Abstract
C75H76N10O23Tb2, triclinic,
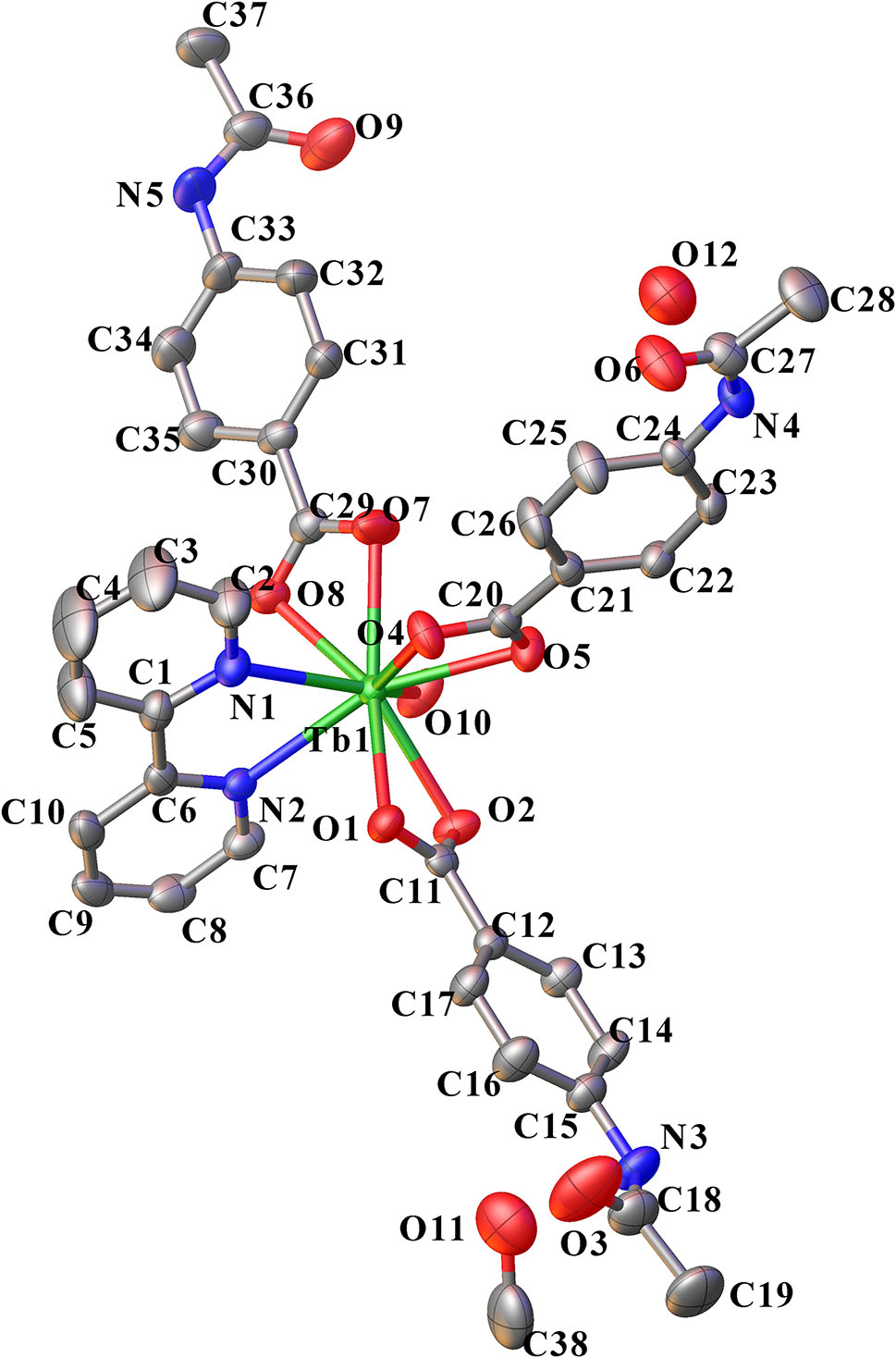
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.22 × 0.20 × 0.18 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 1.94 mm−1 |
| Diffractometer, scan mode: | φ and ω |
| θ max, completeness: | 25.0°, >99 % |
| N(hkl)measured , N(hkl)unique, R int: | 19,697, 6645, 0.027 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 6175 |
| N(param) refined: | 511 |
| Programs: | SHELX [1], [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.7370 (3) | 0.6218 (3) | 0.2255 (2) | 0.0363 (7) |
| C2 | 0.5147 (4) | 0.5380 (3) | 0.1996 (3) | 0.0539 (9) |
| H2 | 0.431205 | 0.538747 | 0.216121 | 0.065* |
| C3 | 0.5278 (5) | 0.4481 (4) | 0.1259 (3) | 0.0803 (15) |
| H3A | 0.454582 | 0.390240 | 0.092448 | 0.096* |
| C4 | 0.6513 (5) | 0.4458 (4) | 0.1027 (4) | 0.0907 (17) |
| H4A | 0.663249 | 0.385814 | 0.053305 | 0.109* |
| C5 | 0.7571 (4) | 0.5328 (3) | 0.1531 (3) | 0.0666 (12) |
| H5A | 0.841620 | 0.531915 | 0.138636 | 0.080* |
| C6 | 0.8460 (3) | 0.7217 (3) | 0.27809 (19) | 0.0314 (6) |
| C7 | 0.9069 (3) | 0.9027 (3) | 0.3841 (2) | 0.0386 (7) |
| H7 | 0.884160 | 0.963728 | 0.425822 | 0.046* |
| C8 | 1.0380 (3) | 0.9127 (3) | 0.3702 (2) | 0.0477 (9) |
| H8 | 1.101405 | 0.979345 | 0.401884 | 0.057* |
| C9 | 1.0722 (3) | 0.8237 (3) | 0.3098 (2) | 0.0483 (9) |
| H9 | 1.159581 | 0.828410 | 0.300311 | 0.058* |
| C10 | 0.9766 (3) | 0.7266 (3) | 0.2627 (2) | 0.0417 (8) |
| H10 | 0.998506 | 0.664974 | 0.221224 | 0.050* |
| C11 | 0.6909 (2) | 0.8099 (2) | 0.54682 (19) | 0.0278 (6) |
| C12 | 0.7295 (3) | 0.8140 (2) | 0.64509 (18) | 0.0291 (6) |
| C13 | 0.7370 (3) | 0.9099 (3) | 0.7218 (2) | 0.0365 (7) |
| H13 | 0.721211 | 0.973892 | 0.712166 | 0.044* |
| C14 | 0.7679 (3) | 0.9111 (3) | 0.8126 (2) | 0.0414 (8) |
| H14 | 0.772006 | 0.975729 | 0.863375 | 0.050* |
| C15 | 0.7928 (3) | 0.8171 (3) | 0.8287 (2) | 0.0372 (7) |
| C16 | 0.7869 (4) | 0.7216 (3) | 0.7526 (2) | 0.0475 (8) |
| H16 | 0.803797 | 0.657871 | 0.762055 | 0.057* |
| C17 | 0.7559 (3) | 0.7216 (3) | 0.6625 (2) | 0.0394 (7) |
| H17 | 0.752665 | 0.657182 | 0.611835 | 0.047* |
| C18 | 0.8376 (4) | 0.7394 (4) | 0.9548 (3) | 0.0566 (10) |
