Abstract
C40H48Cu2N12O12, monoclinic, P21/n (no. 14), a = 10.9519(1) Å, b = 17.4351(2) Å, c = 24.4288(3) Å, β = 102.143(1)°, V = 4560.25(9) Å3, Z = 4, Rgt(F) = 0.0647, wRref(F2) = 0.1904, T = 120 K.
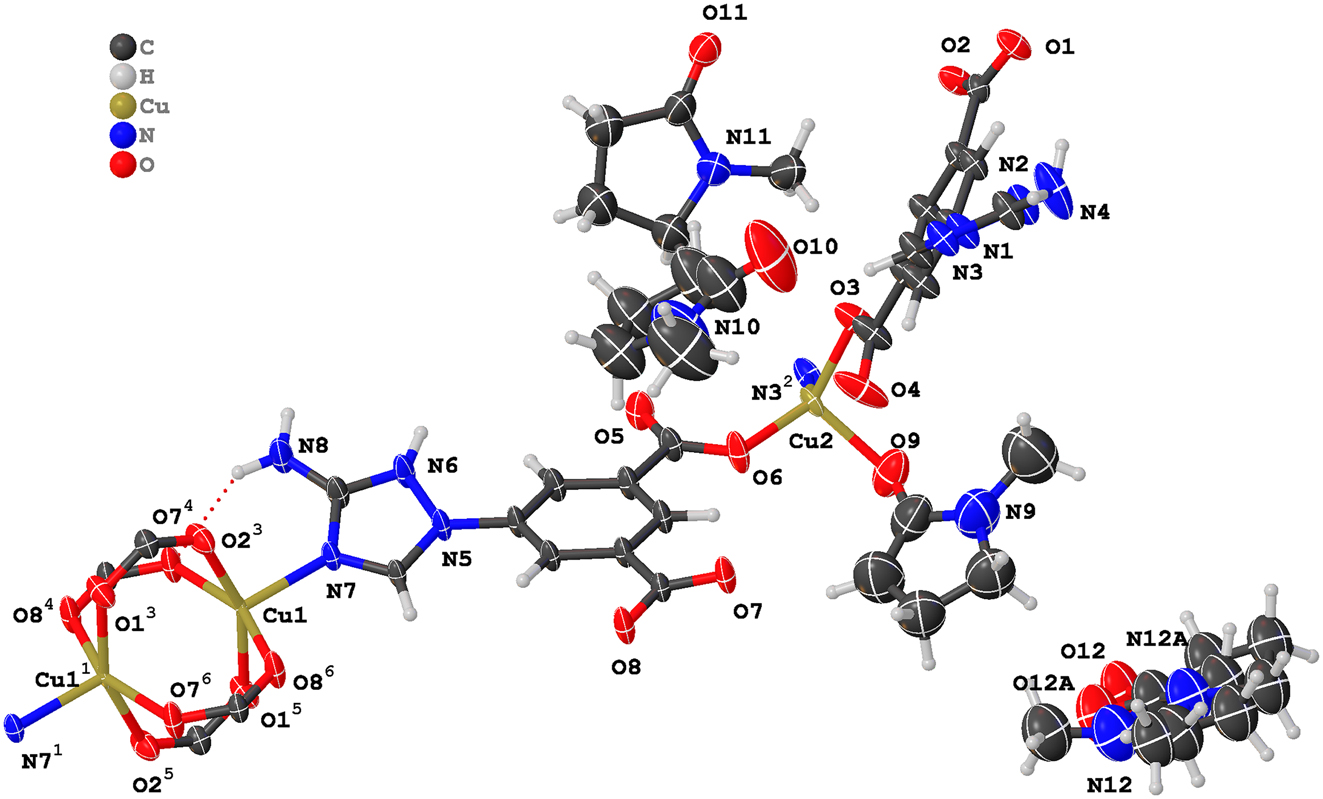
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Blue block |
| Size: | 0.26 × 0.22 × 0.04 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.78 mm−1 |
| Diffractometer, scan mode: | Bruker SMART CCD 6000, φ and ω |
| θmax, completeness: | 68.2°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 28,368, 8311, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 7622 |
| N(param)refined: | 656 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cu1 | −0.38778 (4) | 0.54794 (2) | 0.52401 (2) | 0.01653 (16) |
| Cu2 | 0.51734 (5) | 0.93450 (3) | 0.66025 (2) | 0.03025 (18) |
| O1 | 1.0514 (3) | 0.89476 (17) | 0.95136 (13) | 0.0448 (7) |
| O2 | 0.8909 (3) | 0.96950 (17) | 0.91477 (12) | 0.0447 (7) |
| O3 | 0.6404 (3) | 0.91395 (17) | 0.72876 (14) | 0.0515 (8) |
| O4 | 0.6793 (4) | 0.8115 (2) | 0.68238 (17) | 0.0823 (15) |
| O5 | 0.2298 (3) | 0.88405 (17) | 0.65550 (15) | 0.0507 (8) |
| O6 | 0.4054 (3) | 0.85697 (17) | 0.62653 (15) | 0.0503 (8) |
| O7 | 0.4718 (2) | 0.60520 (16) | 0.55220 (14) | 0.0424 (7) |
| O8 | 0.3080 (2) | 0.53242 (17) | 0.51722 (14) | 0.0427 (7) |
| O9 | 0.6190 (5) | 0.9572 (3) | 0.6030 (2) | 0.0833 (13) |
| N1 | 1.0094 (3) | 0.66584 (19) | 0.82380 (16) | 0.0439 (9) |
| N2 | 1.1337 (3) | 0.6573 (2) | 0.84648 (18) | 0.0494 (9) |
| N3 | 1.0380 (3) | 0.54281 (18) | 0.82897 (17) | 0.0428 (9) |
| N4 | 1.2563 (4) | 0.5457 (3) | 0.8683 (3) | 0.085 (2) |
| H4A | 1.282740 | 0.558833 | 0.903032 | 0.102* |
| H4B | 1.244444 | 0.496450 | 0.866833 | 0.102* |
| N5 | −0.0510 (3) | 0.65666 (18) | 0.58279 (14) | 0.0331 (7) |
| N6 | −0.1131 (3) | 0.6865 (2) | 0.62208 (16) | 0.0448 (9) |
| H6 | −0.084863 | 0.718156 | 0.649899 | 0.054* |
| N7 | −0.2373 (3) | 0.60899 (17) | 0.56077 (13) | 0.0287 (6) |
| N8 | −0.3181 (3) | 0.6658 (2) | 0.63452 (18) | 0.0525 (11) |
