Abstract
C14H20Cl6N8O5Pt, monoclinic, P21/n (no. 14), a = 13.4907(2) Å, b = 13.32240(10) Å, c = 13.8035(2) Å, β = 102.241(1)∘, Z = 4, V = 2424.48(5) Å3, R gt(F) = 0.0256, wR ref(F 2) = 0.0740, T = 290 K.
1 Source of material and general procedures
All chemicals were obtained from commercial sources and used as purchased (Tables 1 and 2).
Data collection and handling.
| Crystal: | Yellow plate |
| Size: | 0.14 × 0.07 × 0.02 mm |
| Wavelength: μ: |
Cu Kα radiation (1.54184 Å) 17.3 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
XtaLAB Synergy, 74.0°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 36111, 4890, 0.032 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4839 |
| N(param) refined: | 318 |
| Programs: | DIAMOND, 1 CrysAlisPRO, 2 SHELX 3 , 4 , 5 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Pt1 | 0.49999 (2) | 0.18203 (2) | 0.23125 (2) | 0.01197 (8) |
| Cl1 | 0.46938 (8) | 0.08039 (8) | 0.35868 (7) | 0.0287 (2) |
| Cl2 | 0.52633 (7) | 0.28561 (6) | 0.10376 (6) | 0.01848 (18) |
| Cl3 | 0.50510 (7) | 0.04200 (6) | 0.13350 (7) | 0.01947 (18) |
| Cl4 | 0.67420 (7) | 0.17041 (6) | 0.29112 (7) | 0.01872 (18) |
| Cl5 | 0.32720 (7) | 0.19171 (7) | 0.16946 (7) | 0.02033 (19) |
| Cl6 | 0.49605 (8) | 0.32525 (7) | 0.32617 (7) | 0.0239 (2) |
| O1 | 0.5517 (2) | 0.2302 (2) | 0.8053 (2) | 0.0199 (5) |
| O2 | 0.8083 (2) | 0.0096 (2) | 0.9296 (2) | 0.0203 (6) |
| O3 | 0.3991 (2) | 0.4420 (2) | 0.6796 (2) | 0.0206 (5) |
| O4 | 0.3137 (2) | 0.5313 (2) | 0.97049 (19) | 0.0193 (5) |
| N1 | 0.6861 (2) | 0.1260 (2) | 0.8672 (2) | 0.0148 (6) |
| N2 | 0.7721 (2) | 0.0343 (2) | 0.7628 (2) | 0.0167 (6) |
| N3 | 0.7204 (2) | 0.0837 (2) | 0.5887 (2) | 0.0184 (6) |
| H31 | 0.760125 | 0.044971 | 0.561845 | 0.022* |
| N4 | 0.6051 (2) | 0.1937 (2) | 0.6036 (2) | 0.0180 (6) |
| H41 | 0.557143 | 0.239412 | 0.588991 | 0.022* |
| N5 | 0.3575 (2) | 0.4908 (2) | 0.8252 (2) | 0.0155 (6) |
| N6 | 0.2195 (2) | 0.5993 (2) | 0.8293 (2) | 0.0158 (6) |
| N7 | 0.1354 (2) | 0.6604 (2) | 0.6646 (2) | 0.0166 (6) |
| H71 | 0.087387 | 0.697952 | 0.680009 | 0.020* |
| N8 | 0.2316 (2) | 0.5857 (2) | 0.5777 (2) | 0.0171 (6) |
| H81 | 0.257139 | 0.565931 | 0.527295 | 0.020* |
| C1 | 0.6207 (3) | 0.1753 (3) | 0.7905 (3) | 0.0157 (7) |
