Abstract
Background. Chaperone activity of α-crystallin in the lens works to prevent protein aggregation and is important to maintain the lens transparency. This study evaluated the effect of hesperetin on lens chaperone activity in selenite-induced cataracts. Methodology. Thirteen-day-old rats were divided into four groups. Animals were given hesperetin (groups G2 and G4) or vehicle (G1 and G3) on Days 0, 1, and 2. Rats in G3 and G4 were administered selenite subcutaneously 4 hours after the first hesperetin injection. On Days 2, 4, and 6, cataract grades were evaluated using slit-lamp biomicroscopy. The amount of a-crystallin and chaperone activity in water-soluble fraction were measured after animals sacrificed. Results. G3 on day 4 had developed significant cataract, as an average cataract grading of 4.6 ± 0.2. In contrast, G4 had less severe central opacities and lower stage cataracts than G3, as an average cataract grading of 2.4 ± 0.4. The a-crystallin levels in G3 lenses were lower than in G1, but the same as G4. Additionally, chaperone activity was weaker in G3 lenses than G1, but the same as in G4. Conclusions. Our results suggest that hesperetin can prevent the decreasing lens chaperone activity and a-crystallin water solubility by administered of selenite.
1 Introduction
The lens contains a high concentration of crystallin, which can be separated into three distinct families (α-, β-, and γ-crystallin). α-crystallin consists of two 20 kDa subunits, αA-and αΒ-crystallin, and exists in the lens as a polydisperse multimeric protein with an average molecular mass of 700 kDa [1, 2]. α-crystallin belongs to a small heat shock protein family and acts as a molecular chaperone [3]. This chaperone activity is critical in vivo to the normal function of the lens because lens proteins are long-lived and have negligible turnover. The chaperone activity of α-crystallin in the lens prevents the formation of protein aggregates, lens opacification, and cataract formation [3, 4]. Numerous studies have shown that a-crystallin chaperone activity protects against protein aggregation that occurs during various stress conditions [5-7].
Subcutaneous injection of sodium selenite (Na2SeO3) into suckling rats (10−18 days old) rapidly induces bilateral nuclear cataracts and this animal model has been used to evaluate anti-cataract agents [8]. Sodium selenite-induced cataracts have characteristics similar to those seen clinically, including reduced lens chaperone activity [9, 10]. In selenite-induced cataracts, the reduction in lens chaperone activity can be reversed with antioxidant flavonoids, including curcumin, lutein, and zeaxanthin [9, 11, 12].
Hesperetin, which is a natural flavonoid that is isolated from orange rinds, has a flavone backbone structure and is known to have strong antioxidant activity [13]. We had been previously reported that hespertin prevent cataract formation assessed by observing the cataract lens and measuring the concentration of lens anti-oxidants such as glutathione (GSH) and ascorbate (AsA) [14]. However, the effect of hesperetin on lens chaperone activity in lenses with cataracts remains unknown. Here, we evaluate the effect of hesperetin on lens chaperone activity in rats with selenite-induced cataracts.
2 Methods
2.1 Animals
Sprague-Dawley (SD) rats were obtained from the Sankyo Labo Service Corporation (Tokyo, Japan) and housed in temperature-controlled cages (25 ± 5°C) with a 12-hour light/dark cycle. Rats were fed balanced commercial rat chow (CE-2, Clea Japan, Tokyo, Japan) and allowed water ad libitum. Rats were sacrificed using an overdose of isoflurane (Wako Pure Chemical Industries, Osaka, Japan). The Keio University Animal Research Committee approved all animal procedures performed in this study, and all animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Selenite-induced cataract and hesperetin treatment
Rats were randomized into the following four groups: control group (normal lens without cataract, no hesperetin; G1), hesperetin-treated group (no cataract, hesperetin treated; G2), selenite cataract group (Se-cataract, no hesperetin; G3), and selenite-cataract with hesperetin treatment group (Se-cataract, hesperetin treatment; G4). Hesperetin (Wako Pure Chemical Industries) and sodium selenite (Wako Pure Chemical Industries) were administered to rats according to the methods described by Nakazawa et al. [14], with minor modifications. Briefly, hesperetin was dissolved in the vehicle, which was a 7% ethanol and 93% olive oil solution. Hesperetin was administered to G2 and G4 (0.4 μg/kg body weight) and vehicle (olive oil-ethanol mixture) was administered to G1 and G3 when rats were 13 days old (Day 0). Hesperetin or vehicle was also administered on Days 1 and 2 (post-natal days 14 and 15). Four hours after the first hesperetin administration on Day 0, sodium selenite (Wako Pure Chemical Industries) was administered subcutaneously at a dose of 20 mmol/kg body weight to G2 and G4. A similar volume of phosphate buffered saline (PBS; 130 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) was administered to G1 and G3. On Day 2, 4, or 6 (post-natal Day 15, 17, or 19), lenses were observed by slit-lamp biomicroscopy, and measured amount of α-crystallin and chaperone activity in the lens.
2.3 Slit-lamp observation and cataract classification
Lenses were evaluated using slit-lamp biomicroscopy (TOPCON corp., Tokyo, Japan) after mydriasis by administration of tropicamide eye drops (Midorin P, Santen Pharmaceutical Company, Osaka, Japan). Cataracts were classified on a scale of stage 1–6 using the Hiraoka system [15] with minor modifications, as stage 1 indicates a normal transparent lens and stage 6 represents a nuclear mature cataract.
Following slit-lamp examination, rats were sacrificed and lenses were removed for further analyses. The amount of a-crystallin in the water-soluble fraction was used for Western blot and chaperone activity.
2.4 Western blot analysis
Each rat lens was homogenized in 0.1 M Tris buffer (pH 8.0) and centrifuged at 20,000 g for 20 minutes at 4oC. Supernatant protein concentrations were measured using the Bradford Protein Assay Kit (Bio-Rad, Hercules, CA). Bovine serum albumin (BSA) was used as the standard. One mg protein in supernatant was loaded on a 12.5% polyacrylamide gel. After electrophoresis, the gel was transferred to a nitrocellulose membrane (Bio-Rad) for Western blot analysis using an anti–αA-crystallin antibody (ab5595; abcam, Cambridge, UK) or an anti-β-actin antibody (C-11 Santa Cruz, Santa Cruz, CA). Proteins were visualized with the horseradish peroxidase/3,3’-diaminobenzidine (DAB) system using DAB tablets (Wako Pure Chemical Industries) [16]. Obtained band intensities were quantified using the National Institutes of Health (Bethesda, MD) image J software.
2.5 Chaperone activity measurement
The chaperone activity of lens water-soluble fractions was measured the light scattering of alcohol dehydrogenase (ALDH) substrate as ∆A360/180 min (Wako Pure Chemical Industries). One mg/ml ALDH in 50 mM sodium phosphate buffer containing 100 mM NaCl (pH = 7.0) was induced to increase the light scattering by adding 100 μM 1,10-phenanthroline (Wako Pure Chemical Industries) at 42°C.