| H8A | −0.306332 | 0.695206 | 0.664474 | 0.063* |
| H8B | −0.390783 | 0.643419 | 0.622497 | 0.063* |
| N9 | 0.7911 (7) | 0.8990 (5) | 0.5774 (4) | 0.117 (2) |
| C1 | 0.9587 (3) | 0.9122 (2) | 0.91419 (16) | 0.0307 (7) |
| C2 | 0.9262 (4) | 0.8597 (2) | 0.86486 (17) | 0.0348 (8) |
| C3 | 0.9873 (4) | 0.7898 (2) | 0.86512 (18) | 0.0366 (8) |
| H3 | 1.053081 | 0.776430 | 0.895599 | 0.044* |
| C4 | 0.9516 (4) | 0.7402 (2) | 0.82081 (19) | 0.0427 (9) |
| C5 | 0.8607 (4) | 0.7597 (2) | 0.7743 (2) | 0.0503 (11) |
| H5 | 0.839746 | 0.725761 | 0.743367 | 0.060* |
| C6 | 0.8009 (4) | 0.8302 (2) | 0.7740 (2) | 0.0474 (10) |
| C7 | 0.8322 (4) | 0.8791 (2) | 0.81968 (18) | 0.0410 (9) |
| H7 | 0.788835 | 0.926272 | 0.819926 | 0.049* |
| C8 | 0.7001 (5) | 0.8521 (3) | 0.7240 (2) | 0.0524 (11) |
| C9 | 0.9543 (4) | 0.5983 (2) | 0.8141 (2) | 0.0471 (10) |
| H9 | 0.868033 | 0.590508 | 0.798741 | 0.056* |
| C10 | 1.1463 (4) | 0.5817 (2) | 0.8486 (2) | 0.0491 (11) |
| C11 | 0.2951 (4) | 0.8422 (2) | 0.63298 (19) | 0.0384 (8) |
| C12 | 0.2446 (3) | 0.7658 (2) | 0.60889 (18) | 0.0352 (8) |
| C13 | 0.1211 (3) | 0.7458 (2) | 0.60812 (18) | 0.0350 (8) |
| H13 | 0.068519 | 0.779220 | 0.623470 | 0.042* |
| C14 | 0.0760 (3) | 0.6763 (2) | 0.58461 (17) | 0.0323 (7) |
| C15 | 0.1505 (3) | 0.6253 (2) | 0.56297 (16) | 0.0312 (7) |
| H15 | 0.117634 | 0.577962 | 0.547098 | 0.037* |
| C16 | 0.2751 (3) | 0.6451 (2) | 0.56500 (16) | 0.0302 (7) |
| C17 | 0.3210 (3) | 0.7149 (2) | 0.58741 (17) | 0.0343 (8) |
| H17 | 0.405450 | 0.728206 | 0.588177 | 0.041* |
| C18 | 0.3580 (3) | 0.5898 (2) | 0.54291 (16) | 0.0290 (7) |
| C19 | −0.1258 (3) | 0.60990 (19) | 0.54784 (15) | 0.0268 (7) |
| H19 | −0.102893 | 0.581665 | 0.518297 | 0.032* |
| C20 | −0.2254 (3) | 0.6553 (2) | 0.60724 (18) | 0.0383 (9) |
| C26 | 0.6635 (8) | 0.9116 (5) | 0.5717 (4) | 0.0968 (18) |
| C27 | 0.6058 (10) | 0.8655 (7) | 0.5284 (5) | 0.131 (2) |
| H27 | 0.517648 | 0.862358 | 0.516125 | 0.157* |
| C28 | 0.6900 (9) | 0.8235 (6) | 0.5039 (4) | 0.117 (2) |