| C2 | 0.7587 (3) | 0.0539 (3) | 0.8575 (3) | 0.0158 (7) |
| C3 | 0.7165 (3) | 0.0871 (3) | 0.6869 (3) | 0.0156 (7) |
| C4 | 0.6525 (3) | 0.1501 (3) | 0.5407 (3) | 0.0205 (8) |
| H4 | 0.640493 | 0.163702 | 0.471632 | 0.025* |
| C5 | 0.6444 (3) | 0.1547 (3) | 0.6971 (3) | 0.0155 (7) |
| C6 | 0.6742 (3) | 0.1478 (3) | 0.9692 (3) | 0.0207 (8) |
| H6A | 0.626125 | 0.100066 | 0.987810 | 0.031* |
| H6B | 0.648496 | 0.216256 | 0.972213 | 0.031* |
| H6C | 0.740023 | 0.141407 | 1.015276 | 0.031* |
| C7 | 0.8467 (3) | −0.0406 (3) | 0.7459 (3) | 0.0232 (8) |
| H7A | 0.899571 | −0.007362 | 0.718630 | 0.035* |
| H7B | 0.812744 | −0.091287 | 0.698877 | 0.035* |
| H7C | 0.877397 | −0.072998 | 0.808797 | 0.035* |
| C8 | 0.3464 (3) | 0.4931 (3) | 0.7223 (3) | 0.0148 (7) |
| C9 | 0.2975 (3) | 0.5403 (3) | 0.8799 (3) | 0.0144 (7) |
| C10 | 0.2070 (3) | 0.6064 (3) | 0.7295 (3) | 0.0144 (7) |
| C11 | 0.1523 (3) | 0.6453 (3) | 0.5729 (3) | 0.0169 (7) |
| H11 | 0.113188 | 0.673291 | 0.513721 | 0.020* |
| C12 | 0.2674 (3) | 0.5596 (3) | 0.6765 (3) | 0.0143 (7) |
| C13 | 0.4376 (3) | 0.4265 (3) | 0.8818 (3) | 0.0202 (8) |
| H13A | 0.494049 | 0.468284 | 0.915941 | 0.030* |
| H13B | 0.461749 | 0.380391 | 0.836491 | 0.030* |
| H13C | 0.410440 | 0.387788 | 0.930686 | 0.030* |
| C14 | 0.1515 (3) | 0.6516 (3) | 0.8823 (3) | 0.0240 (8) |
| H14A | 0.089726 | 0.611819 | 0.878682 | 0.036* |
| H14B | 0.133739 | 0.717377 | 0.851819 | 0.036* |
| H14C | 0.185648 | 0.660502 | 0.951851 | 0.036* |
| O1W | 0.4628 (3) | 0.3273 (2) | 0.5431 (2) | 0.0324 (7) |
| H1W | 0.457 (5) | 0.384 (2) | 0.574 (4) | 0.049* |
| H2W | 0.463 (5) | 0.341 (5) | 0.4808 (15) | 0.049* |
The title compound was synthesized by dissolving 0.18 g (1 mmol) theophylline (180.16 g/mol) and 0.14 PtCl4 (0.5 mmol) in 1 mL concentrated hydrochloric acid. Short-time warming until both components were dissolved, yielded a yellow/orange solution. From the aforementioned solution a large number of block crystals grew upon slow cooling to room temperature within minutes.
The Raman spectra were measured using a Bruker MULTIRAM spectrometer (Nd: YAG-laser at 1064 nm; InGaAs detector) with an apodized resolution of 8 cm−1 in the region of 4000–70 cm−1.