| C19 | 0.8738 (5) | 0.7732 (4) | 1.0596 (3) | 0.0780 (14) |
| H19A | 0.928184 | 0.848481 | 1.084582 | 0.117* |
| H19B | 0.921556 | 0.723067 | 1.073892 | 0.117* |
| H19C | 0.794652 | 0.769706 | 1.087448 | 0.117* |
| C20 | 0.3353 (3) | 0.71162) | 0.41405 (19) | 0.0302 (6) |
| C21 | 0.1972 (3) | 0.6633 (2) | 0.42178 (19) | 0.0304 (6) |
| C22 | 0.1251 (3) | 0.7255 (3) | 0.48031 (19) | 0.0339 (7) |
| H22 | 0.165493 | 0.798102 | 0.520555 | 0.041* |
| C23 | −0.0064 (3) | 0.6804 (3) | 0.4793 (2) | 0.0352 (7) |
| H23 | −0.054052 | 0.723063 | 0.518913 | 0.042* |
| C24 | −0.0689 (3) | 0.5721 (2) | 0.4199 (2) | 0.0318 (6) |
| C25 | 0.0040 (3) | 0.5076 (3) | 0.3631 (3) | 0.0466 (8) |
| H25 | −0.035321 | 0.434067 | 0.324495 | 0.056* |
| C26 | 0.1353 (3) | 0.5542 (3) | 0.3647 (2) | 0.0444 (8) |
| H26 | 0.183727 | 0.511079 | 0.326300 | 0.053* |
| C27 | −0.2851 (3) | 0.4309 (3) | 0.3804 (2) | 0.0420 (8) |
| C28 | −0.4275 (3) | 0.4243 (3) | 0.3931 (3) | 0.0601 (10) |
| H28A | −0.484159 | 0.376665 | 0.335169 | 0.090* |
| H28B | −0.443214 | 0.498078 | 0.410274 | 0.090* |
| H28C | −0.445786 | 0.393841 | 0.441776 | 0.090* |
| C29 | 0.4749 (3) | 0.8155 (2) | 0.1956 (2) | 0.0346 (7) |
| C30 | 0.4170 (3) | 0.8238 (2) | 0.10559 (19) | 0.0349 (7) |
| C31 | 0.2819 (3) | 0.8055 (3) | 0.0819 (2) | 0.0455 (8) |
| H31 | 0.226351 | 0.785567 | 0.121438 | 0.055* |
| C32 | 0.2283 (4) | 0.8163 (4) | −0.0001 (2) | 0.0567 (10) |
| H32 | 0.137079 | 0.804292 | −0.015079 | 0.068* |
| C33 | 0.3088 (4) | 0.8448 (4) | −0.0595 (2) | 0.0579 (10) |
| C34 | 0.4442 (4) | 0.8602 (4) | −0.0380 (2) | 0.0581 (10) |
| H34 | 0.498978 | 0.877522 | −0.078952 | 0.070* |
| C35 | 0.4981 (4) | 0.8498 (3) | 0.0439 (2) | 0.0484 (8) |
| H35 | 0.589222 | 0.860294 | 0.057980 | 0.058* |
| C36 | 0.1660 (4) | 0.9164 (4) | −0.1486 (2) | 0.0532 (9) |
| C37 | 0.1250 (4) | 0.9196 (5) | −0.2447 (3) | 0.0738 (13) |
| H37A | 0.035800 | 0.928062 | −0.250411 | 0.111* |
| H37B | 0.129744 | 0.850902 | −0.291667 | 0.111* |
| H37C | 0.183090 | 0.981747 | −0.253424 | 0.111* |
| C38a | 0.9571 (8) | 0.4811 (7) | 0.9458 (6) | 0.079 (3) |
| H38Aa | 1.034544 | 0.466476 | 0.919417 | 0.119* |
| H38Ba | 0.950664 | 0.454734 | 0.997907 | 0.119* |
| H38Ca | 0.963143 | 0.560343 | 0.967167 | 0.119* |