| H28A | 0.677484 | 0.835102 | 0.463464 | 0.140* |
| H28B | 0.678740 | 0.767776 | 0.508664 | 0.140* |
| C29 | 0.8187 (10) | 0.8486 (6) | 0.5345 (4) | 0.123 (2) |
| H29A | 0.869711 | 0.804248 | 0.551086 | 0.148* |
| H29B | 0.863198 | 0.876334 | 0.509148 | 0.148* |
| C30 | 0.8927 (13) | 0.9333 (8) | 0.6220 (6) | 0.162 (4) |
| H30A | 0.915119 | 0.897202 | 0.653266 | 0.242* |
| H30B | 0.862693 | 0.981265 | 0.635516 | 0.242* |
| H30C | 0.966232 | 0.943849 | 0.606305 | 0.242* |
| O10 | 0.6705 (6) | 0.6302 (5) | 0.8041 (4) | 0.172 (3) |
| N10 | 0.4953 (9) | 0.5959 (5) | 0.7478 (4) | 0.143 (3) |
| C31 | 0.4844 (10) | 0.7131 (7) | 0.7778 (6) | 0.144 (3) |
| H31A | 0.486366 | 0.732020 | 0.816186 | 0.173* |
| H31B | 0.519217 | 0.753553 | 0.757102 | 0.173* |
| C32 | 0.3591 (9) | 0.6971 (5) | 0.7505 (4) | 0.115 (2) |
| H32A | 0.329511 | 0.735222 | 0.720585 | 0.138* |
| H32B | 0.303465 | 0.698872 | 0.777542 | 0.138* |
| C33 | 0.3593 (10) | 0.6206 (6) | 0.7266 (5) | 0.131 (2) |
| H33A | 0.337045 | 0.621983 | 0.685212 | 0.157* |
| H33B | 0.300913 | 0.585998 | 0.740607 | 0.157* |
| C34 | 0.5337 (13) | 0.5210 (7) | 0.7399 (7) | 0.182 (4) |
| H34A | 0.532775 | 0.490524 | 0.773535 | 0.273* |
| H34B | 0.618482 | 0.521830 | 0.732819 | 0.273* |
| H34C | 0.476626 | 0.497986 | 0.707762 | 0.273* |
| C35 | 0.5631 (9) | 0.6407 (6) | 0.7804 (5) | 0.127 (2) |
| O11 | 0.4603 (3) | 0.9464 (2) | 0.93764 (15) | 0.0558 (8) |
| N11 | 0.4282 (5) | 0.9451 (3) | 0.8430 (2) | 0.0695 (13) |
| C21 | 0.4017 (6) | 0.9269 (4) | 0.8923 (3) | 0.0763 (15) |
| C22 | 0.2847 (8) | 0.8768 (7) | 0.8816 (3) | 0.114 (2) |
| H22A | 0.303704 | 0.825388 | 0.898416 | 0.137* |
| H22B | 0.218435 | 0.900623 | 0.897931 | 0.137* |
| C23 | 0.2440 (8) | 0.8708 (6) | 0.8200 (3) | 0.112 (2) |
| H23A | 0.159426 | 0.892898 | 0.807544 | 0.134* |
| H23B | 0.241629 | 0.816392 | 0.808222 | 0.134* |
| C24 | 0.3406 (7) | 0.9163 (6) | 0.7939 (3) | 0.101 (2) |
| H24A | 0.382878 | 0.882361 | 0.771219 | 0.121* |
| H24B | 0.299880 | 0.958996 | 0.770132 | 0.121* |
