Abstract
C8H15NO4S, orthorhombic, P212121 (no. 19), a = 5.0215 (3) Å, b = 7.0392 (5) Å, c = 28.0593 (19) Å, V = 991.82 (11) Å3, Z = 4, R gt(F) = 0.0462, wR ref(F 2) = 0.0899, T = 100 K.
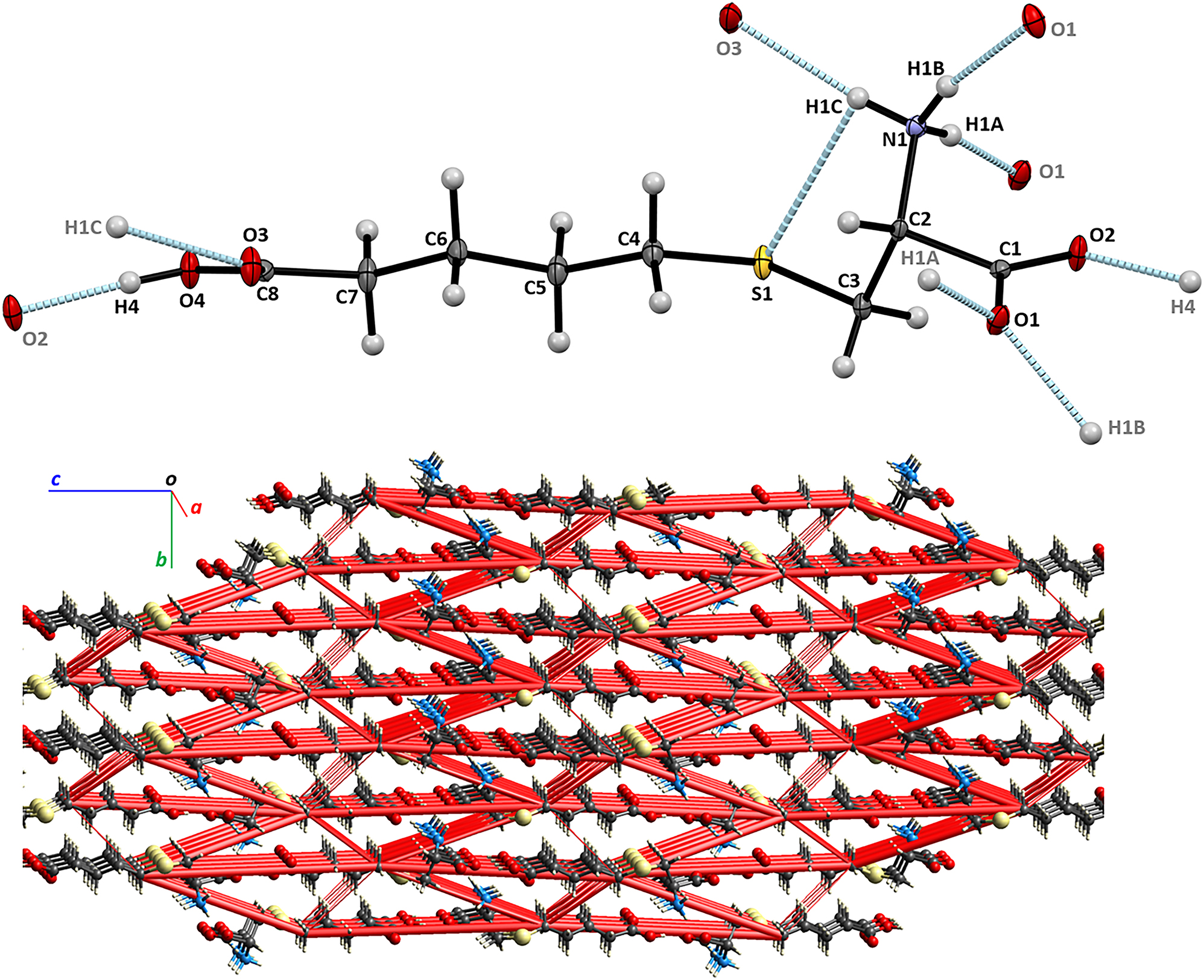
The molecular structure, hydrogen bonds, a part of the title crystal structure and the electrostatic energy framework are shown in the figure. Tables 1 and 2 contain details on the crystal structure as well as measurement conditions and a list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.18 × 0.10 × 0.01 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.32 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
Bruker APEX II, φ and ω
29.0°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 13,433, 2638, 0.048 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2473 |
| N(param)refined: | 174 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| S1 | 0.45191 (15) | 0.04882 (12) | 0.12329 (2) | 0.01319 (17) |
| O1 | 1.1723 (4) | −0.0418 (4) | 0.24142 (7) | 0.0136 (4) |
| O2 | 0.7671 (4) | −0.0568 (4) | 0.27473 (7) | 0.0134 (4) |
| C1 | 0.9256 (6) | −0.0711 (4) | 0.24029 (9) | 0.0087 (5) |
| O3 | 0.8845 (4) | −0.0766 (3) | −0.11118 (7) | 0.0165 (5) |
| O4 | 0.5098 (4) | 0.0376 (4) | −0.14442 (7) | 0.0144 (5) |
| C5 | 0.5356 (7) | 0.0382 (5) | 0.02807 (10) | 0.0139 (6) |
| H5A | 0.345 (8) | −0.003 (5) | 0.0283 (12) | 0.017* |
| H5B | 0.547 (8) | 0.177 (5) | 0.0277 (12) | 0.017* |
| C4 | 0.6598 (7) | −0.0325 (5) | 0.07451 (10) | 0.0128 (6) |
| H4A | 0.842 (7) | 0.014 (5) | 0.0795 (12) | 0.015* |
| H4B | 0.661 (8) | −0.173 (5) | 0.0759 (12) | 0.015* |
| C7 | 0.5341 (7) | 0.0446 (5) | −0.06108 (9) | 0.0141 (6) |
