Abstract
A new two-dimensional (2D) metal–organic complex, [Pb(L)(I)(sba)0.5]2 (1, L = 2-(2,4-difluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline, H2sba = suberic acid), was synthesized under hydrothermal conditions. The single-crystal X-ray analysis indicates that the asymmetric unit of complex 1 has a distorted [:PbIN2O2] octahedral geometry. It is further extended by two π–π stacking interactions and N–H⋯O hydrogen bonding to form and stabilize 2D supramolecular layer.
Coordination complexes are a class of organic hybrid crystalline functional materials that have been studied extensively in recent years due to their intriguing molecular structures and potential applications as functional materials, such as gas adsorption, chemical separation, chemical sensors, fluorescent probes, detection, catalysis, and drug delivery (Andreo et al., 2018; Han et al., 2019; Shao et al., 2020; Song et al., 2021; Zárate and Martínez, 2021). In order to obtain coordination complexes with ideal framework structure and functions, many efforts have been devoted to the rational selection of transition metals with oxidation state +2 and organic N-containing ligands to assemble (Gao et al., 2021; Pachisia and Gupta, 2020; Qi et al., 2003; Song et al., 2021). In this regard, lead(ii) is an ideal candidate for the construction of unusual complexes due to its large radius, unique lone-pair electrons, flexible coordination environment, and variable stereochemical activity (Kowalik et al., 2020; Li et al., 2012; Wang et al., 2010). In addition, 1,10-phenanthroline (phen) and its derivatives, as excellent N-donor chelating ligands, have been widely used to construct novel fascinating molecular architectures because of their rigid planar and electron-poor heteroaromatic system that can easily form π–π interactions (Li et al., 2021; Venkatakrishnan et al., 2007). Thus, the one-dimensional (1D) complexes have been extended to two-dimensional (2D) or three-dimensional (3D) supramolecular structures.
Based on the aforementioned reasons, in this work, we selected suberic acid (H2sba) as an organic linker and 2-(2,4-difluorophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (L) as an N-donor chelating ligand, generating a new dinuclear complex, namely [Pb(L)(I)(sba)0.5]2 (1, Scheme 1).
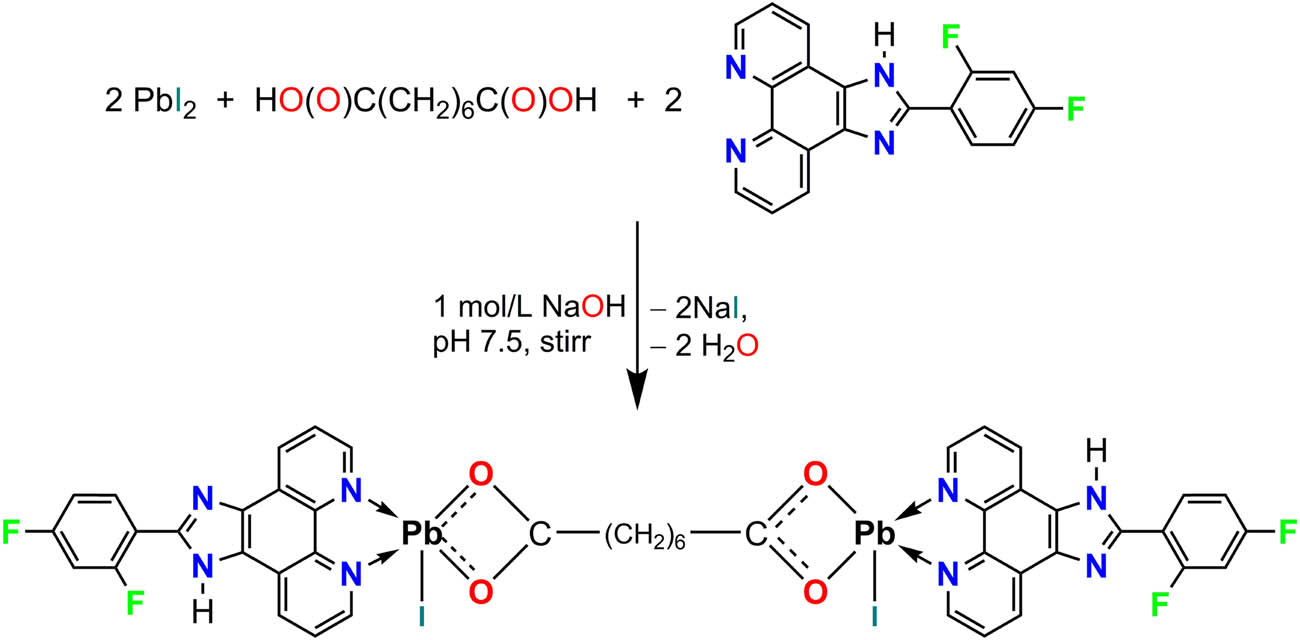
Synthesis of complex 1.