| C25 | 0.5346 (7) | 0.9894 (5) | 0.8372 (3) | 0.089 (2) |
| H25A | 0.587143 | 0.999015 | 0.874275 | 0.134* |
| H25B | 0.506782 | 1.038355 | 0.819129 | 0.134* |
| H25C | 0.582724 | 0.961127 | 0.814221 | 0.134* |
| C36 | 1.0676 (8) | 0.8452 (6) | 0.4389 (4) | 0.1118 (19) |
| O12a | 0.9849 (11) | 0.8936 (9) | 0.4405 (7) | 0.104 (4) |
| N12a | 1.0272 (16) | 0.7641 (11) | 0.4178 (8) | 0.119 (3) |
| C37a | 1.1274 (18) | 0.7079 (17) | 0.4189 (17) | 0.116 (3) |
| H37Aa | 1.123146 | 0.682769 | 0.382194 | 0.140* |
| H37Ba | 1.130819 | 0.668724 | 0.448548 | 0.140* |
| C38a | 1.246 (2) | 0.7735 (14) | 0.4346 (11) | 0.114 (3) |
| H38Aa | 1.311930 | 0.754044 | 0.465642 | 0.137* |
| H38Ba | 1.283715 | 0.781534 | 0.401551 | 0.137* |
| C39a | 1.1936 (19) | 0.8518 (15) | 0.4521 (11) | 0.115 (3) |
| H39Aa | 1.222792 | 0.860785 | 0.492762 | 0.138* |
| H39Ba | 1.221482 | 0.894953 | 0.431458 | 0.138* |
| C40a | 0.9180 (18) | 0.7372 (15) | 0.4197 (11) | 0.131 (4) |
| H40Aa | 0.896638 | 0.751671 | 0.455305 | 0.196* |
| H40Ba | 0.918848 | 0.681205 | 0.416565 | 0.196* |
| H40Ca | 0.855764 | 0.758503 | 0.388626 | 0.196* |
| O12Ab | 0.9590 (11) | 0.8463 (9) | 0.4247 (6) | 0.124 (3) |
| N12Ab | 1.1570 (12) | 0.7963 (10) | 0.4452 (7) | 0.113 (2) |
| C37Ab | 1.2898 (15) | 0.8210 (11) | 0.4517 (9) | 0.113 (3) |
| H37Cb | 1.316385 | 0.825577 | 0.415544 | 0.136* |
| H37Db | 1.348820 | 0.788149 | 0.477873 | 0.136* |
| C38Ab | 1.160 (2) | 0.7284 (13) | 0.4222 (13) | 0.122 (3) |
| H38Cb | 1.080387 | 0.702094 | 0.420887 | 0.183* |
| H38Db | 1.228052 | 0.698052 | 0.444537 | 0.183* |
| H38Eb | 1.174001 | 0.734339 | 0.384125 | 0.183* |
| C39Ab | 1.2659 (14) | 0.9050 (10) | 0.4790 (8) | 0.112 (3) |
| H39Cb | 1.290680 | 0.903465 | 0.520375 | 0.134* |
| H39Db | 1.314194 | 0.945805 | 0.464925 | 0.134* |
| C40Ab | 1.1409 (15) | 0.9179 (11) | 0.4622 (9) | 0.123 (3) |
| H40Db | 1.126962 | 0.958116 | 0.432988 | 0.147* |
| H40Eb | 1.108122 | 0.937197 | 0.494439 | 0.147* |
- a
Occupancy: 0.444 (6), bOccupancy: 0.556 (6).