| N1 | 0.6162 (2) | 0.6243 (2) | 0.24866 (16) | 0.0352 (6) |
| N2 | 0.8121 (2) | 0.8087 (2) | 0.33960 (15) | 0.0297 (5) |
| N3 | 0.8245 (3) | 0.8236 (2) | 0.92279 (18) | 0.0485 (7) |
| H3 | 0.837028 | 0.889036 | 0.964954 | 0.058* |
| N4 | −0.2056 (2) | 0.5357 (2) | 0.41835 (18) | 0.0363 (6) |
| H4 | −0.244010 | 0.587388 | 0.445342 | 0.044* |
| N5 | 0.2556 (4) | 0.8580 (4) | −0.1428 (2) | 0.0862 (14) |
| H5 | 0.283334 | 0.825779 | −0.193760 | 0.103* |
| O1 | 0.6679 (2) | 0.71730 (17) | 0.47911 (13) | 0.0357 (5) |
| O2 | 0.6792 (2) | 0.89745 (17) | 0.53178 (14) | 0.0397 (5) |
| O3 | 0.8186 (4) | 0.6422 (3) | 0.9033 (2) | 0.0978 (12) |
| O4 | 0.4011 (2) | 0.64844 (17) | 0.36719 (16) | 0.0419 (5) |
| O5 | 0.3865 (2) | 0.81601 (17) | 0.45097 (14) | 0.0398 (5) |
| O6 | −0.2464 (2) | 0.3479 (2) | 0.3406 (2) | 0.0589 (7) |
| O7 | 0.3976 (2) | 0.7850 (2) | 0.24712 (16) | 0.0548 (7) |
| O8 | 0.5978 (2) | 0.83788 (19) | 0.21936 (14) | 0.0408 (5) |
| O9 | 0.1211 (3) | 0.9640 (3) | −0.08026 (19) | 0.0765 (9) |
| O10 | 0.6173 (3) | 0.99731 (18) | 0.40177 (15) | 0.0503 (6) |
| H10A | 0.608751 | 1.033407 | 0.460587 | 0.075* |
| H10B | 0.554676 | 1.013137 | 0.368946 | 0.075* |
| O11a | 0.8397 (6) | 0.4228 (5) | 0.8742 (5) | 0.0814 (18) |
| H11a | 0.789970 | 0.464790 | 0.876261 | 0.122* |
| O12 | −0.4135 (3) | 0.1396 (3) | 0.2976 (2) | 0.0779 (8) |
| H12A | −0.372104 | 0.206982 | 0.326121 | 0.117* |
| H12B | −0.493514 | 0.144212 | 0.297781 | 0.117* |
| Tb1 | 0.57563 (2) | 0.79869 (2) | 0.36700 (2) | 0.02488 (5) |
-
aOccupancy: 0.5.
1 Source of material
0.3 mmol p–acetylamino benzoic acid, 0.2 mmol 2,2′-bipyridine (2,2′-bipy) and 0.1 mmol terbium(III) acetate hydrate were dissolved in 15 mL mixed solvent (V methanol:V water = 3:1). The pH value was adjusted to about 6.5 with 0.1 M NaOH solution. The reaction mixture was refluxed for 4 h under stirring. After that, the mixture was placed in a 25 mL hydrothermal reaction kettle. The vessel was sealed and heated at 160 °C for 48 h. Afterwards the system was cooled to room temperature. The resultant solution was filtered and the filtrate was kept untouched and evaporated slowly at room temperature. The colorless and transparent crystals were obtain.