X-ray crystallographic analysis reveals that the asymmetric unit of complex 1 contains one unique Pb(ii) atom, one L ligand, one iodide anion, and one half sba2− anion. As shown in Figure 1, each Pb(ii) is mainly coordinated by two carboxylate oxygen atoms from one half bis-chelating sba2− anion (Pb(1)–O(1) = 2.354(5), Pb(1)–O(2) = 2.627(5) Å), one iodide anion (Pb(1)–I(1) = 3.2109(7) Å), and two nitrogen atoms from one L ligand (Pb(1)–N(1) = 2.573(6), Pb(1)–N(2) = 2.582(6) Å) in a distorted [:PbIN2O2] octahedral geometry (Table 1). The nitrogen atom (N(1)), two O atoms (O(1), O(2)), and the lone pair of electrons comprise the basal plane of octahedral geometry, and the nitrogen atom (N(2)) and the iodide anion (I(1)) are located in the axial positions. The Pb–O and Pb–N distances are near to the reported ones observed in other complexes [Pb(phen)(4-NB)(CH3COO)] (phen = 1,10-phenanthroline, 4-NB = 4-nitrobenzoate) and [Pb2(L2)2] n (H2L2 = 5-(pyridin-4-ylmethoxy)-isophthalic acid) (Zhang et al., 2011, 2021). It is interesting that adjacent Pb(ii) atoms are bridged by sba2− anion to generate a dinuclear complex with a long Pb···Pb distance of 11.458 Å (Figure 2). As shown in Figure 3, because of the presence of conjugated L ligands, adjacent [Pb(L)(I)(sba)0.5]2 dinuclear complexes are linked into a chain structure through π–π stackings between the two phen rings of the L ligands with the centroid-to-centroid distance of 3.547(3) Å, face-to-face distance of 3.333(2) Å, and the dihedral angle between the two planes of ca. 0.00(16)° (the two phen rings are composed of N(1)/N(2)/C(1)–C(12) and N(1)vi/N(2)vi/C(1)vi–C(12)vi, respectively; symmetry code: vi1 − x, 1 − y, 1 − z) (Figure 4). More interestingly, another π–π stacking interaction has been found. It is the aromatic π–π interaction between pyridine rings and benzene rings of L ligands from neighboring layers N(2)/C(6)–C(10), C(18)–C(23) at (−x + 2, −y + 1, −z + 1), a centroid-to-centroid distance of 4.073(5) Å, a face-to-face distance of 3.523(3) Å, a slippage distance of 1.087 Å, and a dihedral angle of 17.8(4)o) (Figure 3). When these interactions are taken into consideration, the 1D chains turn into a 2D supramolecular layer (Figure 5). Additionally, the N–H⋯O hydrogen bond (N(3)–H(3A) = 0.86, H(3A)⋯O(2)v = 2.00, N(3)⋯O(2)v = 2.798(8) Å, ∠N(3)–H(3A)⋯O(2)v = 154.8°, symmetric codes: v1/2 + x, 1/2 − y, 1/2 + z) further stabilizes the 2D supramolecular layer.
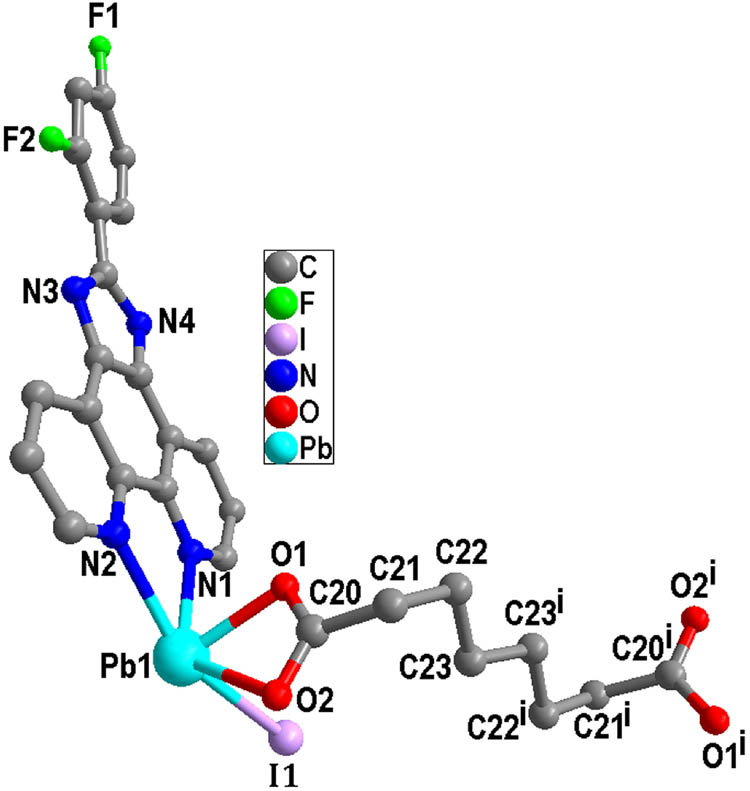
View of the coordination environment of the Pb(ii) atom of 1 (symmetric code: (i)−x + 1, −y + 1, −z).