1 Source of materials
The 5-(3-amino-1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L–NH2) and 1-methyl-2-pyrrolidinone (NMP) were commercial purchased from Shanghai Accela ChemBio Co., Ltd. Other reagents were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. In a 20 mL capped vial, CuCl2·2H2O (0.0170 g, 0.1 mmol) and H2L–NH2 (0.0248 g, 0.1 mmol) were dissolved by NMP and ethyl alcohol (8 mL, V/V = 6:2). The vial was then sealed, transfered to an oven and heated to 110 °C for 72 h and cooled to room temperature at a rate of 15 °C/h. Blue block-like crystals of the title complex were obtained, yielding 41 % (based on the H2L–NH2 ligand).
2 Experimental details
The initial crystal structure was solved by SHELXT program and refined by SHELXL program. Cu-, C-, N- and O-atoms were refined with anisotropic displacement parameters and H-atoms were placed in idealized positions with isotropic thermal parameters. One of the guest NMP molecules was disordered and split treatment were performed with DFIX and SIMU command.
3 Comment
As a ligand bearing two kinds of potential coordinating atoms (carbonyl oxygen atom and triazole nitrogen atom), 5-(1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L) has been applied to construct complexes with a series of metal salts [5], [6], [7], [8]. However, up to now, the metal complexes based on the amino functionalized H2L, i.e., 5-(3-amino-1H-1,2,4-triazol-1-yl)-1,3-benzenedicarboxylic acid (H2L–NH2) has never been reported. Hence, to enrich the family of the metal complexes based on H2L–NH2, the title 3D Cu(II)-complex was synthesized under hydrothermal condition.
The asymmetric unit contains two copper(II) ions, two deprotonated H2L–NH2 ligands, three free NMP molecules and one coordinated NMP molecule. The Cu1(II) cation is penta-coordinated, bonded with four O-atoms (O1−3/2+x,3/2−y,−1/2+z, O21/2−x,−1/2+y,+3/2−z, O7−1+x,y,z, O8−x,1−y,1−z) derived from four different H2L–NH2 ligands and one N-atom (N7) from another H2L–NH2 ligand. The Cu2(II) caion is tetra-coordinated, bonded with three O-atoms (O3, O6, O9) derived from two different H2L–NH2 ligands and the coordinated NMP molecule, and one N-atom (N33/2−x,1/2+y,3/2−z) derived from another H2L–NH2 ligand. The Cu–O and Cu–N bond lengths are within the normal range of 1.893(3)–2.069(3) Å and 2.008(3)–2.017(3) Å, respectively. The bond lengths are comparable to the reported crystal structures containing analogous H2L–NH2 ligand with similar coordination environments [9], [10]. The dihedral angle between the triazole heterocyclic ring and centre benzene ring of the two crystallographic independent H2L–NH2 ligands are 26.77° and 39.97°, respectively. The two H2L–NH2 ligands displayed unified coordination modes, i.e., each ligand coordinated with four copper(II) ions. The two carboxyl groups in each H2L–NH2 ligand revealed different coordination mode. Significantly, two Cu1(II) cations, four carboxyl groups formed classical dinuclear [Cu2(COOR)4] paddle-wheel type secondary building units (SBUs; see the figure). In the end, the copper(II) ions and H2L–NH2 ligands connected with each other and with the help of intramolecular N–H⋯O hydrogen bonding interactions (N4–H4B⋯O53/2−x,−1/2+y,3/2−z, N8–H8A⋯O4−1+x,y,z, N8–H8B⋯O7−1+x,y,z), resulted in the 3D supermolecule framework of the title Cu(II)-complex.