2 Experimental details
The H atoms bound to C atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.96 Å (methylene), with U iso(H) = 1.5U eq(C), and d(C–H) = 0.93 Å (aromatic), with U iso(H) = 1.2U eq(C).
3 Comment
In the domain of rare earth chemistry, the study of rare earth-organic complexes has attracted much attention due to their potential applications in many fields such as organic electroluminescent materials, agricultural light conversion film, high efficiency fluorescent powder for compact lamp, luminescence labeling, magnetic molecular materials, catalysis and so on [4], [5], [6]. Nowadays, much attention has been focused on Tb(III) and Eu(III) complexes with carboxylates as ligands [7], [8], [9]. p–Acetamidobenzoic acid is a widely used aromatic carboxylic acid: it is the raw material for the synthesis of non-barbiturate hypnotic sedative quinazoline-4-one derivatives [10], and also the raw material for the synthesis of fluorescent whitening agent and anti-asthma drug N-cinnamyl anthranilic acid [11]. As a ligand, p-acetaminobenzoate and the paracetaminobenzoic acid can coordinate with transition metal ions as end-group ligand or bridging ligand, and is also the donor and acceptor of hydrogen bond, which is conducive to the formation of structurally stable complexes [12] The ORTEP diagram is presented in the Figure. As shown in Figure, complex 1 consists of one Tb ion, three p-acetamidobenzoate anions (AAB), one 2,2′-bipy molecules, two water molecules and one methanol molecule. The central Tb ion is coordinated with two nitrogen atoms from 2,2′-bipy, and seven oxygen atoms from three AAB anions and one water molecule. The Tb(III) ion adopts a single-capped square antiprism coordination geometry, where the cap position is occupied by O10. Atoms O1, O4, N1 and N2 give the upper plane of the square antiprism, and atoms O2, O5, O7 and O8 determine the plane below. Their plane equations are −3.215x + 12.632y – 1.730z = 8.2531 and −4.989x + 12.392y + −3.405z = 4.3340. The average distance of the atoms in the plane is 0.0310 Å and 0.04631 Å, respectively, and their dihedral angle is 13.5°. The bond lengths Tb1–O1, Tb1–O2, Tb1–O4, Tb1–O5, Tb1–O7, Tb1–O8 and Tb1–O10 are 2.495(2), 2.4509(19), 2.386(2), 2.453(2), 2.428(2), 2.474(2) and 2.395(2) Å, respectively, which are in the normal ranges [11, 12]. The bond lengths of Tb1–N1 and Tb1–N2 are 2.537(2) and 2.527(2) Å, with the average to be 2.532 Å. Otherwise, the shortest center distance between aromatic cycles of 2-(tert-pentyl)anthracene group is 3.736 Å, little longer than 3.700 Å, indicating π–π stacking interaction between the aromatic rings.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. SHELXS2018. Program for the Solution of Crystal Structures; University of Göttingen: Germany, 2018.Suche in Google Scholar
2. Sheldrick, G. M. SHELXL2018. Program for the Refinement of Crystal Structures; University of Göttingen: Germany, 2018.Suche in Google Scholar
3. Sheldrick, G. M. SHELTL. Sturcture Determination Software Suite Version 14; Bruker AXS: Madison, Wisconsin, USA, 2018.Suche in Google Scholar
4. Zong, G. C., Ren, N., Zhang, J. J., Qi, X. X., Gao, J. Lanthanide complexes with 3-bromine-4-methyl benzoic acid and 1,10-phenanthroline. J. Therm. Anal. Calorim. 2016, 123, 105–116; https://doi.org/10.1007/s10973-015-4901-9.Suche in Google Scholar
5. Ye, Y. Z., Wu, X. J., Wang, A. L. Synthesis, structure and property of a novel coordination polymer [Tb(C7H3NO4)(C6H4NO2)(H2O)]. Chin. J. Struct. Chem. 2014, 33, 1860–1864.Suche in Google Scholar
6. Liu, G. X., Zha, X. C., Li, X. L. Synthesis, structure and luminescent properties of a new terbium(III) complex with 5-bromoisophthalic acid. Chin. J. Struct. Chem. 2012, 31, 173–178.10.7312/li--16274-032Suche in Google Scholar