Selected bond lengths (Å) and angles (°) for complex 1
| Pb(1)–N(1) | 2.573(6) |
| Pb(1)–N(2) | 2.582(6) |
| Pb(1)–O(1) | 2.354(5) |
| Pb(1)–O(2) | 2.6927(5) |
| Pb(1)–I(1) | 3.2109(7) |
| O(1)–Pb(1)–I(1) | 88.81(15) |
| N(1)–Pb(1)–I(1) | 88.47(14) |
| N(1)–Pb(1)–O(1) | 75.35(19) |
| N(2)–Pb(1)–I(1) | 151.99(14) |
| N(2)–Pb(1)–O(1) | 78.9(2) |
| N(1)–Pb(1)–N(2) | 64.2(2) |
| O(2)–Pb(1)–I(1) | 88.42(15) |
| O(1)–Pb(1)–O(2) | 52.02(18) |
| N(1)–Pb(1)–O(2) | 127.32(19) |
| N(2)–Pb(1)–O(2) | 103.1(2) |
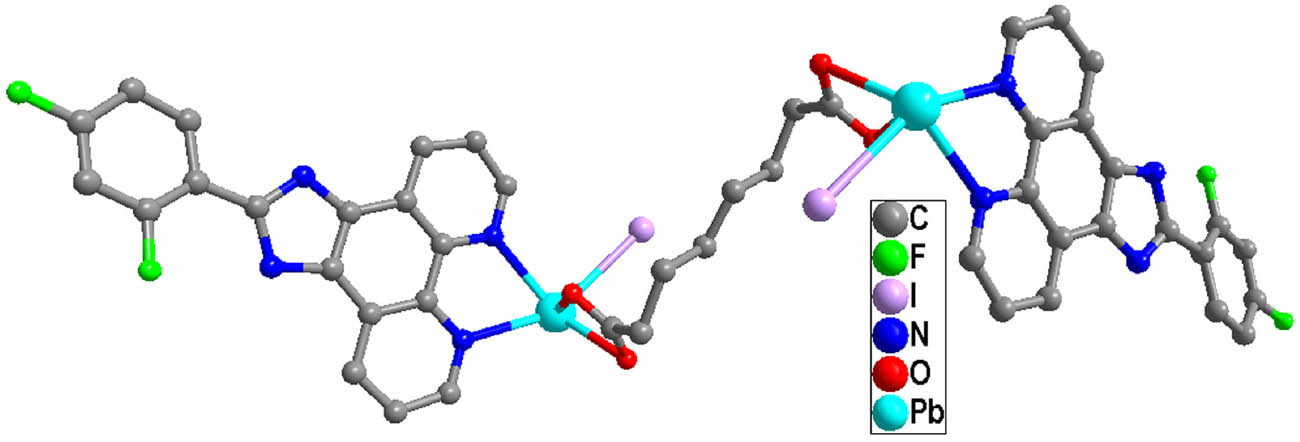
View of the dinuclear structure of 1.
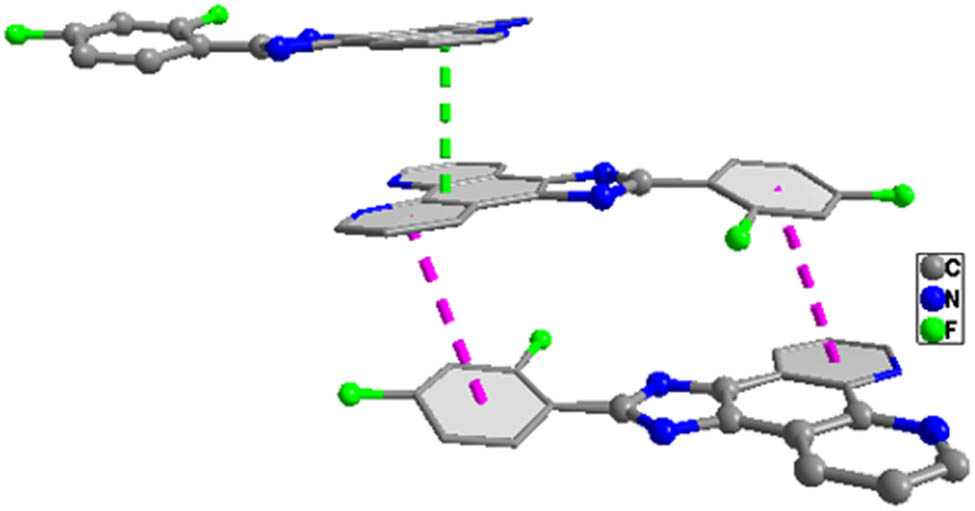
View of the π–π interactions between the two phen rings (green dotted line) and other π–π interactions between pyridine rings and benzene rings (pink dotted line).
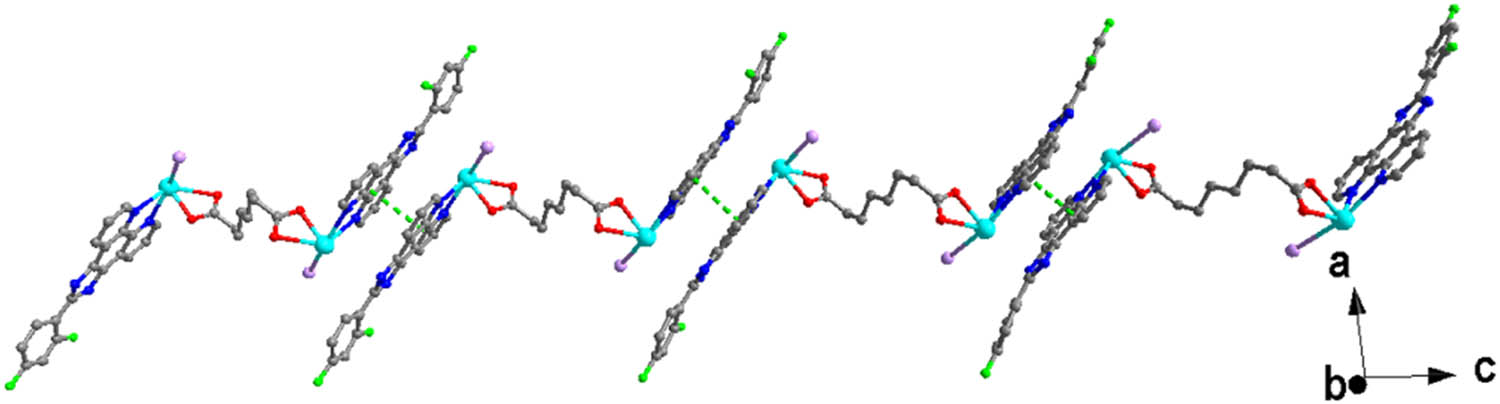
View of the 1D chain structure of 1 constructed by π–π interactions.