Lenses were homogenized in four volume of PBS and centrifuged at 20.000 g for 20 minutes at 4°C. The supernatant proteins were collected as water-soluble proteins. Lens water-soluble protein (8 mg/ml) was added in a 1:1 (vol:vol) to ALDH solution. The extent of aggregation was estimated by measuring light scattering at 360 nm using the microplate reader (Infinite M200: TECAN LTD, Männedorf, Switzerland). Each experiment was independently performed three times.
2.6 Statistical analysis
All data are reported as mean ± S.E. Statistical analysis of the data was performed using one-way ANOVA followed by Dunnett’s multiple comparison. Student’s t-test was used for comparison between two groups. Differences were considered significant at p < 0.05.
3 Results
3.1 Characteristics of selenite-induced cataract rat lens
Lenses were observed with slit-lamp biomicroscope on days 2, 4, and 6 and classified into six grades (grade 1 to 6; as grade 1 indicates a transparent lens and stage 6 represents a nuclear mature cataract). After two days following the selenite injection (Day 2), all rats had transparent lenses (grade 1: 100%). On Day 4, all G1 and G2 rats still had transparent lenses, but G3 rats showed significant cataracts (grade 6: 10%, grade 5: 40%, and grade 4: 50%) and the average cataract grade was of 4.6 ± 0.2 (n = 40 lenses). The lenses of G4 rats were less severe cataracts than those of G3 rats (grade 4: 20%, grade 3: 20%, grade 2: 40%, and grade 1: 40%) and had an average cataract grading of 2.4 ± 0.4 (n = 40 lenses). On Day 6, G1 rats (Figure 1A) and G2 rats (Figure 1B) still showed transparent lenses (grade 1: 100%, G1 n = 30 lenses, G2 n = 30 lenses) and all G3 rats (Figure 1C) showed mature nuclear cataracts, characteristic of selenite administration (grade 6: 100%, n = 40 lenses). The lenses of G4 rats (Figure 1D) were also in mature cataracts, but they were significantly less severe than those in G3 rats (grade 6: 20%, grade 5: 30%, grade 4: 20%, grade 2: 10%, and grade 1: 20%; average grade = 3.9 ± 0.6, 40 lenses; Table 1). Figure 1 shows slit-lamp photographs of each group on Day 6.
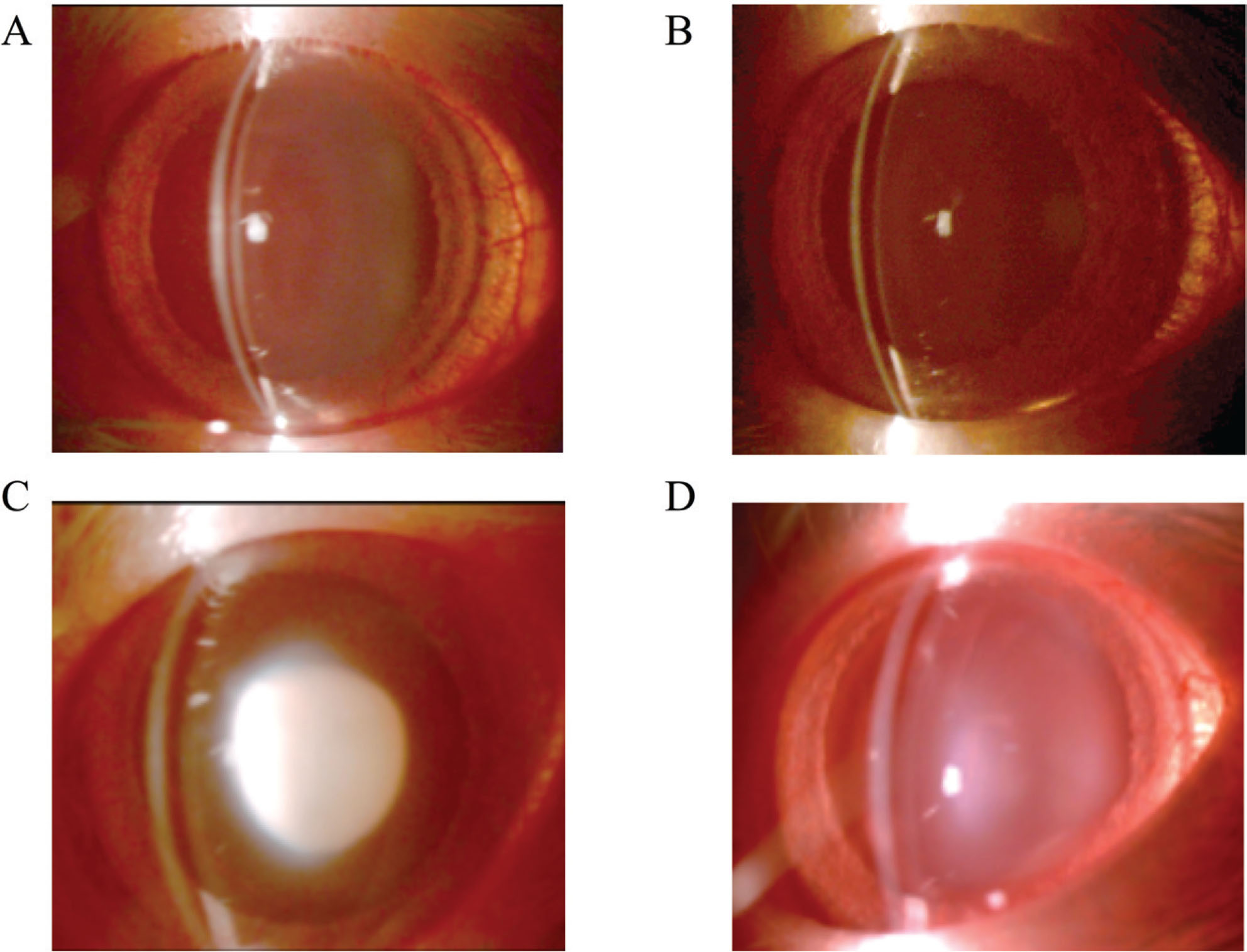
Effect of hespertin for cataractgenesis induced selenite administration. Groups G1 and G2 were injected with vehicle (control) and groups G3 and G4 were injected with sodium selenite subcutaneously into 13-day-old rats (Day 0). Hesperetein was administered to groups G2 and G4 subcutaneously in 13-, 14-, and 15-day-old rats (Day 0, 1, and 2, respectively). All lenses are taken 6 days after selenite or vehicle administration. (A) G1: no selenite and no hesperetin treatment. (B) G2: no selenite and hesperetin treatment. (C) G3: selenite treatment and no hesperetin treatment. (D) G4: selenite and hesperetin treatment. Rats in groups G1 and G2 had transparent lenses. Rats in the G3 group had a mature nuclear cataract, while those in group G4 had milder forms of nuclear cataracts.
Effect of hesperetin to cataract stage in lens of selenite-induced cataract rats.