7. Ma, D. Y., Xiao, J. X., Guo, H. F., Liang, Y. Q., Yan, J. J., Lin, W. J., Ding, W. Q. Magnetic properties in mononuclear gadolinium(III) and terbium(III) complexes based on ethoxylphenylsubstituted nitronyl nitroxide radical and hexafluoroacetylacetonate ligand. Chin. J. Struct. Chem. 2016, 35, 361–370.Suche in Google Scholar
8. Hu, B., Yao, X. Q., Xie, Y. Q., Zhang, Y. M., Wei, T. B. Synthesis, crystal structure and properties of two coordination polymers constructed by lanthanide and [5-amino-1,3,4-thiadiazol-2-yl]thioglycolic acid. Chinese J. Inorg. Chem. 2013, 29, 2422–2432.Suche in Google Scholar
9. Heil, H., Steiger, J., Schmechel, R., Seggern, H. V. Tris(dibenzoylmethane)(monophenanthroline)europium(III) based red emitting organic light emitting diodes. J. Appl. Phys. 2001, 90, 5357–5362; https://doi.org/10.1063/1.1410319.Suche in Google Scholar
10. Li, W., Li, C. H., Yang, Y. Q., Kuang, Y. F. Hydrothermal synthesis, crystal structure and thermal stability properties of complex [Zn(p–ABA)2(phen) (H2O)]H2O. Chin. J. Inorg. Chem. 2007, 23, 2023–2027.Suche in Google Scholar
11. Wang, Z. H., Fan, J., Zhang, W. G. Studies of radii-dependent lanthanide coordination behavior with 4-acetamidobenzoate and 1,10-phenanthroline. Z. Anorg. Allg. Chem. 2009, 635, 2333–2339; https://doi.org/10.1002/zaac.200801412.Suche in Google Scholar
12. Li, W., Tan, X. W., Li, C. H., Yang, Y. Q. Crystal structure of aqua[4-carboxylamino-benzoic acid−k2:O1:O2]bis[2,2′-bipyridine-k2:N1:N2]copper(II) complex, [Cu(2,2′-bipy) (C8H6O4N)2]⋯43(H2O). Z. Kristallogr. N. Cryst. Struct. 2011, 225, 715–716.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Solvothermal synthesis and crystal structure of aqua-tris(p-acetamidobenzoate-κ2O,O′)-(2,2′-bipyridine-κ2N,N′)terbium(III) - water - methanol (1/1/1)
- Crystal structure of hexaaquazinc(II) catena-poly[bis(1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ2O,O′)-bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ2O:O′)trizinc(II)] hexahydrate C26H36N4O20Zn2
- The crystal structure of valinyl-N-ium-4-(5-(thiophen-2-yl)isoxazol-3-yl)phenyl trifluoroacetate
- Crystal structure of bis(3,5-diisopropyl-1H-pyrazol-4-ammonium) tetrafluoroterephthalate, 2[C9H18N3][C8F4O4]
- Crystal structure of aqua-octakis(μ3-salicylato)-(1,10-phenanthroline)-(acetonitrile)-dicobalt(II)-trititanium(IV), C70H45N3O25Co2Ti3
- Crystal structure of catena-poly[aqua-(μ2-4,4′-diimidazole diphenyl ether-κ2N:N′)-(sulfato-κ1O)-cobalt(II)] – dimethylformamide (2/1), C39H37CoN9O8S
- Crystal structure of (5R,8R,9R,10R,12R, 13R,14R,17S)-2-(E-3-fluorobenzylidene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C37H53FO3
- Crystal structure of (Z)-4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenyl diphenylphosphinate, C30H25O7P
- Crystal structure of 3-((5-methylpyridin-2-yl)amino)-1-phenylpropan-1-one, C15H16N2O
- The crystal structure of (R)-9-(5-methoxy-2-methyl-2,3-dihydro-1H-cyclopenta[a]naphthalen-1-ylidene)-9H-thioxanthene, C28H22OS
- Crystal structure of diaqua-bis[1-(1-(hydroxymethyl)-1H-pyrazol-3-yl)-5-methyl-1H-1,2,3-triazole-4-carboxylato-κ2N,O)] manganese(II), C16H20MnN10O8
- The crystal structure of t-butyl 7-[4-(4-fluorophenyl)-2-[(methanesulfonyl)(methyl)amino]-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate, C26H36FN3O6S
- The crystal structure of samarium sulfate pentahydrate, Sm2(SO4)3(H2O)5
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ 4 N,O,O,O)-zinc(II)] monohydrate, C12H15NO9Zn
- The crystal structure of 2,3-difluoro-11H-benzo-[4,5]imidazo[2,1-a]isoindol-11-one, C14H6F2N2O