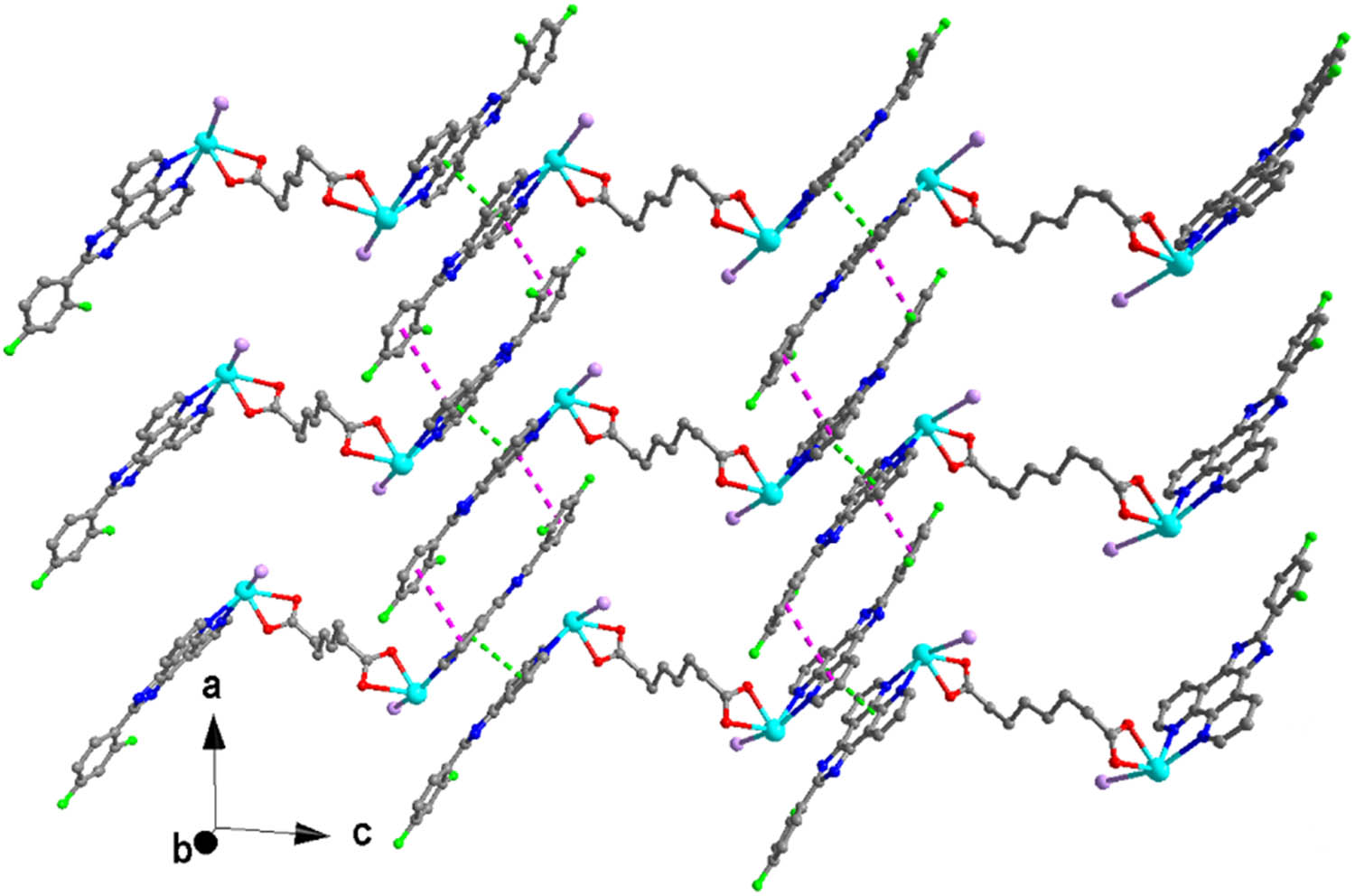
View of the 2D supramolecular layer structure of 1 formed by two π–π interactions.
The infrared (IR) spectrum of 1 is shown in the 4,000–400 cm−1 region (Figure 6). The IR spectrum of 1 shows a broad band centered around 3,422 cm−1 attributable to the N–H stretching frequency of the L ligand. The peak at 1,639 cm−1 for 1 can be attributed to the stretching vibrations of the carboxylate groups. The C–N and C═N stretching vibrations of the L ligand are observed at 1,119 and 1,407 cm−1.