| H7A | 0.341 (8) | 0.011 (5) | −0.0629 (12) | 0.017* |
| H7B | 0.534 (8) | 0.183 (5) | −0.0596 (12) | 0.017* |
| C6 | 0.6665 (6) | −0.0385 (5) | −0.01705 (10) | 0.0130 (6) |
| H6A | 0.861 (7) | −0.009 (5) | −0.0169 (12) | 0.016* |
| H6B | 0.642 (7) | −0.180 (5) | −0.0183 (12) | 0.016* |
| N1 | 0.5859 (5) | −0.2606 (4) | 0.19879 (9) | 0.0088 (5) |
| C2 | 0.7987 (6) | −0.1157 (4) | 0.19187 (10) | 0.0085 (5) |
| H2 | 0.928 (7) | −0.169 (5) | 0.1700 (11) | 0.010* |
| C3 | 0.6849 (6) | 0.0706 (5) | 0.17204 (10) | 0.0114 (6) |
| H3A | 0.827 (7) | 0.151 (5) | 0.1641 (12) | 0.014* |
| H3B | 0.582 (7) | 0.124 (5) | 0.1962 (12) | 0.014* |
| C8 | 0.6645 (6) | −0.0048 (4) | −0.10798 (10) | 0.0105 (6) |
| H1A | 0.461 (8) | −0.207 (5) | 0.2132 (12) | 0.012 (9)* |
| H1B | 0.657 (8) | −0.362 (6) | 0.2149 (14) | 0.026 (11)* |
| H1C | 0.524 (10) | −0.301 (6) | 0.1713 (16) | 0.039* |
| H4 | 0.608 (9) | 0.034 (7) | −0.1716 (15) | 0.039* |
1 Source of materials
l–Cysteine (12.1 g, 0.1 mol) and 4-pentenoic acid (11 g, 0.11 mol) were dissolved in 500 mL of water containing 4.4 g (0.11 mol) of NaOH and left overnight at room temperature. The solution was further acidified with 6.6 mL (0.11 mol) of glacial acetic acid and allowed to stand at 4°C for next 3 days. Colourless plates of chromatographically pure crystalline CBC have formed during this time; the crystalline mass was filtered out, washed with cold 95 % ethanol, dried on air and used for subsequent diffraction studies. Element analysis. Calc. for C8H15NO4S: N, 6.33 %. Found: N, 6.37 %. Exact mass of the [M+H]+ ion in ESI mass-spectrum. Calc. for C6H12NO4S: m/z 222.07. Found: m/z 222.08.