G1 = no selenite and no hesperetin treatment; G2 = no selenite and hesperetin treatment; G3 = selenite treatment and no hesperetin treatment; G4 = selenite and hesperetin treatment. Means were calculated using data from 30 – 40 lenses.
| Group | Cataract stage | ||
|---|---|---|---|
| Day 2 | Day 4 | Day 6 | |
| G1 | 1.0 | 1.0 | 1.0 |
| G2 | 1.0 | 1.0 | 1.0 |
| G3 | 1.0 | 4.6 ± 0.2 | 6.0 |
| G4 | 1.0 | 2.4 ± 0.4 | 3.9 ± 0.6 |
3.2 Western blot analyses of α-crystallin
The amount of a-crystallin in the water-soluble fraction was measured using image of a western blot analysis on days 2, 4, and 6. On Day 2, amount of aA-crystallin was not significantly different between groups (Figure 2A). On Days 4 and 6, water-soluble aA-crystallin levels were significantly lower in G3 (Se-cataract) lenses than in G1 (control) lenses (Figure 2B, lane G3; Figure 2C, lane G3). On Day 6, the intensity of the aA-crystallin in G4 (Se-cataract, hesperetin-treated) lenses was not different from G1 or G2 lenses, but was significantly higher than that in G3 (Se-cataract) lenses (Figure 2C, lane G4). aA-crystallin levels were normalized by β-actin levels, as measured with densitometry (Figure 2D). On Day 6, aA-crystallin levels were significantly lower in G3 lenses than in G1 and G2 lenses. To the contrast, hesperetin treatment prevented water-solubility decreasing of this protein in lens with selenite-induced cataracts (G4).
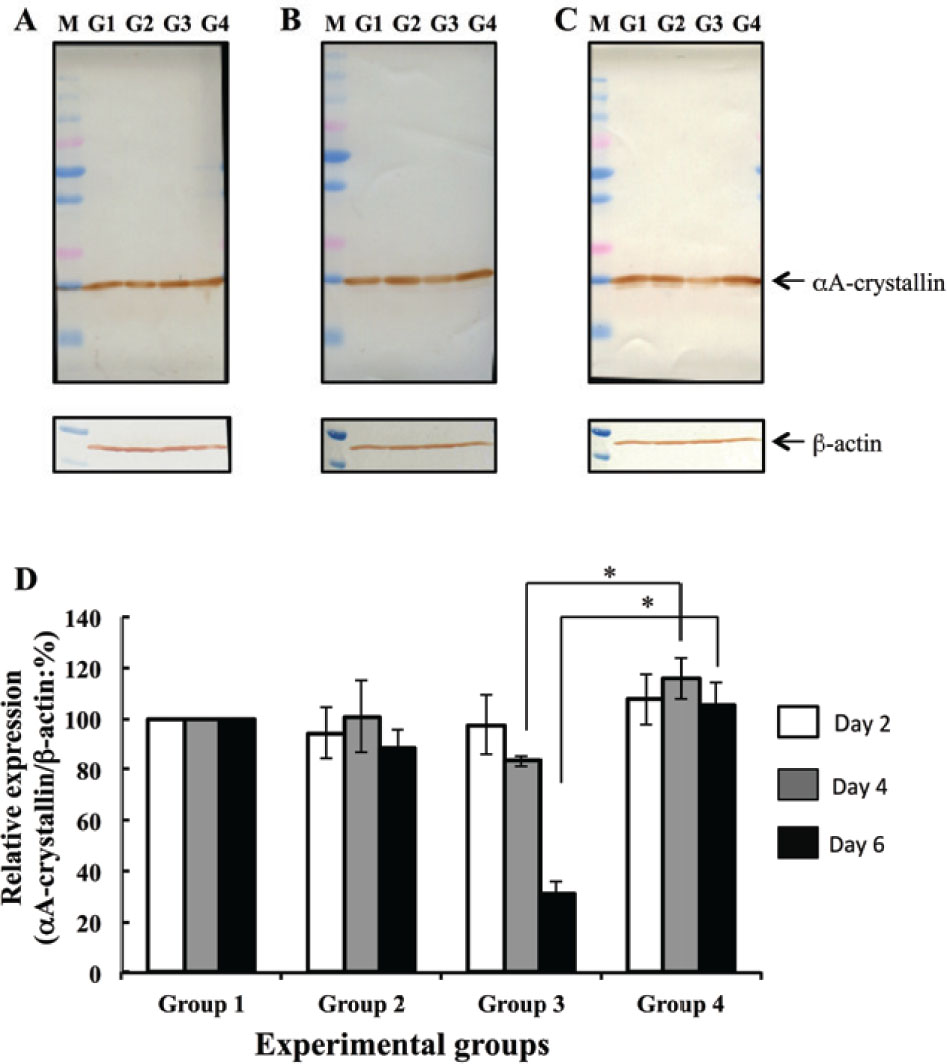
Western blot analyses to determine αA-crystallin levels. aA-crystallin levels were analyzed 2 days (A), 4 days (B), and 6 days (C) following selenite or placebo administration. b-actin was used as an internal control. Lane G1 was loaded water-soluble fraction of G1 lens (no selenite and no hesperetin treatment). Lane G2 was loaded water-soluble fraction of G2 lens (no selenite and hesperetin treatment). Lane G3 was loaded water-soluble fraction of G3 lens (selenite treatment and no hesperetin treatment). Lane G4 was loaded water-soluble fraction of G4 lens (selenite and hesperetin treatment). Lane M shows molecular weight markers. (D) Bar diagraph of aA-crystallin levels, as determined with densitometry-measured band intensity, at each time point examined. All values are relative to that measured in group G1. Data represent mean values and error bars represent one S.E. * indicates P < 0.05 for significant differences.
3.3 Chaperone activity of lens protein
Lens chaperone activity was evaluated by measuring the time course of the light scattering of ALDH at 360 nm (Figure 3). The light scattering of ALDH was elevated in the absence of lens proteins (Figure 3, curve 1). This elevation of light scattering was suppressed by immixing lens water-soluble protein from G1 (Figure 3, curve 5) and G2 (Figure 3, curve 4). Light scattering was not suppressed in the presence of G3 water-soluble proteins (Figure 3, curve 2), but elevation of light scatting was inhibited in the presence of G4 water-soluble proteins (Figure 3, curve 3). The elevation of light scattering was not occurred in lens water-soluble proteins without ALDH (Figure 3, curve 6).