- The crystal structure of 2,3-di(9H-carbazol-9-yl)-9H-fluoren-9-one, C37H22N2O
- The crystal structure of 5-(2-chloro-3-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)-10,11-dihydro-5H-dibenzo[b,f]azepine, C40H39ClN2
- Crystal structure of 2-bromo-1-hydroxy-3-(3-methylbut-2-enyloxy)-9H-xanthen-9-one, C18H15BrO4
- Crystal structure of bis(μ2-benzenesulfonato-κ2O:O′)-bis(μ2-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))-bis(2-methoxyphenolato-κ6O,O′:O′,N,N′,O′′:O′′,O′′′))disodium(I)dicopper(II)
- The crystal structure of (E)-1,2-bis(benzo[e][1,2]azaborinin-2(1H)-yl)ethene, C18H16B2N2
- Crystal structure of 3-oxo-urs-12-en-28-benzyl ester, C37H52O3
- The crystal structure of ethyl (E)-1-chloro-3-(4-chloro-1-methyl-1H-indole-2-carbonyl)-4-oxo-2-phenylcyclooct-2-ene-1-carboxylate, C27H25Cl2NO4
- The crystal structure of 4,4′-((5-bromo-2-iodo-1,3-phenylene)bis(oxy))bis(tert-butylbenzene) ─ ethanol (2/1), C26H28BrIO2
- Crystal structure of (E)-1-(4-(benzyloxy)-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C18H19NO3
- The crystal structure of N1,N3-bis(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)\ propanediamide hydrate, C25H26N6O4, 2(H2O)
- The crystal structure of 2,5-bis[(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino]cyclohexa-2,5-diene-1,4-dione, C28H26N6O4
- Crystal structure of 3,4-bis[2-(hydroxymethyl)-pyrrolidin-1-yl] cyclobut-3-ene-1,2-dione hydrate, C14H22N2O5
- The crystal structure of 2-(3,4–dichlorobenzyl)-1H-benzimidazole, C14H10Cl2N2
- The crystal structure of 2-(2-((4,6-dimethoxypyrimidin-2-yl)oxy)phenyl)-4-(piperidin-1-yl)-5H-chromeno[2,3-d]pyrimidine, C28H27N5O4
- The crystal structure of 6-(benzofuran-2-yl)-2-oxo-4,5-diphenyl-3,4-dihydro-2H-pyran-3-carbonitrile, C26H17NO3
- Crystal structure of N-(4-bromobenzyl)-3-(difluoromethyl)-1-methyl-N-(pyridin-2-yl)-1H-pyrazole-4-carboxamide, C18H15BrF2N4O
- The crystal structure of the host-guest complex: N′-{5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide-diethyl ether (2/1)
- The crystal structure of (Z)-4-amino-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H17N3O2
- The crystal structure of diethyl 1,4-dihydro-2,6-dimethyl-4-(3-cyanophenyl)-3,5-pyridinedicarboxylate, C20H22N2O4
- Crystal structure of 3-(5-((4-(difluoromethoxy)phenyl) sulfonyl)-3,4,5,6-tetrahydropyrrolo[3,4-c]pyrrol-2(1H)-yl) oxetane-3-carboxamide, C17H19F2N3O5S
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)-N-(2-(4-methylpiperazin-1-yl)ethyl)benzamide hydrate, C25H37Cl2N5O6
- Crystal structure of 3-(benzo[d]thiazol-2-yl)-5-bromo-2-hydroxybenzaldehyde, C14H8BrNO2S
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(pyridin-2-yl)-1H-pyrazole-4-carboxamide, C11H10F2N4O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-isopropyl-1H-imidazol-3-ium hexafluoridophosphate(V), C20H34F12N4P2
- Crystal structure of ethyl 5,6-dichloro-2-methyl-2,3-dihydro-1 H-benzo[d]imidazole-2-carboxylate, C11H12Cl2N2O2
- The crystal of structure of (OC-6-22)-pentakis(acetonitrile)bromidoruthenium(II)bromide monohydrate, C10H15Br2N5Ru
- Crystal structure of (2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-2-(((4aS,5R,6S)-1-oxo-5-vinyl-4,4a,5,6-tetrahydro-1H,3H-pyrano[3,4-c]pyran-6-yl)oxy)tetrahydro-2H-pyran-3-yl 2,3-dihydroxybenzoate hydrate, C23H26O12·H2O
- The crystal structure of (E)-4-amino-N′-(1-(4-fluorophenyl)propylidene)benzohydrazide, C16H16FN3O
- The crystal structure of 2′-(9H-carbazol-9-yl)-[1,1′-binaphthalen]-2-amine, C32H22N2
- Crystal structure of poly[μ3-diiodido-[μ2-di(1H-pyrazol-1-yl)methane-κ2N,N′)]dicopper(I)], C7H8Cu2I2N4
- Crystal structure of 3-amino-N′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 1,3-diacetyltetrahydroimidazo[4,5-d]imidazole-2,5(1H,3H)-dione, C8H10O4N4