2 Experimental details
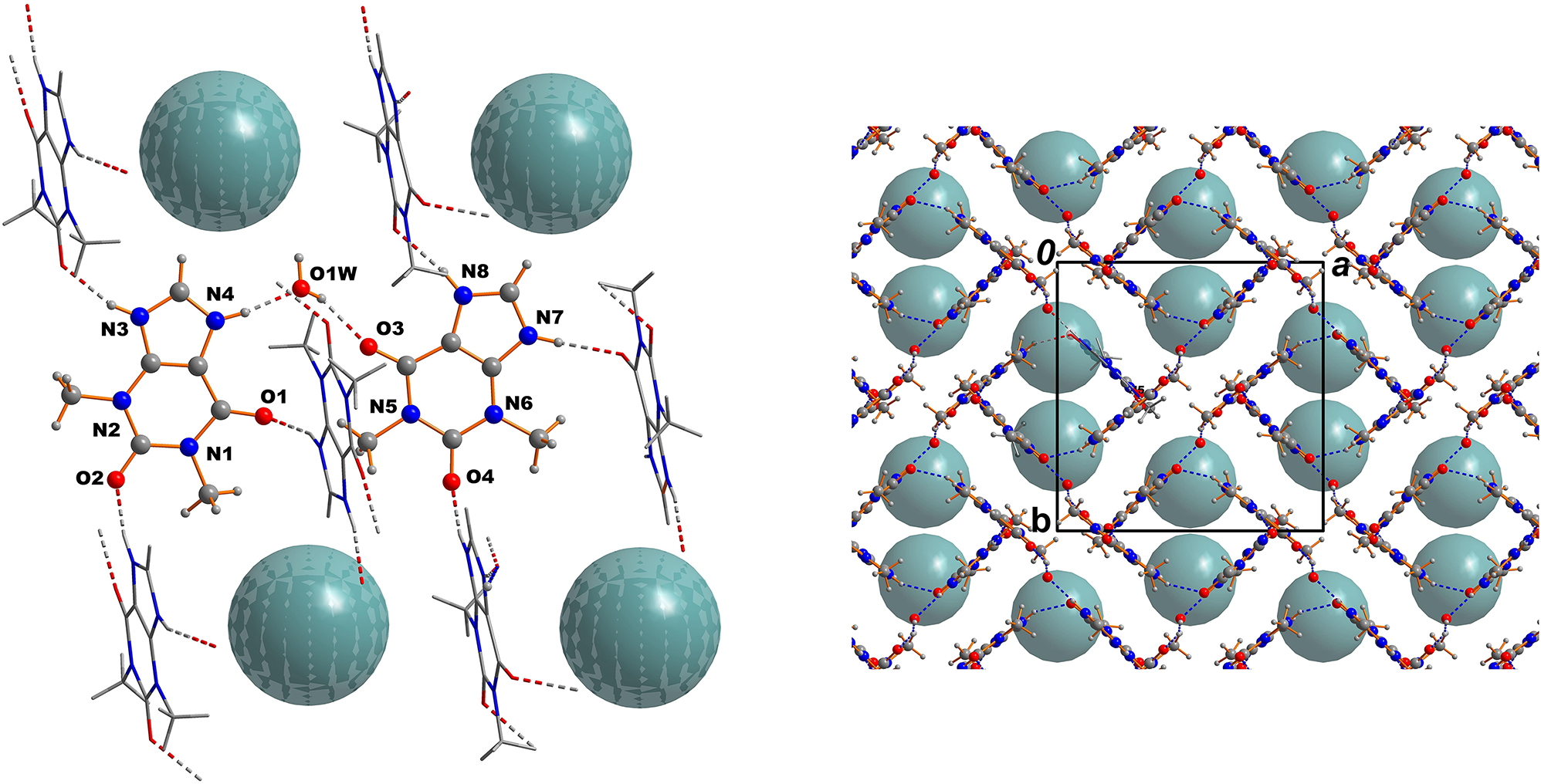
A single crystal of the title compound was directly selected from the mother liquor and rapidly transferred to the Xcalibur four-circle diffractometer equipped with an EOS detector. 2 An absorption correction (Gaussian method) was applied. 2 The structure solution and the refinement were successfully carried out using the SHELX program system. 3 , 4 , 5 Pseudosymmetry is present in this structure, as the complex [PtCl6]2− anions almost occupy positions that meet the conditions of a higher symmetry (right part of the figure). 6 , 7
All hydrogen atoms were seen in the Fourier map after all non-hydrogen atoms were located. C-bound hydrogen atoms were included using a riding model. Coordinates of nitrogen- and oxygen bound atoms were refined using distance restraints and individually refined Uiso parameters.