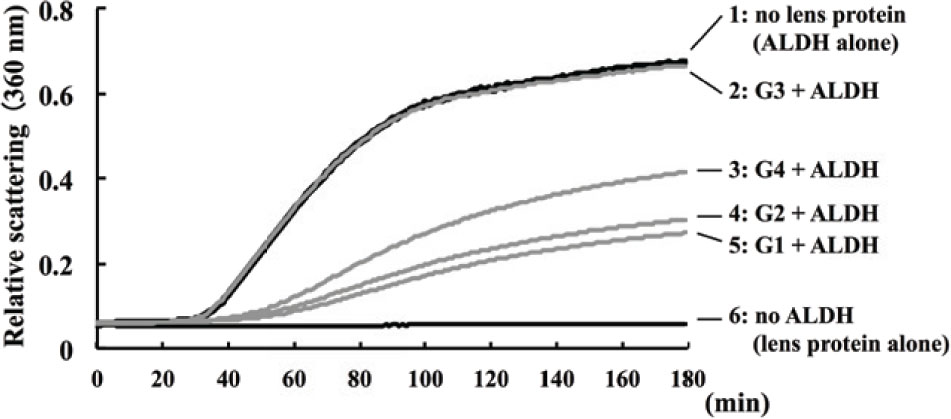
Chaperone activity of lens protein. The chaperone activity of water-soluble lens fractions was measured by a light scattering of ALDH at 360 nm, following the rat lens (day 6) administration of selenite or placebo. Curve 1 represents the light scattering of ALDH in the absence of lens protein. Curve 2 represents the light scattering of mixture of G3 lens protein (selenite treatment and no hesperetin treatment) and ALDH. Curve 3 shows the light scattering of mixture of G4 lens protein (selenite and hesperetin treatment) and ALDH. Curve 4 shows the light scattering of mixture of G2 lens protein (no selenite and hesperetin treatment) and ALDH. Curve 5 was shown the light scattering of mixture of G1 lens protein (no selenite and no hesperetin treatment) and ALDH. Curve 6 was shown the light scattering of lens protein without ALDH. Increasing of light scatting indicates the ALDH aggregation, and inhibition of light scattering depends on chaperone activity.
The depression activity of light scatting in lens watersoluble protein was measured on days 2, 4, and 6. Data were represented relative ∆A360/180 minutes values to light scattering of ALDH without lens protein that was defined as 100% and indicated the bar graph in Figure 4. On Day 2 (white bar), samples from G3 and G4 rats with preclinical cataracts showed similar light scatting as samples from G1 and G2 rats without cataracts (no Se-cataract control groups). On Day 4 (gray bar), the light scattering of ALDH in the presence of G3 was increased compared with in the presence of G1 and G2, but water-soluble proteins from G4 lenses retained protection against light scatting increasing. On Day 6 (black bar), G3 lenses had mature nuclear cataracts and completely lacked the inhibition of light scattering decreasing, but G4 lenses (hesperetin treated group) had less severe cataracts and retained protection against light scatting increasing (Figure 4). Increasing of light scatting indicated ALDH aggregation, and inhibition of protein aggregation was considered to depend on chaperone activity. These data suggested that hesperetin treatment prevent protein aggregation by maintaining the chaperone activity in lens water-soluble proteins.
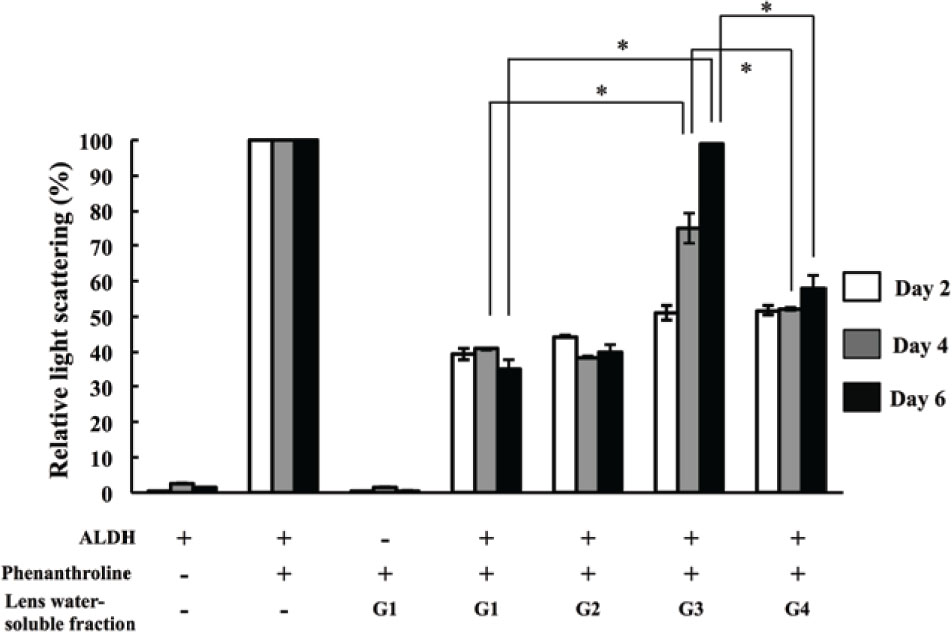
Relative change of light scattering. The change of ALDH scattering during 180 min after adding 1,10-phenanthroline at 42°C was calculated. The change of ALDH scattering in the sample without lens water-soluble fraction indicates for as 100% in the bar graph. The scatterings were measured on day 2 (white bar), day 4 (gray bar), and day 6 (black bar) following selenite or placebo administration. Bar represent mean values (n =9 lenses in each group) and error bars represent S.E. * indicates P < 0.05 for significant differences.
4 Discussion
Alpha-crystallin acts as a molecular chaperone to prevent protein aggregation and cataract formation. In this study, we hypothesized that chaperone activity might be reduced in the presence of a selenite-induced cataract because the water solubility of α-crystallin decreases in selenite-induced cataracts. To test this idea, we assessed lens chaperone activity using heat-aggregated ALDH measured the light scatting of ALDH (∆A360 / 180 minutes).
Chaperone activity was drastically reduced in lenses of selenite-induced cataracts, but hesperetin treatment rescued this activity. Furthermore, decreasing water solubility of a-crystallin by selenite administration was associated with lens chaperone activity. Chaperone activity was known to modulate by antioxidants (e.g., curcumin and cumin) [17, 18]. In agreement, we found that the antioxidant hesperetin also affects chaperone activity, as assessed with heat-aggregated ALDH in this report. Chaperone activity was also evaluated using a-lactalbumin aggregated by DTT and b-crystallin aggregated by heat. a-lactalbumin and b-crystallin aggregation assays also showed that lens chaperone activity reduced in proteins from lenses with mature selenite-induced cataracts and this activity rescued by hesperetin treatment (data not shown). All results showed that lens chaperone activity is correlated with lens transparency.
It has been reported that lens α-crystallin chaperone activity is known to be altered by several posttranslational modifications, including oxidation, racemization, deamidation, glycation, phosphorylation, and kynureniration [19-24]. In particular, α-crystallin in selenite-induced cataracts lenses have been reported to be modified by oxidative stress [11]. In the current study, amount of water soluble α-crystallin apparently decreased in selenite-induced cataract lens (G3, Figure 2). This result indicated that water solubility of α-crystallin was reduced in lenses of selenite-induced cataracts. These results suggest that selenite administration induced oxidative modifications of lens α-crystallin that make the reduction of lens α-crystallin water-solubility in addition to reduced lens chaperone activity. This diminution of water soluble α-crystallin that have chaperone activity ultimately resulted in cataract formation.