- Crystal structure of catena-poly[aqua-(μ2-1,4-diazabicyclo[2.2.2]octane-k2N: N′)-bis(sorbato-κ1O)-copper(II), C18H28CuN2O5
- Crystal structure of catena-poly[triaqua-(μ2 -1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ3O,O′:O′′)manganese(II)], C12H12N2O8Mn
- The crystal structure of [hexaaquamagnesium(II)] 4-[(pyridine-4-carbonyl)-amino]-phthalate trihydrate, C14H26N2O14Mg
- Crystal structure of 1-(p-tolylphenyl)-4-(2-furoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O3
- The crystal structure of bis(1,4,7,10,13-pentaoxacyclopentadecane)-potassium(I) dichloridocopper(I), C20H40Cl2CuKO10
- The crystal structure of tris(tetra-n-butylammonium) hexanitrato-κ2O,O′-lanthanium(III) C48H108N9O18La
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Solvothermal synthesis and crystal structure of aqua-tris(p-acetamidobenzoate-κ2O,O′)-(2,2′-bipyridine-κ2N,N′)terbium(III) - water - methanol (1/1/1)
- Crystal structure of hexaaquazinc(II) catena-poly[bis(1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ2O,O′)-bis(μ2-1-(3-carboxyphenyl)-5-methyl-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ2O:O′)trizinc(II)] hexahydrate C26H36N4O20Zn2
- The crystal structure of valinyl-N-ium-4-(5-(thiophen-2-yl)isoxazol-3-yl)phenyl trifluoroacetate
- Crystal structure of bis(3,5-diisopropyl-1H-pyrazol-4-ammonium) tetrafluoroterephthalate, 2[C9H18N3][C8F4O4]
- Crystal structure of aqua-octakis(μ3-salicylato)-(1,10-phenanthroline)-(acetonitrile)-dicobalt(II)-trititanium(IV), C70H45N3O25Co2Ti3
- Crystal structure of catena-poly[aqua-(μ2-4,4′-diimidazole diphenyl ether-κ2N:N′)-(sulfato-κ1O)-cobalt(II)] – dimethylformamide (2/1), C39H37CoN9O8S
- Crystal structure of (5R,8R,9R,10R,12R, 13R,14R,17S)-2-(E-3-fluorobenzylidene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a]phenanthren-3-one, C37H53FO3
- Crystal structure of (Z)-4-((4,5,6-trimethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)phenyl diphenylphosphinate, C30H25O7P
- Crystal structure of 3-((5-methylpyridin-2-yl)amino)-1-phenylpropan-1-one, C15H16N2O
- The crystal structure of (R)-9-(5-methoxy-2-methyl-2,3-dihydro-1H-cyclopenta[a]naphthalen-1-ylidene)-9H-thioxanthene, C28H22OS
- Crystal structure of diaqua-bis[1-(1-(hydroxymethyl)-1H-pyrazol-3-yl)-5-methyl-1H-1,2,3-triazole-4-carboxylato-κ2N,O)] manganese(II), C16H20MnN10O8
- The crystal structure of t-butyl 7-[4-(4-fluorophenyl)-2-[(methanesulfonyl)(methyl)amino]-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoate, C26H36FN3O6S
- The crystal structure of samarium sulfate pentahydrate, Sm2(SO4)3(H2O)5
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylate-κ 4 N,O,O,O)-zinc(II)] monohydrate, C12H15NO9Zn
- The crystal structure of 2,3-difluoro-11H-benzo-[4,5]imidazo[2,1-a]isoindol-11-one, C14H6F2N2O
- The crystal structure of 2,3-di(9H-carbazol-9-yl)-9H-fluoren-9-one, C37H22N2O
- The crystal structure of 5-(2-chloro-3-(3,6-di-tert-butyl-9H-carbazol-9-yl)phenyl)-10,11-dihydro-5H-dibenzo[b,f]azepine, C40H39ClN2
- Crystal structure of 2-bromo-1-hydroxy-3-(3-methylbut-2-enyloxy)-9H-xanthen-9-one, C18H15BrO4
- Crystal structure of bis(μ2-benzenesulfonato-κ2O:O′)-bis(μ2-6,6′-((ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))-bis(2-methoxyphenolato-κ6O,O′:O′,N,N′,O′′:O′′,O′′′))disodium(I)dicopper(II)
- The crystal structure of (E)-1,2-bis(benzo[e][1,2]azaborinin-2(1H)-yl)ethene, C18H16B2N2
- Crystal structure of 3-oxo-urs-12-en-28-benzyl ester, C37H52O3
- The crystal structure of ethyl (E)-1-chloro-3-(4-chloro-1-methyl-1H-indole-2-carbonyl)-4-oxo-2-phenylcyclooct-2-ene-1-carboxylate, C27H25Cl2NO4