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Shandong Province Natural Science Foundation (No. ZR2022QB202).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL – Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
5. Yuan, F., Ma, H.-X., Yuan, C.-M., Zhou, C.-S., Hu, H.-M., Kumar, A., Muddassir, M. Syntheses of a series of lanthanide metal-organic frameworks for efficient UV-light-driven dye degradation: experiment and simulation. CrystEngComm 2021, 23, 2404–2413; https://doi.org/10.1039/d0ce01245a.Search in Google Scholar
6. Zhu, Z.-X., Wang, C.-J., Luo, D., Liu, C., Liu, D.-N., Xiao, Y.-M., Chen, S., Wang, Y.-Y. Six new lanthanide metal-organic frameworks as luminescent sensors for the detection of 1–N, TDGA, UA, and HA in urine. J. Coord. Chem. 2019, 72, 3526–3543; https://doi.org/10.1080/00958972.2019.1702646.Search in Google Scholar
7. Sun, J., Xi, Y., Gao, L., Hu, M., Liu, W., Ma, E., Huang, R., Qin, W., Wu, G. Two isostructural Ln–MOFs containing triazole groups as luminescent probes for efficient sensing of NACs and Fe3+. Inorg. Chim. Acta 2023, 547, 121376; https://doi.org/10.1016/j.ica.2022.121376.Search in Google Scholar
8. Yuan, F., Li, Y., Yuan, C.-M., Liu, Y.-L., Zhou, C.-S., Chen, F.-Y., Cao, B.-Y., Li, Z.-J., Li, K.-B., Yu, H.-S. Effect of pH on the construction of zinc coordination polymers based on carboxylate functionalized triazole derivative ligand. J. Mol. Struct. 2019, 1198, 126905; https://doi.org/10.1016/j.molstruc.2019.126905.Search in Google Scholar
9. Wu, M. Z., Ma, Z. L., Shi, J. Y., Tian, L. A Zn(II) metal-organic framework based on bimetallic paddle wheels as a luminescence indicator for carcinogenic organic pollutants: phthalate esters. RSC Adv. 2019, 9, 37101–37108; https://doi.org/10.1039/c9ra08417g.Search in Google Scholar PubMed PubMed Central
10. Ma, Z. L., Liu, P. X., Liu, Z. Y., Wang, J. J., Li, L. B., Tian, L. A thermally and chemically stable copper(II) metal-organic framework with high performance for gas adsorption and separation. Inorg. Chem. 2021, 60, 6550–6558; https://doi.org/10.1021/acs.inorgchem.1c00357.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial 2024 – New developments and changes of Zeitschrift für Kristallographie – New Crystal Structures
- New Crystal Structures
- Hydrogen bonding and π⋅⋅⋅halogen interactions in the crystal structure of bis(theophyllinium) hexachloridoplatinate(IV) monohydrate
- The crystal structure of 6-amino-2-carboxypyridin-1-ium perchlorate, C6H7ClN2O6
- Crystal structure of poly[(μ4-(3-amino-1H-1,2,4-triazol-1-yl)benzene-1,3-dicarboxylato-κ 4 N:O:O':O')(1-methylpyrroldin-2-one-κ1O)dicopper(II)] – 1-methylpyrroldin-2-one (1/3), C40H48Cu2N12O12