The lens contains high concentrations of antioxidants, including GSH and AsA. Both of these compounds maintain a reductive state in the lens and protect lens against photo-oxidative damage and cataract formation. The concentrations of GSH and AsA are frequently used as markers of cataract formation in both humans and animal models [25-27]. Hesperetin administration has been previously shown to prevent selenite-induced cataract formation [14]. This study provided the positive effects of hesperetin on selenite-induced cataracts for GSH and AsA levels and degradation of filensin, a lens-specific beaded filament, in rat lenses. But hesperetin was not detected in the lens within 4 hr after subcutaneous injection using HPLC (data not shown). These results suggested that administration of hesperetin maintained the lens in a reductive state indirectly, inhibited oxidative α-crystallin modification, maintained chaperone activity, keeped the a-crystallin water solubility, and preserved lens transparency. Hesperetin is a cytoprotective agent against oxidative stress and an effective medicine for cataract.
In summary, our findings demonstrated that hesperetin could delay the progression of lens opacification in selenite-induced cataract rats by maintenance of chaperone activity in lens water-soluble proteins. Hesperetin might be the drug lead compound to prevent the cataract formation.
Conflict of interest
No authors have any conflict of interest to disclose.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-supported Program for Strategic Research Foundation at Private Universities (S1101003).
References
[1] Spector A, Li L.K., Augusteyn R.C., Schneider A., Freund T., alpha-Crystallin. The isolation and characterization of distinct macromolecular fractions, Biochem J., 1971, 124, 337-34310.1016/0002-9394(71)90413-2Suche in Google Scholar
[2] Kramps H.A., Stols A.L., Hoenders H.J., On the quaternary structure of high-molecular-weight proteins from the bovine eye lens, Eur. J. Biochem., 1975, 50, 503-50910.1111/j.1432-1033.1975.tb09889.xSuche in Google Scholar
[3] Horwitz J., Alpha-crystallin can function as a molecular chaperone, Proc. Natl. Acad. Sci. USA., 1992, 89, 10449-1045310.1073/pnas.89.21.10449Suche in Google Scholar
[4] Hook D.W., Harding J.J., Protection of enzymes by alpha-crystallin acting as a molecular chaperone, Int. J. Biol. Macromol., 1998, 22, 295-30610.1016/S0141-8130(98)00027-0Suche in Google Scholar
[5] Derham B.K., Harding J.J., Alpha-crystallin as a molecular chaperone, Prog. Retin. Eye Res., 1999, 18, 463-50910.1016/S1350-9462(98)00030-5Suche in Google Scholar
[6] Horwitz J., Alpha-crystallin, Exp. Eye Res., 2003, 76, 145-15310.1016/S0014-4835(02)00278-6Suche in Google Scholar
[7] Sun Y., MacRae T.H., Small heat shock proteins: molecular structure and chaperone function, Cell Mol. Life Sci., 2005, 62, 2460-247610.1007/s00018-005-5190-4Suche in Google Scholar
[8] Ostádalová I., Babický A., Obenberger J., Cataract induced by administration of a single dose of sodium selenite to suckling rats, Experientia 1978, 34, 222-22310.1007/BF01944690Suche in Google Scholar
[9] Kelley M.J., David L.L., Iwasaki N., Wright J., Shearer T.R., alpha-Crystallin chaperone activity is reduced by calpain II in vitro and in selenite cataract, J. Biol. Chem., 1993, 268, 18844-1884910.1016/S0021-9258(17)46704-4Suche in Google Scholar
[10] Derham B.K., Harding J.J., Effect of aging on the chaperone-like function of human alpha-crystallin assessed by three methods, Biochem. J., 1997, 328, 763-76810.1042/bj3280763Suche in Google Scholar PubMed PubMed Central
[11] Rooban B.N., Sasikala V., Sahasranamam V., Abraham A., Analysis on the alterations of lens proteins by Vitex negundo in selenite cataract models, Mol. Vis., 2011, 17, 1239-1248Suche in Google Scholar
[12] Sasikala V., Rooban B.N., Sahasranamam V., Abraham A., Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of alpha-crystallin, Graefes. Arch. Clin. Exp. Ophthalmol., 2013, 251, 1747-175510.1007/s00417-013-2281-zSuche in Google Scholar
[13] Hwang S.L., Yen G.C., Effect of hesperetin against oxidative stress via ER- and TrkA-mediated actions in PC12 cells, J. Agric. Food Chem., 2011, 59, 5779-578510.1021/jf104632aSuche in Google Scholar
[14] Nakazawa Y., Oka M., Bando M., Takehana M., Hesperetin prevents selenite-induced cataract in rats, Mol. Vis., 2015, 21, 804-810Suche in Google Scholar
[15] Hiraoka T., Clark J.I., Inhibition of lens opacification during the early stages of cataract formation, Invest. Ophthalmol. Vis. Sci., 1995, 36, 2550-2555Suche in Google Scholar
[16] Oka M., Kudo H., Sugama N., Asami Y., Takehana M., The function of filensin and phakinin in lens transparency, Mol. Vis., 2008, 14, 815-822Suche in Google Scholar
[17] Kumar P.A., Suryanarayana P., Reddy P.Y., Reddy G.B., Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin, Mol. Vis., 2005, 11, 561-568Suche in Google Scholar
[18] Kumar P.A., Reddy P.Y., Srinivas P.N., Reddy G.B., Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of alpha-crystallin chaperone activity, J. Nutr, Biochem., 2009, 20, 553-56210.1016/j.jnutbio.2008.05.015Suche in Google Scholar
[19] Finley E.L., Dillon J., Crouch R.K., Schey K.L., Identification of tryptophan oxidation products in bovine alpha-crystallin, Protein Sci., 1998, 7, 2391-239710.1002/pro.5560071116Suche in Google Scholar
[20] Fujii N., Hiroki K., Matsumoto S., Masuda K., Inoue M., Tanaka Y., Awakura M., Akaboshi M., Correlation between the loss of the chaperone-like activity and the oxidation, isomerization and racemization of gamma-irradiated alpha-crystallin, Photochem. Photobiol., 2001, 74, 477-48210.1562/0031-8655(2001)074<0477:CBTLOT>2.0.CO;2Suche in Google Scholar
[21] Gupta R., Srivastava O.P., Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin, J. Biol. Chem., 2004, 279, 44258-4426910.1074/jbc.M405648200Suche in Google Scholar
[22] Kumar P.A., Kumar M.S., Reddy G.B., Effect of glycation on alpha-crystallin structure and chaperone-like function, Biochem. J., 2007, 408, 251-25810.1042/BJ20070989Suche in Google Scholar
[23] Kamei A., Hamaguchi T., Matsuura N., Masuda K., Does post-translational modification influence chaperone-like activity of alpha-crystallin? I. Study on phosphorylation, Biol. Pharm. Bull., 2001, 24, 96-9910.1248/bpb.24.96Suche in Google Scholar