- The crystal structure of 4,4′-((5-bromo-2-iodo-1,3-phenylene)bis(oxy))bis(tert-butylbenzene) ─ ethanol (2/1), C26H28BrIO2
- Crystal structure of (E)-1-(4-(benzyloxy)-2-hydroxyphenyl)-3-(dimethylamino)prop-2-en-1-one, C18H19NO3
- The crystal structure of N1,N3-bis(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)\ propanediamide hydrate, C25H26N6O4, 2(H2O)
- The crystal structure of 2,5-bis[(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino]cyclohexa-2,5-diene-1,4-dione, C28H26N6O4
- Crystal structure of 3,4-bis[2-(hydroxymethyl)-pyrrolidin-1-yl] cyclobut-3-ene-1,2-dione hydrate, C14H22N2O5
- The crystal structure of 2-(3,4–dichlorobenzyl)-1H-benzimidazole, C14H10Cl2N2
- The crystal structure of 2-(2-((4,6-dimethoxypyrimidin-2-yl)oxy)phenyl)-4-(piperidin-1-yl)-5H-chromeno[2,3-d]pyrimidine, C28H27N5O4
- The crystal structure of 6-(benzofuran-2-yl)-2-oxo-4,5-diphenyl-3,4-dihydro-2H-pyran-3-carbonitrile, C26H17NO3
- Crystal structure of N-(4-bromobenzyl)-3-(difluoromethyl)-1-methyl-N-(pyridin-2-yl)-1H-pyrazole-4-carboxamide, C18H15BrF2N4O
- The crystal structure of the host-guest complex: N′-{5-[2-(2,6-dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-(2-oxo-1,3-diazinan-1-yl)butanamide-diethyl ether (2/1)
- The crystal structure of (Z)-4-amino-N′-(1-(4-hydroxyphenyl)propylidene)benzohydrazide, C16H17N3O2
- The crystal structure of diethyl 1,4-dihydro-2,6-dimethyl-4-(3-cyanophenyl)-3,5-pyridinedicarboxylate, C20H22N2O4
- Crystal structure of 3-(5-((4-(difluoromethoxy)phenyl) sulfonyl)-3,4,5,6-tetrahydropyrrolo[3,4-c]pyrrol-2(1H)-yl) oxetane-3-carboxamide, C17H19F2N3O5S
- Crystal structure of 2-((2,6-dichloro-4-(3,5-dimethylisoxazol-4-yl)phenyl)amino)-N-(2-(4-methylpiperazin-1-yl)ethyl)benzamide hydrate, C25H37Cl2N5O6
- Crystal structure of 3-(benzo[d]thiazol-2-yl)-5-bromo-2-hydroxybenzaldehyde, C14H8BrNO2S
- Crystal structure of 3-(difluoromethyl)-1-methyl-N-(pyridin-2-yl)-1H-pyrazole-4-carboxamide, C11H10F2N4O
- Crystal structure of 3-(2-ethoxy-2-oxoethyl)-1-isopropyl-1H-imidazol-3-ium hexafluoridophosphate(V), C20H34F12N4P2
- Crystal structure of ethyl 5,6-dichloro-2-methyl-2,3-dihydro-1 H-benzo[d]imidazole-2-carboxylate, C11H12Cl2N2O2
- The crystal of structure of (OC-6-22)-pentakis(acetonitrile)bromidoruthenium(II)bromide monohydrate, C10H15Br2N5Ru
- Crystal structure of (2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-2-(((4aS,5R,6S)-1-oxo-5-vinyl-4,4a,5,6-tetrahydro-1H,3H-pyrano[3,4-c]pyran-6-yl)oxy)tetrahydro-2H-pyran-3-yl 2,3-dihydroxybenzoate hydrate, C23H26O12·H2O
- The crystal structure of (E)-4-amino-N′-(1-(4-fluorophenyl)propylidene)benzohydrazide, C16H16FN3O
- The crystal structure of 2′-(9H-carbazol-9-yl)-[1,1′-binaphthalen]-2-amine, C32H22N2
- Crystal structure of poly[μ3-diiodido-[μ2-di(1H-pyrazol-1-yl)methane-κ2N,N′)]dicopper(I)], C7H8Cu2I2N4
- Crystal structure of 3-amino-N′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 1,3-diacetyltetrahydroimidazo[4,5-d]imidazole-2,5(1H,3H)-dione, C8H10O4N4
- Crystal structure of catena-poly[aqua-(μ2-1,4-diazabicyclo[2.2.2]octane-k2N: N′)-bis(sorbato-κ1O)-copper(II), C18H28CuN2O5
- Crystal structure of catena-poly[triaqua-(μ2 -1-(4-carboxylatophenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylato-κ3O,O′:O′′)manganese(II)], C12H12N2O8Mn
- The crystal structure of [hexaaquamagnesium(II)] 4-[(pyridine-4-carbonyl)-amino]-phthalate trihydrate, C14H26N2O14Mg
- Crystal structure of 1-(p-tolylphenyl)-4-(2-furoyl)-3-methyl-1H-pyrazol-5-ol, C16H14N2O3
- The crystal structure of bis(1,4,7,10,13-pentaoxacyclopentadecane)-potassium(I) dichloridocopper(I), C20H40Cl2CuKO10
- The crystal structure of tris(tetra-n-butylammonium) hexanitrato-κ2O,O′-lanthanium(III) C48H108N9O18La