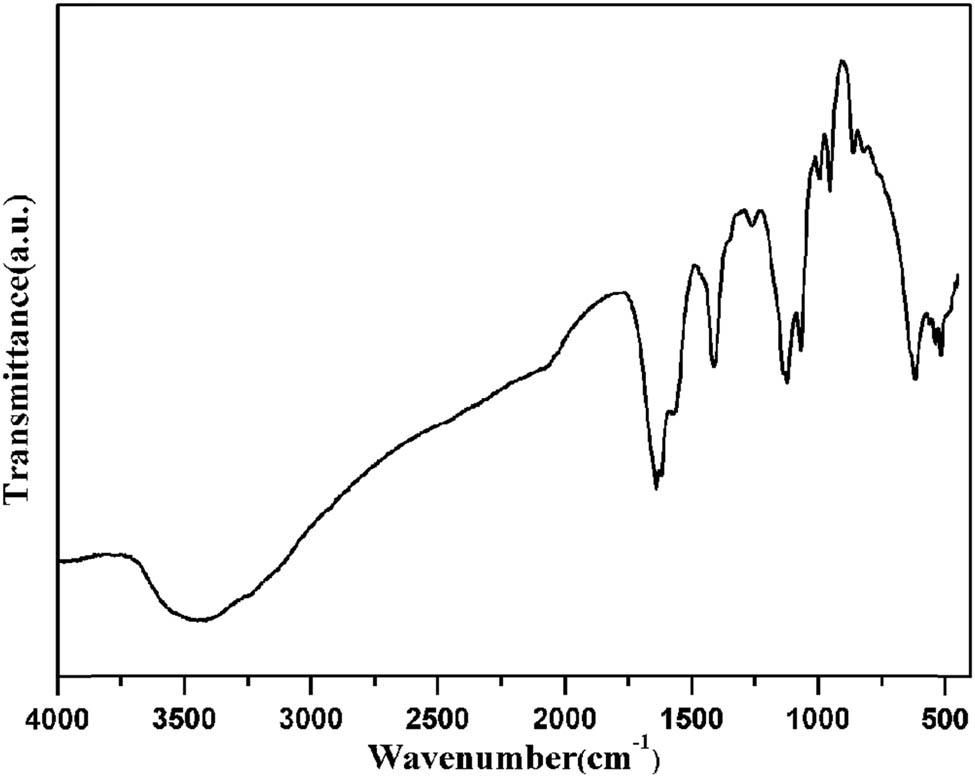
View of the IR spectrum of 1.
All calculations were carried out using the Gaussian09 program with the parameters of the molecular structure from the experimental data of 1. Natural bond orbital analysis (Reed et al., 1988) was performed by density functional theory (Parr and Yang, 1989) with the B3LYP (Lee et al., 1988) hybrid functional and the LANL2DZ basis set (Dunning and Hay, 1976).
As shown in Table 2, the electronic configurations of Pb(ii) ion, O atoms, N and iodide atoms are 6s1.896p0.647p0.01, 2s1.73,1.352p5.12–5.213p0.01, 2s1.36–1.382p4.20–4.243p0.02, and 5s1.965p5.78, respectively. Based on the aforementioned results, one can conclude that the lone pair electrons consisting of the 6s-character were located at the Pb ions and occupied the coordination position, and other can extrapolate that the Pb(ii) ion coordination with O and N atoms is mainly on the 6s and 6p orbitals. Iodide atoms supply electrons of 5s and 5p to the Pb(ii) ion and form coordination bonds. O and N atoms form coordination bonds with the Pb(ii) ion using 2s and 2p orbitals. Therefore, the Pb(ii) ion obtained some electrons from iodide atoms, O atoms of sba2− anion, and N atoms of L ligand. Thus, according to valence-bond theory the atomic net charge distribution in the compound shows obvious covalent interactions between the coordinated atoms and Pb(ii) ion.
Selected natural atomic charges and natural electron configuration of 1
| Atom | Net charge | Electron configuration |
|---|---|---|
| Pb(1) | 1.183 | [core]6s(1.89)6p(0.64)7p(0.01) |
| O(1) | −0.684 | [core]2s(1.73)2p(5.21)3p(0.01) |
| O(2) | −0.604 | [core]2s(1.73)2p(5.12)3p(0.01) |
| N(1) | −0.414 | [core]2s(1.36)2p(4.24)3p(0.02) |
| N(2) | −0.411 | [core]2s(1.38)2p(4.20)3p(0.02) |
| I(1) | −0.648 | [core]5s(1.96)5p(5.78) |
As shown in Figure 7, the highest occupied molecular orbital (HOMO) and lowest occupied molecular orbital (LUMO) are mainly composed of the s orbital of Pb(ii) ion, p orbital of one I atom, and p orbitals of two O and two N atoms coordinated with Pb(ii) ion. The HOMO − 1 is located mostly on the I atom and sba2− anion. The LUMO + 1 is mainly composed of the π orbital of the L ligand.