[24] Garner B., Shaw D.C., Lindner R.A., Carver J.A., Truscott R.J., Non-oxidative modification of lens crystallins by kynurenine: a novel post-translational protein modification with possible relevance to ageing and cataract, Biochim. Biophys. Acta., 2000, 1476, 265-27810.1016/S0167-4838(99)00234-4Suche in Google Scholar
[25] Fernando M.R., Satake M., Monnier V.M., Lou M.F., Thioltranferase mediated ascorbate recycling in human lens epithelial cells, Invest. Ophthalmol. Vis. Sci., 2004, 45, 230-23710.1167/iovs.03-0545Suche in Google Scholar
[26] Yoshida M., Takashima Y., Inoue M., Iwasaki M., Otani T., Sasaki S., Tsugane S., Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population, Eur. J. Nutr., 2007, 46, 118-12410.1007/s00394-006-0641-8Suche in Google Scholar PubMed
[27] Nakazawa Y., Oka M., Bando M., Inoue T., Takehana M., The role of ascorbic acid transporter in the lens of streptozotocin-induced diabetic rat, Biomed. Prev. Nutr., 2011, 1, 43-4810.1016/j.bionut.2010.09.008Suche in Google Scholar
© 2016 Yosuke Nakazawa et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Research Article
- The possible molecular regulation mechanism of CIK cells inhibiting the proliferation of Human Lung Adenocarcinoma NCL-H157 Cells
- Case Report
- Urethral stone of unexpected size: case report and short literature review
- Case Report
- Complete remission through icotinib treatment in Non-small cell lung cancer epidermal growth factor receptor mutation patient with brain metastasis: A case report
- Research Article
- FPL tendon thickness, tremor and hand functions in Parkinson’s disease
- Research Article
- Diagnostic value of circulating tumor cells in cerebrospinal fluid
- Research Article
- A meta-analysis of neuroprotective effect for traditional Chinese medicine (TCM) in the treatment of glaucoma
- Research Article
- MiR-218 increases sensitivity to cisplatin in esophageal cancer cells via targeting survivin expression
- Research Article
- Association of HOTAIR expression with PI3K/Akt pathway activation in adenocarcinoma of esophagogastric junction
- Research Article
- The role of interleukin genes in the course of depression
- Case Report
- A rare case of primary pulmonary diffuse large B cell lymphoma with CD5 positive expression
- Research Article
- DWI and SPARCC scoring assess curative effect of early ankylosing spondylitis
- Research Article
- The diagnostic value of serum CEA, NSE and MMP-9 for on-small cell lung cancer
- Case Report
- Dysphonia – the single symptom of rifampicin resistant laryngeal tuberculosis
- Review Article
- Development of epidermal growth factor receptor tyrosine kinase inhibitors against EGFR T790M. Mutation in non small-cell lung carcinoma
- Research Article
- Negative regulation of CDC42 expression and cell cycle progression by miR-29a in breast cancer
- Research Article
- Expression analysis of the TGF-β/SMAD target genes in adenocarcinoma of esophagogastric junction
- Research Article
- Blood cells in thyroid cancer patients: a possible influence of apoptosis
- Research Article
- Detected EGFR mutation in cerebrospinal fluid of lung adenocarcinoma patients with meningeal metastasis
- Mini-review
- Pathogenesis-oriented approaches for the management of corticosteroid-resistant or relapsedprimary immune thrombocytopenia
- Research Article
- GSTP1 A>G polymorphism and chemosensitivity of osteosarcoma: A meta-analysis
- Research Article
- A meta-analysis of adiponectin gene rs22411766 T>G polymorphism and ischemic stroke susceptibility
- Research Article
- The diagnosis and pathological value of combined detection of HE4 and CA125 for patients with ovarian cancer
- Research Article
- SOX7 inhibits tumor progression of glioblastoma and is regulated by miRNA-24
- Research Article
- Sevoflurane affects evoked electromyography monitoring in cerebral palsy
- Case Report
- A case report of hereditary spherocytosis with concomitant chronic myelocytic leukemia
- Case Report
- A case of giant saphenous vein graft aneurysm followed serially after coronary artery bypass surgery
- Research Article
- LncRNA TUG1 is upregulated and promotes cell proliferation in osteosarcoma
- Review Article
- Meningioma recurrence
- Case Report
- Endobronchial amyloidosis mimicking bronchial asthma: a case report and review of the literature
- Case Report
- A confusing case report of pulmonary langerhans cell histiocytosis and literature review
- Research Article
- Effect of hesperetin on chaperone activity in selenite-induced cataract
- Research Article
- Clinical value of self-assessment risk of osteoporosis in Chinese
- Research Article
- Correlation analysis of VHL and Jade-1 gene expression in human renal cell carcinoma
- Research Article
- Is acute appendicitis still misdiagnosed?
- Retraction
- Retraction of: application of food-specific IgG antibody detection in allergy dermatosis
- Review Article
- Platelet Rich Plasma: a short overview of certain bioactive components
- Research Article
- Correlation between CTLA-4 gene rs221775A>G single nucleotide polymorphism and multiple sclerosis susceptibility. A meta-analysis
- Review Article
- Standards of anesthesiology practice during neuroradiological interventions
- Research Article
- Expression and clinical significance of LXRα and SREBP-1c in placentas of preeclampsia
- Letter to the Editor
- ARDS diagnosed by SpO2/FiO2 ratio compared with PaO2/FiO2 ratio: the role as a diagnostic tool for early enrolment into clinical trials
- Research Article
- Impact of sensory integration training on balance among stroke patients: sensory integration training on balance among stroke patients
- Review Article
- MicroRNAs as regulatory elements in psoriasis
- Review Article
- Influenza A(H1N1)pdm09 and postpandemic influenza in Lithuania
- Review Article
- Garengeot’s hernia: two case reports with CT diagnosis and literature review
- Research Article
- Concept of experimental preparation for treating dentin hypersensitivity
- Research Article
- Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy
- Research Article
- Xanthogranuloma of the sellar region diagnosed by frozen section
- Case Report
- Laparoscopic antegrade cholecystectomy: a standard procedure?