3 Comment
3.1 Introduction
Theophylline is a natural product and was first described by Kossel after its isolation from tea leaves which is responsible for the naming up to now. 8 , 9 There is still a fundamental interest in this compound, the corresponding solid state phases 10 , 11 and its co-crystals. 12 , 13 , 14 , 15 Nowadays theophylline is often used as pharmaceutical agent due to its effects on the respiratory system. 16 , 17 , 18 , 19 We have already shown that methylxanthines like caffeine 20 and especially theophylline 21 , 22 , 23 , 24 are excellent tectons to construct hydrogen bonded networks. A database check (Cambridge Structural database 25 ) showed that not more than about twenty crystal structures have been deposited so far, that contain a theophyllinium cation. From the reaction of theophylline (systematic name: 1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione) with hydrochloric in the presence of one half of an equivalent of PtCl4, block crystals of the title compound were obtained.
3.2 Structural comments
The asymmetric unit of the title structure consists of two N-protonated theophyllinium cations (TheoH), one hexachloridoplatinate(IV) dianion and one water molecule, which are all located in general positions. Bond lengths within the TheoH cation are all in the expected ranges. 21 , 22 , 23 , 24 The same is true for the hexachloridoplatinate(IV) anion. 26 The TheoH cation I (N1···N4) is connected to three neighboring TheoH cations and one water molecule by NH···O hydrogen bonds (see left part of the figure). The TheoH cation II (N5···N8) accepts one OH···O hydrogen bond from a water molecule and it is involved in hydrogen bonds with three neighboring TheoH cations. The water molecule furthermore donates a OH···Cl hydrogen bond (H···Cl: 2.28(2) Å; O···Cl: 3.121(3) Å; O–H···Cl = 160°). These connections create a network of the cationic sub structure with the flat theophyllinium cations to be oriented perpendicular to each other (see the left part of the Figure). It should not go unmentioned that the C–H group of the five membered ring of each TheoH cation forms a weak hydrogen bond to neigboring hexachloridoplatinate(IV) anions (C4···Cl4: 3.530(4) Å; C11···Cl2: 3.574(4) Å) Finally it should be mentioned that there is a halogen-π interaction between the chlorido ligand Cl3 and the N7 atom of the cation II (3.198(3) Å). All the aforementioned intermolecular interactions create a complex hydrogen bonded framework. In the right part of the figure the packing is shown with view along [001]. It is clearly visible that the TheoH cations are oriented almost perpendicular (82.1°) to each other to create the network including the water molecules.
3.3 Raman spectroscopy
There are three very strong signals in the Raman spectrum at 346, 319 and 163 cm−1 respectively. These signals must be assigned to the [[PtCl6]2− anion and perfectly agree with reported spectra. 27 , 28
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: Ministry of Innovation, Science and Research of North–Rhine Westphalia and the German Research Foundation (DFG) for financial support (Xcalibur diffractometer; INST 208/533–1, project no. 162659349). I. S. K. thanks the Alexander von Humboldt Foundation and European Commission for her MSCA4Ukraine grant. Finally, funding by the open access fund of the Heinrich-Heine-Universität Düsseldorf is also gratefully acknowledged.
