.; Lin, Y. X.; Yang, H. Y.; Chen, J.; Qiu, T. Crystal Structure of 3,3′-Dimethoxy-4,4′-Oxy-Di-Benzaldehyde, C16H14O5. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 611–612. https://doi.org/10.1515/ncrs-2024–0133.10.1515/ncrs-2024-0095Search in Google Scholar
12. Koval’chukova, O. V.; Strashnova, S. B.; Avramenko, O. V.; Ryabov, M. A.; Dorovatovskii, P. V.; Zubavichus, Y. V.; Khrustalev, V. N. Coordination Compounds of Bivalent Metals with (Z)-4-(2–Hydroxy-5-Nitrophenyl)Hydrazono-3-Methyl-1-Phenyl-1h-Pyrazol-5(4h)-One: Crystal and Molecular Structure of C16H13N5O4. Russ. J. Inorg. Chem. 2018, 63, 874–880. https://doi.org/10.1134/s0036023618070112.Search in Google Scholar
13. Qian, H.-F.; Geng, J.; Xu, D.; Huang, W. Hydrazone to Deprotonated Azo/Azo-Enol Transformation for Isomeric Pyrazolone Based Heterocyclic Dyes via Metal-Ion Complexation. Dyes Pigments 2019, 160, 853–862. https://doi.org/10.1016/j.dyepig.2018.09.018.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