- Case Report
- Maxillary fibrous dysplasia associated with McCune-Albright syndrome. A case study
- Regular Article
- Sialoendoscopy, sialography, and ultrasound: a comparison of diagnostic methods
- Research Article
- Antibody Response to Live Attenuated Vaccines in Adults in Japan
- Conference article
- Excellence and safety in surgery require excellent and safe tutoring
- Conference article
- Suggestions on how to make suboptimal kidney transplantation an ethically viable option
- Regular Article
- Ectopic pregnancy treatment by combination therapy
- Conference article
- Use of a simplified consent form to facilitate patient understanding of informed consent for laparoscopic cholecystectomy
- Regular Article
- Cusum analysis for learning curve of videothoracoscopic lobectomy
- Regular Article
- A meta-analysis of association between glutathione S-transferase M1 gene polymorphism and Parkinson’s disease susceptibility
- Conference article
- Plastination: ethical and medico-legal considerations
- Regular Article
- Investigation and control of a suspected nosocomial outbreak of pan-drug resistant Acinetobacter baumannii in an intensive care unit
- Regular Article
- Multifactorial analysis of fatigue scale among nurses in Poland
- Regular Article
- Smoking cessation for free: outcomes of a study of three Romanian clinics
- Regular Article
- Clinical efficacy and safety of tripterygium glycosides in treatment of stage IV diabetic nephropathy: A meta-analysis
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Prevention and treatment of peritoneal adhesions in patients affected by vascular diseases following surgery: a review of the literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Surgical treatment of recidivist lymphedema
- Special Issue on Italian Society for the Study of Vascular Anomalies
- CT and MR imaging of the thoracic aorta
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Role of FDG-PET scan in staging of pulmonary epithelioid hemangioendothelioma
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Sternal reconstruction by extracellular matrix: a rare case of phaces syndrome
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Prenatal diagnosis, 3-D virtual rendering and lung sparing surgery by ligasure device in a baby with “CCAM and intralobar pulmonary sequestration”
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Serum levels of inhibin B in adolescents after varicocelelectomy: A long term follow up
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Our experience in the treatment of Malignant Fibrous Hystiocytoma of the larynx: clinical diagnosis, therapeutic approach and review of literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Delayed recurrent nerve paralysis following post-traumatic aortic pseudoaneurysm
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Integrated therapeutic approach to giant solitary fibrous tumor of the pleura: report of a case and review of the literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Celiac axis compression syndrome: laparoscopic approach in a strange case of chronic abdominal pain in 71 years old man
- Special Issue on Italian Society for the Study of Vascular Anomalies
- A rare case of persistent hypoglossal artery associated with contralateral proximal subclavian stenosis
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Contralateral risk reducing mastectomy in Non-BRCA-Mutated patients
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Professional dental and oral surgery liability in Italy: a comparative analysis of the insurance products offered to health workers
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Informed consent in robotic surgery: quality of information and patient perception
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Malfunctions of robotic system in surgery: role and responsibility of surgeon in legal point of view
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Medicolegal implications of surgical errors and complications in neck surgery: A review based on the Italian current legislation
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Iatrogenic splenic injury: review of the literature and medico-legal issues
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Donation of the body for scientific purposes in Italy: ethical and medico-legal considerations
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Cosmetic surgery: medicolegal considerations
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Voluntary termination of pregnancy (medical or surgical abortion): forensic medicine issues
- Review Article
- Role of Laparoscopic Splenectomy in Elderly Immune Thrombocytopenia
- Review Article
- Endoscopic diagnosis and treatment of neuroendocrine tumors of the digestive system
- Review Article
- Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia
- Research Article
- Relationship between gastroesophageal reflux disease and Ph nose and salivary: proposal of a simple method outpatient in patients adults
- Case Report
- Idiopathic pleural panniculitis with recurrent pleural effusion not associated with Weber-Christian disease
- Research Article
- Morbid Obesity: treatment with Bioenterics Intragastric Balloon (BIB), psychological and nursing care: our experience
- Research Article
- Learning curve for endorectal ultrasound in young and elderly: lights and shades
- Case Report
- Uncommon primary hydatid cyst occupying the adrenal gland space, treated with laparoscopic surgical approach in an old patient
- Research Article
- Distraction techniques for face and smile aesthetic preventing ageing decay
- Research Article
- Preoperative high-intensity training in frail old patients undergoing pulmonary resection for NSCLC
- Review Article
- Descending necrotizing mediastinitis in the elderly patients
- Research Article
- Prophylactic GSV surgery in elderly candidates for hip or knee arthroplasty
- Research Article
- Diagnostic yield and safety of C-TBNA in elderly patients with lung cancer
- Research Article
- The learning curve of laparoscopic holecystectomy in general surgery resident training: old age of the patient may be a risk factor?
- Research Article
- Self-gripping mesh versus fibrin glue fixation in laparoscopic inguinal hernia repair: a randomized prospective clinical trial in young and elderly patients
- Research Article
- Anal sphincter dysfunction in multiple sclerosis: an observation manometric study
Artikel in diesem Heft
- Research Article
- The possible molecular regulation mechanism of CIK cells inhibiting the proliferation of Human Lung Adenocarcinoma NCL-H157 Cells
- Case Report
- Urethral stone of unexpected size: case report and short literature review
- Case Report
- Complete remission through icotinib treatment in Non-small cell lung cancer epidermal growth factor receptor mutation patient with brain metastasis: A case report
- Research Article
- FPL tendon thickness, tremor and hand functions in Parkinson’s disease
- Research Article
- Diagnostic value of circulating tumor cells in cerebrospinal fluid
- Research Article
- A meta-analysis of neuroprotective effect for traditional Chinese medicine (TCM) in the treatment of glaucoma
- Research Article
- MiR-218 increases sensitivity to cisplatin in esophageal cancer cells via targeting survivin expression
- Research Article
- Association of HOTAIR expression with PI3K/Akt pathway activation in adenocarcinoma of esophagogastric junction
- Research Article
- The role of interleukin genes in the course of depression
- Case Report
- A rare case of primary pulmonary diffuse large B cell lymphoma with CD5 positive expression
- Research Article
- DWI and SPARCC scoring assess curative effect of early ankylosing spondylitis
- Research Article
- The diagnostic value of serum CEA, NSE and MMP-9 for on-small cell lung cancer
- Case Report
- Dysphonia – the single symptom of rifampicin resistant laryngeal tuberculosis
- Review Article
- Development of epidermal growth factor receptor tyrosine kinase inhibitors against EGFR T790M. Mutation in non small-cell lung carcinoma
- Research Article
- Negative regulation of CDC42 expression and cell cycle progression by miR-29a in breast cancer
- Research Article
- Expression analysis of the TGF-β/SMAD target genes in adenocarcinoma of esophagogastric junction
- Research Article
- Blood cells in thyroid cancer patients: a possible influence of apoptosis
- Research Article
- Detected EGFR mutation in cerebrospinal fluid of lung adenocarcinoma patients with meningeal metastasis
- Mini-review
- Pathogenesis-oriented approaches for the management of corticosteroid-resistant or relapsedprimary immune thrombocytopenia
- Research Article
- GSTP1 A>G polymorphism and chemosensitivity of osteosarcoma: A meta-analysis
- Research Article
- A meta-analysis of adiponectin gene rs22411766 T>G polymorphism and ischemic stroke susceptibility
- Research Article
- The diagnosis and pathological value of combined detection of HE4 and CA125 for patients with ovarian cancer
- Research Article
- SOX7 inhibits tumor progression of glioblastoma and is regulated by miRNA-24
- Research Article
- Sevoflurane affects evoked electromyography monitoring in cerebral palsy
- Case Report
- A case report of hereditary spherocytosis with concomitant chronic myelocytic leukemia
- Case Report
- A case of giant saphenous vein graft aneurysm followed serially after coronary artery bypass surgery
- Research Article
- LncRNA TUG1 is upregulated and promotes cell proliferation in osteosarcoma
- Review Article
- Meningioma recurrence
- Case Report
- Endobronchial amyloidosis mimicking bronchial asthma: a case report and review of the literature
- Case Report
- A confusing case report of pulmonary langerhans cell histiocytosis and literature review
- Research Article
- Effect of hesperetin on chaperone activity in selenite-induced cataract
- Research Article
- Clinical value of self-assessment risk of osteoporosis in Chinese
- Research Article
- Correlation analysis of VHL and Jade-1 gene expression in human renal cell carcinoma
- Research Article
- Is acute appendicitis still misdiagnosed?