- The crystal structure of 18-crown-6-k6O6(2,4,5-trinitroimidazol-1-ido-k1O)potassium(I)
- Crystal structure of poly[tetraaqua-bis(μ2-5-bromoisophthalato-κ3O,O′:O″)-(μ2-1,5-bis(imidazol-2-methyl)pentane-κ2N:N′)dicadmium(II)] dihydrate
- Crystal structure of (5R,6S,E)-5-acetoxy-2-methyl-6-((2aR,3R,5aS,5bS,11aR,12aS)-2a,5a,8,8-tetramethyl-9-oxotetradecahydro-1H,12H-cyclopenta[a]cyclopropa[e]phenanthren-3-yl)hept-2-enoic acid, C32H48O5
- The crystal structure of poly[diaqua-bis(μ2 -thiocyanato-κ2N:O)cobalt(II) monohydrate
- The crystal structure of 1,3,5-tri(1H-imidazol-1-yl)benzene–2,3,5,6-tetrachlorobenzene-1,4-dicarboxylic acid (1/1)
- Crystal structure of dichlorido-bis(1-[(2-ethyl-benzimidazole-1-yl)methyl]-1H–benzotriazole) cadmium(II), C32H32CdN10OCl2
- The crystal structure of N′-(tert-butyl)-N′-(3,5-dimethylbenzoyl)-3-methoxy-N,2-dimethylbenzohydrazide, C23H30N2O3
- Crystal stucture of 3-benzamido-N-(2-bromo-4-(perfluoropropan-2-yl)-6-(trifluoromethyl)phenyl)-2-fluorobenzamide
- Crystal structure of bis(μ-benzeneselenolato)-(tetracarbonyl)-{μ-[N-(diphenylphosphanyl)-N-(3-ethynylphenyl)-P,P-diphenylphosphinous amide]} diiron, C48H35Fe2NO4P2Se2
- The crystal structure of 2′-(p-tolyl)-4′H-spiro[isochromane-1,1′-naphthalene]-3,4′-dione, C25H18O3
- The crystal structure of poly[hexaqua-tetrakis(μ4-pyridine-2,4-dicarboxylate-κ5N: O: O′: O″: O‴)-bi(μ2-pyridine-2,4-dicarboxylate-κ3N: O: O′)-digadolinium(III)tricopper (II)], [Gd2Cu3(C7H3NO4)6(H2O)6] n
- Crystal structure of poly[bis(4-(4-(pyridin-4-yl)phenyl)pyridin-1-ium-κ1N)-(μ4-benzene-1,2,4,5-tetracarboxylato-κ5O:O′: O″:O‴:O⁗)-(μ2-2,5-dicarboxyterephthalato-κ2O:O′)dizinc(II)], C52H32N4O16Zn2
- The crystal structure of 4-(3-carboxy-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium 2-carboxy-6-nitrobenzoate monohydrate, C24H25FN4O10
- Crystal structure of dichlorido-(1-((3,5-dimethyl-2,3-dihydro-1H-1,2,3-triazol-1-yl)methyl)-1H-benzo[d][1,2,3]triazole-k1N)zinc(II), C22H24ZnN12Cl2
- The crystal structure of (3-chlorothiophene-2-carboxylato-κ2O, O′)-(2,2′-dipyridyl-κ2N,N′)lead(II), C20H12Cl2N2O4S2Pb
- Synthesis and crystal structure of (Z)-4-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one, C19H14FN5O
- The crystal structure of the coordination compound catena-poly[(18-crown-6-ether-κ6O6)(4,5-dinitroimidazolato-κ1O)potassium(I)]
- Crystal structure of 7-(diethylamino)-3-(trifluoroacetyl)-2H-chromen-2-one, C15H14F3NO3
- Crystal structure of dichlorido-1-[(2-ethylimidazole-1-yl)methyl]-1H–benzotriazole κ1N zinc(II), C24H26ZnN10Cl2
- Crystal and molecular structure of 5-bromopyridine-2,3-diamine
- Crystal structure of catena-poly[bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-k3-O,O′:O″)hexaqua-dicobalt tetrahydrate], C26H36N4O20Co2
- Crystal structure of thiocyanate-κ1N-bis(μ1-2,6-diformyl-4-methylphenol oxime-κ2N,O)-manganese(III) acetonitrile solvate, C21H21MnN6O6S
- The crystal structure of pyrrolidin-1-yl pivalate, C9H13NO4
- The crystal structure of 2,2′-(2,2-diphenylethene-1,1-diyl)bis(1,4-dimethoxybenzene), C30H28O4