2 Experimental details
The Flack absolute structure parameter determined (−0.02 (3) for 1277 quotients 5 ) is consistent with the (2R) configuration, which was assigned for this molecule on the basis of the known configuration for the starting material l-cysteine. Data were corrected for Lorentz, polarization, and absorption effects. The hydroxyl and ammonium hydrogen atoms were located in difference Fourier maps and were allowed to refine freely. The remaining H-atoms were placed at calculated positions and included in the refinement using a riding model. All hydrogen atom thermal parameters were constrained to ride on the carrier atoms (U iso(methine H) = 1.2 U eq and U iso(methyl H) = 1.5 U eq).
3 Comment
The title compound, S-(4-carboxybutyl)- l -cysteine (CBC), is a synthetic analog of two important biologically active S-carboxyalkylcysteines, a mucolytic drug S-carboxymethyl–l-cysteine (carbocisteine, CMC) and a natural insecticide from legumes S-(2-carboxyethyl)–l-cysteine (β–CEC). 6 Both CMC and β–CEC are thioether-containing amino acids and, similarly to methionine, possess the antioxidant capacity that could be employed in clinics. 7 Recently, 8 , 9 we have demonstrated that CMC and β–CEC can protect DNA from oxidative degradation, protect airway and kidney cells from cytotoxic platinum drugs or copper oxide nanoparticles, mitigate pro-inflammatory signaling in the cells. In continuation of our studies on antioxidant amino acids, 8 , 9 , 10 we have synthesized CBC and report here its molecular and crystal structure.
The asymmetric unit of the title structure contains one molecule of CBC, shown in the Figure generated by Mercury. 11 The valence bond lengths and angles are in the expected ranges. The molecule of this dicarboxylic amino acid exists as a zwitterion, with positively charged protonated α-amino group and negatively charged deprotonated α-carboxylic group. Most of non-hydrogen atoms, with exception of N1, O1, and C3, are located within 0.3 Å from a molecular plane. There is a weak intramolecular hydrogen bond between the ammonium donor and thioether acceptor heteroatoms (N1⃛S1 = 3.112 (3) Å, H1C⃛S1 = 2.83 (4) Å, N1–H1C⃛S1 = 101 (3)°). The conventional hydrogen bonding in crystal structure of CBC is extensive, involves all heteroatoms and forms network layers propagating in parallel to (001). Each layer is formed by a system of intercrossing chains. In the [100] direction, the heterodromic chain ⃛H1C–N1–H1B⃛O1–C1–O2⃛H4–O4–C8–O3⃛H1C–N1–H1B⃛ incorporates the shortest heteroatom contact formed between the α- and η-carboxylic groups (O4⃛O2 = 2.534 (3) Å, H4⃛O2 = 1.64 (4) Å, O4–H4⃛O2′ = 168 (4)°, ’ = 3/2−x, −y, −1/2 + z). In the [010] direction, the antidromic chains ⃛H1A–N1–H1B⃛O1⃛H1A–N1–H1B⃛O1⃛ feature bifurcated H-bonds at the O1 atoms. The network is reinforced by the short C3–H3A⃛O4 and C4–H4B⃛O3 contacts contributing to the polar interactions in the crystal structure, as well.