References
1. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.5.2; Crystal Impact: Bonn, Germany, 2018.Search in Google Scholar
2. Oxford Diffraction: CrysAlisPRO, (version 1.171.33.42)Oxford Diffraction Ltd.: Oxford, UK, 2009.Search in Google Scholar
3. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B. ShelXle: a Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284; https://doi.org/10.1107/s0021889811043202.Search in Google Scholar PubMed PubMed Central
6. Reiss, G. J. A Reinvestigation of Wilm’s Salt, (NH4)4[RhCl6]NO3 – Structure, Spectroscopy and Thermal Analysis. Z. für Kristallogr. – Cryst. Mater. 2002, 217, 550–556; https://doi.org/10.1524/zkri.217.10.550.20794.Search in Google Scholar
7. Megen, M.; Reiss, G. J. The Pseudosymmetric Structure of Bis(pentane-1,5-Diaminium) Iodide Tris(triiodide). Acta Crystallogr. 2012, E68, o1331–o1332; https://doi.org/10.1107/s1600536812014420.Search in Google Scholar PubMed PubMed Central
8. Kossel, A. Ueber eine neue Base aus dem Pflanzenreich. Ber. Dtsch. Chem. Ges. 1888, 21, 2164; https://doi.org/10.1002/cber.188802101422.Search in Google Scholar
9. Kossel, A. Ueber das Theophyllin, einen neuen Bestandtheil des Thees. Z. Physiolog. Chem. 1889, 13, 298–308; https://doi.org/10.1515/bchm1.1889.13.3.298.Search in Google Scholar
10. Fucke, K.; McIntyre, G. J.; Wilkinson, C.; Henry, M.; Howard, J. A.; Steed, J. W. New Insights into an Old Molecule: Interaction Energies of Theophylline Crystal Forms. Cryst. Growth Des. 2012, 12, 1395–1401; https://doi.org/10.1021/cg201499s.Search in Google Scholar
11. Matsuo, K.; Matsuoka, M. Solid-state Polymorphic Transition of Theophylline Anhydrate and Humidity Effect. Cryst. Growth Des. 2007, 7, 411–415; https://doi.org/10.1021/cg060299i.Search in Google Scholar
12. Darwish, S.; Zeglinski, J.; Krishna, G. R.; Shaikh, R.; Khraisheh, M.; Walker, G. M.; Croker, D. M. A New 1:1 Drug-Drug Cocrystal of Theophylline and Aspirin: Discovery, Characterization, and Construction of Ternary Phase Diagrams. Cryst. Growth Des. 2018, 18, 7526–7532; https://doi.org/10.1021/acs.cgd.8b01330.Search in Google Scholar
13. Wang, L.; Luo, M.; Li, J.; Wang, J.; Zhang, H.; Deng, Z. Sweet Theophylline Cocrystal with Two Tautomers of Acesulfame. Cryst. Growth Des. 2015, 15, 2574–2578; https://doi.org/10.1021/acs.cgd.5b00207.Search in Google Scholar
14. McTague, H.; Rasmuson, A. C. Nucleation of the Theophylline : Salicylic Acid 1:1 Cocrystal. Cryst. Growth Des. 2021, 21, 2711–2719; https://doi.org/10.1021/acs.cgd.0c01594.Search in Google Scholar PubMed PubMed Central
15. Lange, L.; Sadowski, G. Polymorphs, Hydrates, Cocrystals, and Cocrystal Hydrates: Thermodynamic Modeling of Theophylline Systems. Cryst. Growth Des. 2016, 16, 4439–4449; https://doi.org/10.1021/acs.cgd.6b00554.Search in Google Scholar
16. Persson, C. G. A. Overview of Effects of Theophylline. J. Allergy Clin. Immunol. 1986, 78, 780–787; https://doi.org/10.1016/0091-6749(86)90061-8.Search in Google Scholar PubMed
17. Sofian, Z. M.; Benaouda, F.; Wang, J. T. W.; Lu, Y.; Barlow, D. J.; Royall, P. G.; Farag, D. B.; Rahman, K. M.; Al Jamal, K. T.; Forbes, B.; Jones, S. A. A Cyclodextrin Stabilized Spermine Tagged Drug Triplex that Targets Theophylline to the Lungs Selectively in Respiratory Emergency. Adv. Therap. 2020, 5, 2000153. https://doi.org/10.1002/adtp.202000153.Search in Google Scholar PubMed PubMed Central