- Crystal structure of bis(benzyltrimethylammonium) tetrathiotungstate(VI), {(C6H5CH2)(CH3)3N}2[WS4]
- The crystal structure of ethyl (Z)-2-(ethoxymethylene)-3-oxobutanoate, C9H14O4
- The crystal structure of (E)-6-bromo-3,5-dimethyl-2-(1-phenylprop-1-en-2-yl)-3Himidazo[4,5b]pyridine, C17H16BrN3
- Crystal structure of (3S,3′S,4R,4′S)-3′-(furan-3-yl)-3-hydroxy-4′-methyl-3,5,6′,7′-tetrahydro-1H,3′H-4,5′-spirobi[isobenzofuran]-1,1′(4′H)-dione-methanol (1/1), C21H22O7
- Cocrystal structure of progesterone-isophthalic acid, C25H33O4
- The crystal structure of 3-(6-fluoro-1H-indol-3-yl)-1-methylquinoxalin-2(1H)-one, C17H12FN3O
- Crystal structure of S-(4-carboxybutyl)- l -cysteine
- The cocrystal of 2,2′-(hydrazine-1,1-diyl)bis(1H-imidazole-4,5-dicarbonitrile)– methanol (2/3)
- Crystal structure of (1′R,2′S,4′R,6′S)-4,6-dihydroxy-1′,8′,8′-trimethyl-3-(3-methylbutanoyl)-4′,8′,6′,1′,7,2′-hexahydro-1H-4′,6′-methanoxanthene-8-carbaldehyde, C23H30O5
- Crystal structure of (3,6-di(2-pyridyl)-4-methylphenyl pyridazine-k 2 N,N′)-bis(1-phenyl-pyrazole-κ 2 C,N) iridium(III) hexafluorophosphate, C39H29F6IrN8P
- Crystal structure of 1,5-bis[(E)-1-(2-hydroxyphenyl)ethylidene]thiocarbonohydrazide dimethyl sulfoxide monosolvate, C17H18N4O2S·C2H6OS
- Crystal structure of (S)-4-(2-(4-(2-acetyl-5-chlorophenyl)-3-methoxy-6-oxopyridazin-1(6H)-yl)-3-phenylpropanamido)benzoic acid monohydrate, C29H26ClN3O7
- The crystal structure of 1,3-bis(2,4-dinitro-1H-imidazol-1-yl)propane
- Crystal structure of 4-chlorobenzyl (S)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H19ClO3
- Crystal structure of 1-(5-(benzo[d][1,3]dioxol-5-yl)-4-benzyl-1-(4-bromophenyl)-4,5-dihydro-1H-1,2,4-triazol-3-yl)ethan-1-one, C24H20BrN3O3
- The crystal structure of (Z)-3′-(2-(1-(3,4-dimethyl-phenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene)hydrazinyl)-2′-hydroxy-[1,1′-biphenyl]-3-carboxylicacid ─ methanol (1/1), C26H26N4O5
- Crystal structure of (S)-1-phenylpropan-1-aminium (S)-(1-phenylpropyl)carbamate C19H26N2O2
- Synthesis and crystal structure of methyl 2-((5-bromo-4-(4-cyclopropylnaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)thio)acetate, C18H16BrN3O2S
- The crystal structure of trichlorobis(pyridine-2,6-dithio-κS-carbomethylamido)antimony(III), [SbCl3(C9H11N3S2)2]
- Crystal structure of 1,8-dihydroxy-3-{[(triphenylstannyl)oxy]carbonyl} anthracene-9,10-dione, C33H22O6Sn
- The crystal structure of (E)-4-(2-(pyridin-4-ylmethylene)hydrazine-1-carbonyl)pyridin-1-ium-2-olate dihydrate, C12H14N4O4
- The crystal structure of 6-amino-pyridinium-2-carboxylate, C6H6N2O2
- The crystal structure of catena-poly[aqua-nitrato-κ3O,O:O′′-(1,10-phenanthroline-κ2N,N′)sodium(I)], C24H18N6O7Na2
- Retractions
- Retraction of: Crystal structure of bis[diaquaisonicotinatosamarium(III)]-µ-isonicotinato-[diisonicotinatocopper(II)], CuSm2(C6H4NO2)8(H2O)4
- Retraction of: Crystal structure of aqua(2,2′-bipyridine-k 2 N:N′)(nitrato)-(4-aminobenzoato)cadmium(II) nitrate, [Cd(H2O)(NO3)(C10H8N2)(C7H7NO2)][NO3]