To account for all interactions contributing to the crystal structure, we have performed DFT calculations, at the B3LYP/6–31 G(d,p) theory level, 12 , 13 of the electrostatic, dispersion, polarization, and repulsion energies in the CBC crystal structure. According to the calculations, the interactions between pairs of molecules linked via the N1–H1B⃛O1″ hydrogen bond (” = 2−x, −1/2 + y, 1/2−z) provided the largest contribution, about 30 %, to the lattice energy, with the electrostatic interactions contributing the most for the attractive forces between neighbouring molecules of CBC (i.e. Eelstat = −90.3 kJ/mol, Eenergy-dispersive = −15 kJ/mol for 2 − x, −1/2 + y, 1/2 − z). The spatial distribution of the energetically most significant interactions is illustrated in the Figure, showing the electrostatic energy framework as red cylinders penetrating the crystal lattice of CBC. The cylinders connect centroids of the interacting molecules, and their diameters are proportional to the total energies of the interactions, with the 10 kJ/mol cut-off, for clarity. The most extensive intermolecular interactions occur in the directions parallel to [001]. To estimate the crystal structure energy, the total energies of unique pairwise interactions between molecules were integrated, thus yielding Ecryst = −353.5 kJ/mol for the CBC crystal.
Funding source: University of Missouri Agriculture Experiment Station Chemical Laboratories and by the National Institute of Food and Agriculture
Award Identifier / Grant number: Hatch project 1023929
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: University of Missouri Agriculture Experiment Station Chemical Laboratories and by the National Institute of Food and Agriculture (Hatch project 1023929).
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. Apex3 v. 2016.9–0 and SAINT v. 8.37A. Bruker Axs Inc.: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Parsons, S.; Flack, H. D.; Wagner, T. Use of Intensity Quotients and Differences in Absolute Structure Refinement. Acta Crystallogr. 2013, B69, 249–259; https://doi.org/10.1107/s2052519213010014.Search in Google Scholar
6. Romeo, J. T.; Simmonds, M. S. J. Nonprotein Amino Acid Feeding Deterrents from Calliandra. ACS Symp. Ser. 1989, 387, 59–68; https://doi.org/10.1021/bk-1989-0387.ch005.Search in Google Scholar
7. Maccio, A.; Madeddu, C.; Panzone, F.; Mantovani, G. Carbocysteine: Clinical Experience and New Perspectives in the Treatment of Chronic Inflammatory Diseases. Expert Opin. Pharmacother. 2009, 10, 693–703; https://doi.org/10.1517/14656560902758343.Search in Google Scholar PubMed
8. Waters, J. K.; Kelley, S. P.; Mossine, V. V.; Mawhinney, T. P. Structure, Antioxidant and Anti-inflammatory Activities of the (4R)- and (4S)-Epimers of S-Carboxymethyl–L-Cysteine Sulfoxide. Pharmaceuticals 2020, 13, 270; https://doi.org/10.3390/ph13100270.Search in Google Scholar PubMed PubMed Central
9. Waters, J. K.; Mossine, V. V.; Kelley, S. P.; Mawhinney, T. P. Structural and Functional Studies of S-(2-carboxyethyl)–L-cysteine and S-(2-carboxyethyl)–L-cysteine Sulfoxide. Molecules 2022, 27, 5317; https://doi.org/10.3390/molecules27165317.Search in Google Scholar PubMed PubMed Central
10. Mawhinney, T. P.; Li, Y.; Chance, D. L.; Kelley, S. P.; Mossine, V. V. Crystal Structure of (R,S)-2-hydroxy-4-(methylsulfanyl)butanoic Acid. Acta Crystallogr. 2020, E76, 562–566; https://doi.org/10.1107/s2056989020003138.Search in Google Scholar
11. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez–Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A. Mercury CSD 2.0 – New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470; https://doi.org/10.1107/s0021889807067908.Search in Google Scholar
12. Spackman, P. R.; Turner, M. J.; McKinnon, J. J.; Wolff, S. K.; Grimwood, D. J.; Jayatilaka, D.; Spackman, M. A. CrystalExplorer: a Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011; https://doi.org/10.1107/s1600576721002910.Search in Google Scholar
13. Thomas, S. P.; Spackman, P. R.; Jayatilaka, D.; Spackman, M. A. Accurate Lattice Energies for Molecular Crystals from Experimental Crystal Structures. J. Chem. Theor. Comput. 2018, 14, 1614–1623; https://doi.org/10.1021/acs.jctc.7b01200.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