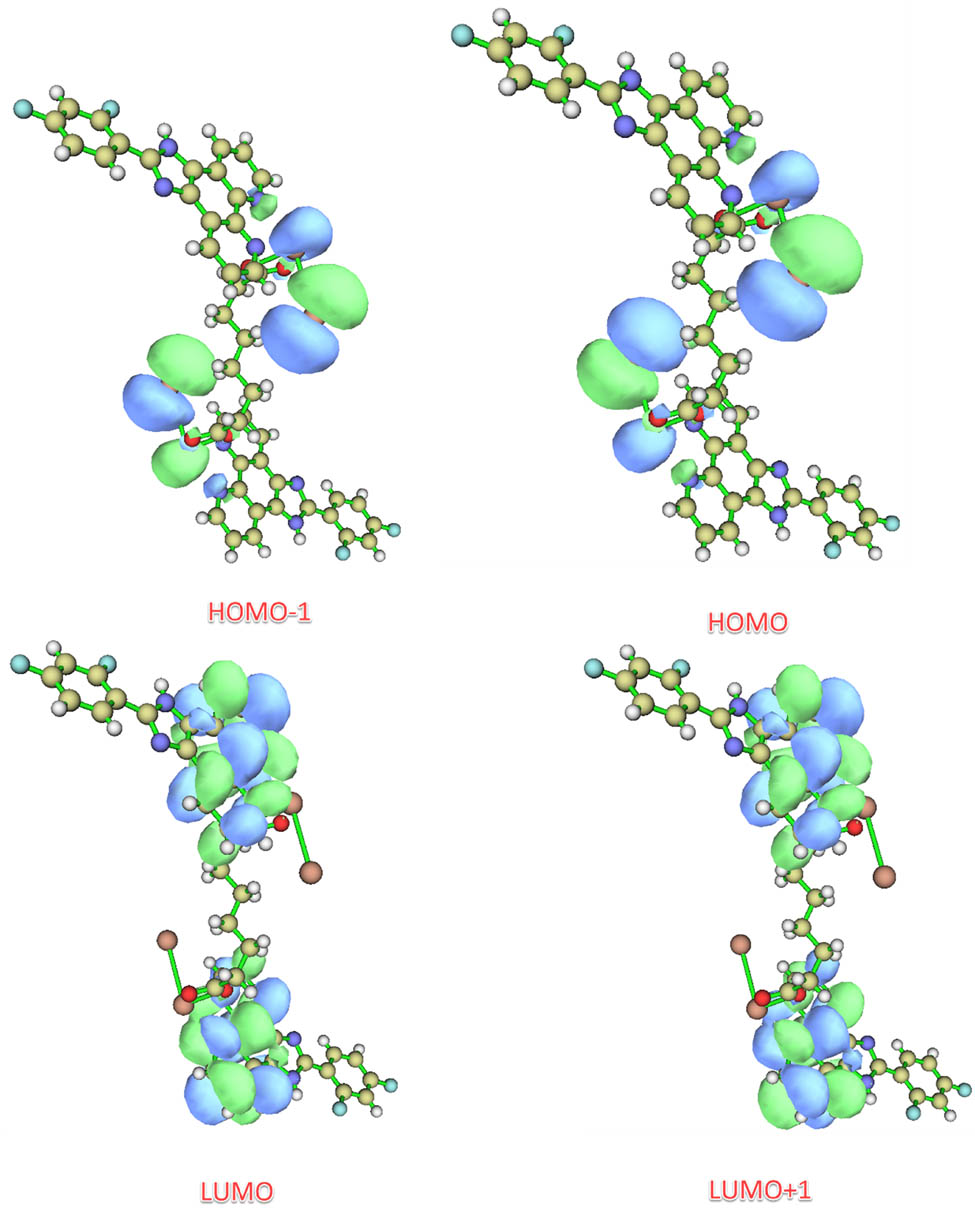
View of the frontier molecular orbitals of 1.
In summary, a novel 2D supramolecular complex [Pb(L)(I)(sba)0.5]2 (1) has been successfully synthesized and characterized under hydrothermal conditions. The central Pb(ii) ion in 1 is coordinated with five atoms, forming distorted [:PbIN2O2] octahedral geometry. The Pb(ii) atoms are alternately bridged by sba2− anion to generate a dinuclear complex, affording a 1D chain structure by π–π stacking interactions. These adjacent 1D chains are glued together via the aromatic π–π interactions, leading to a 2D supramolecular framework. Moreover, the N–H⋯O hydrogen bonding further stabilizes the 2D supramolecular structure.
Experimental
All reagents and solvents used in the synthesis procedure were bought from Shanghai Yiyan Biological Technology Co. Ltd and Tianjin Yuzhou Chemical Sales Co. Ltd (China). Elemental analyses of C, H, and N were performed using a Perkin-Elmer 240 CHN elemental analyzer (Perkin Elmer, North Waltham, USA). The IR spectrum was recorded using an Alpha Centauri FTIR spectrophotometer (Mattson Technology, USA).
Preparation of [Pb(L)(I)(sba)0.5]2 (1)
Reaction mixture of PbI2 (0.2 mmol, 0.0922 g), L (0.1 mmol, 0.0332 g), H2sba (0.2 mmol, 0.0348 g), 1 mL of anhydrous ethanol, and H2O (8 mL) was adjusted to the pH value of 7.5 with 1 mol·L−1 NaOH solution. The mixture was then sealed into a 16 mL Teflon-lined stainless-steel container and heated at 180°C for 4 days, and then cooled down at a rate of 5°C·h−1. Yellow block crystals were obtained in 46% yield based on L. Analytical calculated for C46H32F4I2N8O4Pb2, %: C, 36.71; H, 2.14; N, 7.45; found, %: C, 36.22; H, 2.10; N, 7.31.
X-ray crystallography
The intensity data for the X-ray diffraction analysis of 1 were measured at 298 (2) K using a Bruker-AXS Smart CCD diffractometer with graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å) using the ϕ and ω scan technique. The structure was solved by direct methods using SIR2014 (Burla et al., 2015) and refined by a full-matrix least squares technique on F2 using SHELXL2018/3 program (Sheldrick, 2015). All H atoms were found by generated calculations with refining as riding, and the non-hydrogen atoms were refined with anisotropic temperature parameters. Crystallographic characteristics and the X-ray data and structure-refinement parameters of 1 are summarized in Table 1. The crystallographic parameters and refinements are summarized in Table 3.