18. Benaouda, F.; Jones, S. A.; Chana, J.; Dal Corno, B. M.; Barlow, D. J.; Hider, R. C.; Page, C. P.; Forbes, B. Ion-pairing with Spermine Targets Theophylline to the Lungs via the Polyamine Transport System. Mol. Pharm. 2018, 15, 861–870; https://doi.org/10.1021/acs.molpharmaceut.7b00715.Search in Google Scholar PubMed
19. Tanaka, R.; Hattori, Y.; Otsuka, M.; Ashizawa, K. Application of Spray Freeze Drying to Theophylline-Oxalic Acid Cocrystal Engineering for Inhaled Dry Powder Technology. Drug Dev. Ind. Pharm. 2020, 46, 179–187; https://doi.org/10.1080/03639045.2020.1716367.Search in Google Scholar PubMed
20. Merkelbach, J.; Majewski, M. A.; Reiss, G. J. Crystal Structure of Caffeinium triiodide – Caffeine (1/1), C16H21I3N8O4. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 941–944; https://doi.org/10.1515/ncrs-2018-0125.Search in Google Scholar
21. Reiss, G. J. A Cyclic I2−10 Anion in the Layered Crystal Structure of Theophyllinium pentaiodide, C7H9I5N4O2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 737–739; https://doi.org/10.1515/ncrs-2019-0082.Search in Google Scholar
22. Wyshusek, M.; Reiss, G. J.; Frank, W. The Triple Salt 2(C7H9N4O2) [MoOCl4(H2O)]·2(C7H9N4O2)Cl·(H17O8)Cl Containing a C2-Symmetrical Unbranched H+(H2O)8 Zundel Type Species in a Framework Composed of Theophyllinium, Aquatetrachloridooxidomolybdate and Chloride Ions. Z. Anorg. Allg. Chem. 2021, 647, 575–581; https://doi.org/10.1002/zaac.202100007.Search in Google Scholar
23. Reiss, G. J.; Wyshusek, M.; Rheinländer, J. C. An I2−6 Anion in the Crystal Structure of Theophyllinium triiodide monohydrate, C7H11I3N4O3. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 1017–1020; https://doi.org/10.1515/ncrs-2022-0358.Search in Google Scholar
24. Reiss, G. J.; Wyshusek, M. Cones with a Three-fold Symmetry Constructed from Three Hydrogen Bonded Theophyllinium Cations that Coat [FeCl4]− Anions in the Crystal Structure of Tris(theophyllinium) bis(tetrachloridoferrate(III)) chloride trihydrate, C21H33Cl9Fe2N12O9. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 121–124. https://doi.org/10.1515/ncrs-2021–0399.10.1515/ncrs-2021-0399Search in Google Scholar
25. Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179; https://doi.org/10.1107/s2052520616003954.Search in Google Scholar
26. Ha, K. Diacridinium hexachloridoplatinate(IV) dihydrate. Acta Crystallogr. 2010, E66, m425; https://doi.org/10.1107/s1600536810009566.Search in Google Scholar
27. Woodward, L. A.; Creigrton, J. A. Raman Spectra of the Hexachloropalladate, Hexachloroplatinate and Hexabromoplatinate Ions in Aqueous Solution. Spectrochim. Acta 1961, 17, 594–599; https://doi.org/10.1016/0371–1951(61)80119-7.10.1016/0371-1951(61)80119-7Search in Google Scholar
28. Ermakova, T. G.; Shaulina, L. P.; Kuznetsova, N. P.; Volkova, L. I.; Pozdnyakov, A. S.; Prozorova, G. F. Sorption of Noble Metal Compounds by Cross-Linked Copolymer of 1-Vinyl-1,2,4-triazole with Acrylic acid. Russ. J. Appl. Chem. 2012, 85, 35–40; https://doi.org/10.1134/S1070427212010077.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