- Retraction
- Retraction of: application of food-specific IgG antibody detection in allergy dermatosis
- Review Article
- Platelet Rich Plasma: a short overview of certain bioactive components
- Research Article
- Correlation between CTLA-4 gene rs221775A>G single nucleotide polymorphism and multiple sclerosis susceptibility. A meta-analysis
- Review Article
- Standards of anesthesiology practice during neuroradiological interventions
- Research Article
- Expression and clinical significance of LXRα and SREBP-1c in placentas of preeclampsia
- Letter to the Editor
- ARDS diagnosed by SpO2/FiO2 ratio compared with PaO2/FiO2 ratio: the role as a diagnostic tool for early enrolment into clinical trials
- Research Article
- Impact of sensory integration training on balance among stroke patients: sensory integration training on balance among stroke patients
- Review Article
- MicroRNAs as regulatory elements in psoriasis
- Review Article
- Influenza A(H1N1)pdm09 and postpandemic influenza in Lithuania
- Review Article
- Garengeot’s hernia: two case reports with CT diagnosis and literature review
- Research Article
- Concept of experimental preparation for treating dentin hypersensitivity
- Research Article
- Hydrogen water reduces NSE, IL-6, and TNF-α levels in hypoxic-ischemic encephalopathy
- Research Article
- Xanthogranuloma of the sellar region diagnosed by frozen section
- Case Report
- Laparoscopic antegrade cholecystectomy: a standard procedure?
- Case Report
- Maxillary fibrous dysplasia associated with McCune-Albright syndrome. A case study
- Regular Article
- Sialoendoscopy, sialography, and ultrasound: a comparison of diagnostic methods
- Research Article
- Antibody Response to Live Attenuated Vaccines in Adults in Japan
- Conference article
- Excellence and safety in surgery require excellent and safe tutoring
- Conference article
- Suggestions on how to make suboptimal kidney transplantation an ethically viable option
- Regular Article
- Ectopic pregnancy treatment by combination therapy
- Conference article
- Use of a simplified consent form to facilitate patient understanding of informed consent for laparoscopic cholecystectomy
- Regular Article
- Cusum analysis for learning curve of videothoracoscopic lobectomy
- Regular Article
- A meta-analysis of association between glutathione S-transferase M1 gene polymorphism and Parkinson’s disease susceptibility
- Conference article
- Plastination: ethical and medico-legal considerations
- Regular Article
- Investigation and control of a suspected nosocomial outbreak of pan-drug resistant Acinetobacter baumannii in an intensive care unit
- Regular Article
- Multifactorial analysis of fatigue scale among nurses in Poland
- Regular Article
- Smoking cessation for free: outcomes of a study of three Romanian clinics
- Regular Article
- Clinical efficacy and safety of tripterygium glycosides in treatment of stage IV diabetic nephropathy: A meta-analysis
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Prevention and treatment of peritoneal adhesions in patients affected by vascular diseases following surgery: a review of the literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Surgical treatment of recidivist lymphedema
- Special Issue on Italian Society for the Study of Vascular Anomalies
- CT and MR imaging of the thoracic aorta
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Role of FDG-PET scan in staging of pulmonary epithelioid hemangioendothelioma
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Sternal reconstruction by extracellular matrix: a rare case of phaces syndrome
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Prenatal diagnosis, 3-D virtual rendering and lung sparing surgery by ligasure device in a baby with “CCAM and intralobar pulmonary sequestration”
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Serum levels of inhibin B in adolescents after varicocelelectomy: A long term follow up
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Our experience in the treatment of Malignant Fibrous Hystiocytoma of the larynx: clinical diagnosis, therapeutic approach and review of literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Delayed recurrent nerve paralysis following post-traumatic aortic pseudoaneurysm
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Integrated therapeutic approach to giant solitary fibrous tumor of the pleura: report of a case and review of the literature
- Special Issue on Italian Society for the Study of Vascular Anomalies
- Celiac axis compression syndrome: laparoscopic approach in a strange case of chronic abdominal pain in 71 years old man
- Special Issue on Italian Society for the Study of Vascular Anomalies
- A rare case of persistent hypoglossal artery associated with contralateral proximal subclavian stenosis
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Contralateral risk reducing mastectomy in Non-BRCA-Mutated patients
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Professional dental and oral surgery liability in Italy: a comparative analysis of the insurance products offered to health workers
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Informed consent in robotic surgery: quality of information and patient perception
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Malfunctions of robotic system in surgery: role and responsibility of surgeon in legal point of view
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Medicolegal implications of surgical errors and complications in neck surgery: A review based on the Italian current legislation
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Iatrogenic splenic injury: review of the literature and medico-legal issues
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Donation of the body for scientific purposes in Italy: ethical and medico-legal considerations
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Cosmetic surgery: medicolegal considerations
- Focus on Medico-Legal and Ethical Topics in Surgery in Italy
- Voluntary termination of pregnancy (medical or surgical abortion): forensic medicine issues
- Review Article
- Role of Laparoscopic Splenectomy in Elderly Immune Thrombocytopenia
- Review Article
- Endoscopic diagnosis and treatment of neuroendocrine tumors of the digestive system
- Review Article
- Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia
- Research Article
- Relationship between gastroesophageal reflux disease and Ph nose and salivary: proposal of a simple method outpatient in patients adults
- Case Report
- Idiopathic pleural panniculitis with recurrent pleural effusion not associated with Weber-Christian disease
- Research Article
- Morbid Obesity: treatment with Bioenterics Intragastric Balloon (BIB), psychological and nursing care: our experience
- Research Article
- Learning curve for endorectal ultrasound in young and elderly: lights and shades
- Case Report
- Uncommon primary hydatid cyst occupying the adrenal gland space, treated with laparoscopic surgical approach in an old patient
- Research Article
- Distraction techniques for face and smile aesthetic preventing ageing decay
- Research Article
- Preoperative high-intensity training in frail old patients undergoing pulmonary resection for NSCLC
- Review Article
- Descending necrotizing mediastinitis in the elderly patients
- Research Article
- Prophylactic GSV surgery in elderly candidates for hip or knee arthroplasty
- Research Article
- Diagnostic yield and safety of C-TBNA in elderly patients with lung cancer
- Research Article
- The learning curve of laparoscopic holecystectomy in general surgery resident training: old age of the patient may be a risk factor?
- Research Article
- Self-gripping mesh versus fibrin glue fixation in laparoscopic inguinal hernia repair: a randomized prospective clinical trial in young and elderly patients
- Research Article
- Anal sphincter dysfunction in multiple sclerosis: an observation manometric study