Crystalline data and refinement parameters for complex 1
| Empirical formula | C46H32F4I2N8O4Pb2 |
| Formula weight | 1,504.97 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 11.2285(15) |
| b (Å) | 11.4117(15) |
| c (Å) | 17.205(2) |
| β (°) | 94.634(2) |
| Volume (Å3) | 2197.4(5) |
| Z | 2 |
| D c (g·cm−3) | 2.275 |
| µ (mm−1) | 9.129 |
| F(000) | 1,404 |
| θ range (°) | 2.091–25.010 |
| Crystal size (mm) | 0.214 × 0.185 × 0.143 |
| Tot. reflections | 11,826 |
| Uniq. reflections, R int | 3,844, 0.0262 |
| GOF on F 2 | 1.132 |
| R 1 indices [I > 2σ(I)] | 0.0372 |
| wR 2 indices (all data) | 0.0972 |
| ∆ρ min, ∆ρ max (e·Å–3) | −1.395, 1.902 |
| CCDC No. | 2130661 |
-
Funding information: This research was supported by the National Key R&D Program of China (No. 2021YFE0205300) and the Graduate Innovation Project of Jilin Normal University (No. 202030).
-
Author contributions: Xiuyan Wang: writing – original draft, writing – review and editing; methodology; Ziru Li: writing – original draft, data curation, visualization; Jie Wang: conceptualization, investigation, resources; Lina Zhao: writing – review and editing, software.
-
Conflict of interest: The authors certify that that there is no conflict of interest regarding the publication.
-
Supplementary material: Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 2130661. Copies of this information can be obtained free of charge using the link: www.ccdc.cam.ac.uk or from the CCDC, 12 union Road, Cambridge CB2 1EZ, UK (fax: 0044 1223 336 033; e-mail: deposit@ccdc.cam.ac.uk).
References
Andreo A., Priola E., Alberto G., Benzi P., Marabello D., Proserpio D.M., et al., Autoluminescent metal–organic frameworks (MOFs): self-photoemission of a highly stable thorium MOF. J. Am. Chem. Soc., 2018, 140(43), 14144–14149. 10.1021/jacs.8b07113.Search in Google Scholar PubMed
Burla M.C., Caliandro R., Carrozzini B., Cascarano G.L., Cuocci C., Giacovazzo C., et al., Crystal structure determination and refinement via SIR2014. J. Appl. Cryst., 2015, 48, 306–309. 10.1107/S1600576715001132.Search in Google Scholar
Dunning T.H.Jr, Hay P.J., Modern theoretical chemistry (HF III). Plenum Press, New York, 1976, pp. 1–28.Search in Google Scholar
Han X., Yang J., Liu Y.Y., Xu G.H., Ma J.F., Metal ion induced single-crystal-to-single-crystal transformation and luminescent sensing properties of resorcinarene-based metal-organic frameworks. Polyhedron., 2019, 16(115), 145–153. 10.1016/j.poly.2018.12.051.Search in Google Scholar
Kowalik M., Masternak J., Kazimierczuk K., Kupcewicz B., Khavryuchenko O.V., Barszcz B., Exploring thiophene-2-acetate and thiophene-3-acetate binding modes towards the molecular and supramolecular structures and photoluminescence properties of Pb(II) polymers. CrystEngComm., 2020, 22, 7025–7035. 10.1039/D0CE01224F.Search in Google Scholar
Lee C.T., Yang W.T., Parr R.G., Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B., 1988, 37(2), 785–789. 10.1103/PhysRevB.37.785.Search in Google Scholar
Li X.Q., Zhang H.B., Wu S.T., Lin J.D., Lin P., Li Z.H., et al., Synthesis, structures and luminescent properties of new Pb(II)/M(I) (M = K, Rb and Cs) frameworks based on dicarboxylic acids: a novel icosahedral Pb6-M6 SBU. CrystEngComm., 2012, 14, 936–944. 10.1039/C1CE06111A.Search in Google Scholar
Li X.Y., Gao L., Che G.B., Yan Y.S., Li C.X., A Fe(II) coordination polymer based on 1,10-phenanthroline derivative and oxalic acid: synthesis, structure, and properties. Chinese J. Struct. Chem., 2021, 40(3), 317–323 (in Polish).Search in Google Scholar
Gao Y.X., Li X.M., Zhou S., Liu B., Synthesis, structure and theoretical calculation of metal coordination polymers with pyrazine-2,3-dicarboxylic acid and 3-(2-pyridyl)pyrazole as Co-ligands. Chinese J. Struct. Chem., 2021, 40(6), 753–758 (in Polish).Search in Google Scholar
Pachisia S., Gupta R., Architectural and catalytic aspects of designer materials built using metalloligands of pyridine-2,6-dicarboxamide based ligands. Dalton Trans., 2020, 49, 14731–14748. 10.1039/D0DT03058A.Search in Google Scholar PubMed
Parr R.G., Yang W., Density functional theory of atoms and molecules. Oxford University Press, New York, 1989.Search in Google Scholar
Qi Y.J., Wang Y.H., Hu C.W., Cao M.H., Mao L., Wang E., A new type of single-helix coordination polymer with mixed ligands [M2(phen)2(e,a-cis-1,4-chdc)2(H2O)2]n (M = Co and Ni; phen = 1,10-phenanthroline; chdc = cyclohexanedicarboxylate). Inorg. Chem., 2003, 42(25), 8519–8523. 10.1021/ic034796p.Search in Google Scholar PubMed
Reed A.E., Curtiss L.A., Weinhold F., Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev., 1988, 88(6), 899–926. 10.1021/cr00088a005. Search in Google Scholar
Shao L., Hua B., Hu X.Q., Stalla D., Kelley S.P., Atwood J.L., Construction of polymeric metal–organic nanocapsule networks via supramolecular coordination-driven self-assembly. J. Am. Chem. Soc., 2020, 142(16), 7270–7275. 10.1021/jacs.0c00640.Search in Google Scholar PubMed
Sheldrick, G.M., Crystal structure refinement with SHELXL. Acta Cryst., 2015, C71, 3–8. 10.1107/S2053229614024218.Search in Google Scholar PubMed PubMed Central
Song Y., Xu L.P., Li G.T., Wang X.Y., A 3D supramolecular polymer involving hydrogen bonds based on nickel(II), 1,3-benzenedicarboxylic acid, and a nitrogen-containing ligand. J. Struct. Chem., 2021, 62 (7), 1182–1188. 10.26902/JSC_id74462.Search in Google Scholar
Song Y., Wang H.L., Yang J.Y., Zhang X.T., Wang X.Y., A cobalt(II) polymer constructed by N,N’-bis(3-pyridinecarboxamide)-1,4-benzene: synthesis and structural characterization. Crystallogr. Rep., 2021, 66(7), 1286–1289. 10.1134/S106377452107018X.Search in Google Scholar
Venkatakrishnan T.S., Rajamani R., Ramasesha S., Sutter J.P., Synthesis, crystal structure, and magnetic properties of hexanuclear [{MnL2}4{Nb(CN)8}2] and nonanuclear [{MnL2}6{Nb(CN)8}3] heterometallic clusters (L = bpy, phen). Inorg. Chem., 2007, 46(23), 9569–9574. 10.1021/ic062383t.Search in Google Scholar PubMed
Wang X.L., Chen Y.Q., Gao Q., Lin H.Y., Liu G.C., Zhang J.X., et al., Coordination behavior of 5,6-substituted 1,10-phenanthroline derivatives and structural diversities by coligands in the construction of lead(ii) complexes. Cryst. Growth Des., 2010, 10(5), 2174–2184. 10.1021/cg901431r.Search in Google Scholar
Zárate R.L., Martínez J.V., Controlling π–π interactions through coordination bond formation: assembly of 1-D chains of acac-based coordination compounds. Cryst. Growth Des., 2021, 21(7), 3756–3769. 10.1021/acs.cgd.1c00083.Search in Google Scholar
Zhang L.L., Tang S.L., Li, D.J., Cheng Y.Z., Zhang L.P., Synthesis and crystal structure of one new Pb(II) complex constructed by 1,10-phenanthroline and two carboxylate ligands. Main Group Met. Chem., 2021, 44, 157–164. 10.1515/mgmc-2021-0019.Search in Google Scholar
Zhang X., Cheng J., Chen F., Sun M., Yao Y., Isomeric photoluminescent lead(ii) coordination polymers based on designed pyridinecarboxylate ligands. Inorg. Chem. Commun., 2011, 14, 358–361. 10.1016/j.inoche.2010.11.032.Search in Google Scholar
© 2022 Xiuyan Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure
Articles in the same Issue
- Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties
- Research Articles
- Syntheses and crystal structures of ethyltin complexes with ferrocenecarboxylic acid
- Ultra-fast and effective ultrasonic synthesis of potassium borate: Santite
- Synthesis and structural characterization of new ladder-like organostannoxanes derived from carboxylic acid derivatives: [C5H4N(p-CO2)]2[Bu2Sn]4(μ3-O)2(μ2-OH)2, [Ph2CHCO2]4[Bu2Sn]4(μ3-O)2, and [(p-NH2)-C6H4-CO2]2[Bu2Sn]4(μ3-O)2(μ2-OH)2
- HPA-ZSM-5 nanocomposite as high-performance catalyst for the synthesis of indenopyrazolones
- Conjugation of tetracycline and penicillin with Sb(v) and Ag(i) against breast cancer cells
- Simple preparation and investigation of magnetic nanocomposites: Electrodeposition of polymeric aniline-barium ferrite and aniline-strontium ferrite thin films
- Effect of substrate temperature on structural, optical, and photoelectrochemical properties of Tl2S thin films fabricated using AACVD technique
- Core–shell structured magnetic MCM-41-type mesoporous silica-supported Cu/Fe: A novel recyclable nanocatalyst for Ullmann-type homocoupling reactions
- Synthesis and structural characterization of a novel lead coordination polymer: [Pb(L)(1,3-bdc)]·2H2O
- Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells
- In silico ADMET, molecular docking study, and nano Sb2O3-catalyzed microwave-mediated synthesis of new α-aminophosphonates as potential anti-diabetic agents
- Synthesis, structure, and cytotoxicity of some triorganotin(iv) complexes of 3-aminobenzoic acid-based Schiff bases
- Rapid Communications
- Synthesis and crystal structure of one new cadmium coordination polymer constructed by phenanthroline derivate and 1,4-naphthalenedicarboxylic acid
- A new cadmium(ii) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: Synthesis and crystal structure
- Synthesis and structural characterization of a novel lead dinuclear complex: [Pb(L)(I)(sba)0.5]2
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Computation of edge- and vertex-degree-based topological indices for tetrahedral sheets of clay minerals
- Structures devised by the generalizations of two graph operations and their topological descriptors
- On topological indices of zinc-based metal organic frameworks
- On computation of the reduced reverse degree and neighbourhood degree sum-based topological indices for metal-organic frameworks
- An estimation of HOMO–LUMO gap for a class of molecular graphs
- On k-regular edge connectivity of chemical graphs
- On arithmetic–geometric eigenvalues of graphs
- Mostar index of graphs associated to groups
- On topological polynomials and indices for metal-organic and cuboctahedral bimetallic networks
- Finite vertex-based resolvability of supramolecular chain in dialkyltin
- Expressions for Mostar and weighted Mostar invariants in a chemical structure

