External validation of a non-invasive vaginal tool to assess the risk of intra-amniotic inflammation in pregnant women with preterm labor and intact membranes
-
Teresa Cobo
, Xavier P. Burgos-Artizzu
Abstract
Objectives
To prospectively validate the diagnostic performance of a non-invasive point-of-care tool (Rapid IAI System), including vaginal alpha-fetoprotein and interleukin-6, to predict the occurrence of intra-amniotic inflammation in a Spanish cohort of patients admitted with a diagnosis of preterm labor and intact membranes.
Methods
From 2017 to 2022, we prospectively evaluated a cohort of pregnant women diagnosed with preterm labor and intact membranes admitted below 34+0 weeks who underwent amniocentesis to rule-in/out intra-amniotic infection and/or inflammation. Vaginal sampling was performed at the time of amniocentesis or within 24–48 h. Amniotic fluid IL-6, vaginal alpha-fetoprotein and vaginal IL-6 concentrations were measured using a point-of-care tool provided by Hologic Inc., “Rapid IAI System”. We defined intra-amniotic inflammation when amniotic fluid IL-6 values were greater than 11.3 ng/mL. During recruitment, clinicians were blinded to the results of the point-of-care tool. The original prediction model proposed by Hologic Inc. to predict intra-amniotic inflammation was validated in this cohort of patients.
Results
We included 151 patients diagnosed with preterm labor and intact membranes. Among these, 29 (19.2 %) had intra-amniotic inflammation. The algorithm including vaginal IL-6 and alpha-fetoprotein showed an area under curve to predict intra-amniotic inflammation of 80.3 % (±5.3 %) with a sensitivity of 72.4 %, specificity of 84.6 %, positive predictive valuve (PPV) of 52.5 %, negative predictive value (NPV) of 92.9 %, and a positive likelihood ratio (LR+) of 4.6 and negative likelihood ratio (LR−) of 0.33.
Conclusions
External validation of a non-invasive rapid point-of-care tool, including vaginal alpha-fetoprotein and IL-6, showed very good diagnostic performance for predicting the absence of intra-amniotic inflammation in women with preterm labor and intact membranes.
Introduction
Among women with preterm labor (PTL) and intact membranes, those with intra-amniotic inflammation present the highest risk to deliver in the following seven days [1]. This is the group with the worse neonatal outcome, probably due to the very short latency (of days) and the early gestational age at delivery of these patients [2], 3]. In addition, regardless the occurrence of intra-amniotic infection, the presence of an intra-amniotic inflammatory status is related to a higher risk of cerebral palsy [4] and neurological impairment in premature infants [5]. Finally, there is growing evidence showing latency to delivery and gestational age at delivery are longer in the absence of intra-amniotic inflammation [6]. This might question current management of PTL, with steroids, tocolysis and magnesium sulfate, that does not discriminate according to the occurrence of this inflammatory status. Although more evidence is needed to determine whether antenatal antibiotic treatment in this subclinical stage improves perinatal outcomes [7], it is clear that these patients are who might most benefit from being transferred to neonatal intensive care units and be managed with antenatal treatments that have shown to improve neonatal outcomes of premature newborns, such as antenatal steroids or magnesium sulfate [8], 9].
The main concern with targeting this high-risk group is the need to perform an invasive procedure. Whereas the risk of amniocentesis late in the second and third trimester is very low [10], even in patients with preterm prelabor rupture of membranes (PPROM) (<0.7 %) [11], many physicians are reluctant to perform this procedure in the absence of symptoms of clinical chorioamnionitis. However, clinical chorioamnionitis is the tip of the iceberg. Majority of intra-amniotic infection occurs in a subclinical stage in women without clinical signs of chorioamnionitis. In this scenario, the development of a non-invasive point-of-care tool to alert clinicians of the occurrence of this infectious/inflammatory condition would be of significant clinical relevance. Similarly, with a negative non-invasive test, clinicians can identify pregnancies in which inflammation is highly unlikely and in which amniocentesis is not justified.
In this regard, some point-of care tests, alone [12], 13] or in combination [14], [15], [16] have been proposed to target patients at high-risk of intra-amniotic inflammation including proteins, metabolites and ultrasound cervical length. Thus, Oh et al. [17] proposed a multivariable prediction model including cervical fetal fibronectin, maternal serum C-reactive protein, cervical dilatation, and gestational age to predict intra-amniotic infection and/or inflammation in patients with preterm labor and intact membranes with good diagnostic performance.
However, these models have not yet been implemented in the clinical setting, probably due to the lack of external validation.
Alpha-fetoprotein (AFP) is a tumor-associated oncofetal protein which has been associated with fetal defects and malignant tumor growth. During pregnancy AFP has been shown to be a marker of acute inflammation. Thus, AFP interacts and binds to caspase-3,9 enzymes (cysteine proteases) which constitute key components of molecular complexes called inflammosomes. Inflammosomes triggers the maturation of the proinflammatory cytokine interleukin-1β to encage innate immune defense processes [18], 19]. Interleukin (IL)-6 has been widely reported as modulator of host immune response and considered a key cytokine for the identification of intra-amniotic inflammation, infection [20], 21], and spontaneous delivery within seven days [22].
Hologic, Inc. has shown interest on biomarkers or combinations of biomarkers that can be used for non-invasive diagnosis of intra-amniotic infection [23]. In a cohort of 196 women, they observed that patterns of cervical-vaginal protein concentrations, particularly AFP and IL-6, differed between patients with intra-amniotic inflammation (defined by the presence of amniotic fluid IL-6 concentrations ≥11.3 ng/mL) vs. those that did not. The receiver-operating characteristic (ROC) curve of vaginal AFP and IL-6 for classifying intra-amniotic inflammation was 0.88 and improved to 0.91 with the addition of gestational age, showing a sensitivity of 83 %, a specificity of 85 % a positive predictive value (PPV) of 56 % and a negative predictive value (NPV) of 96 % (unpublished data). Using this data, Hologic Inc. constructed an algorithm based on vaginal fluid IL-6 and AFP and developed a non-invasive point-of-care tool (Rapid IAI System) to classify patients with intra-amniotic inflammation vs. those without. The clinical utility of this non-invasive rapid tool including vaginal AFP and IL-6 was early identification of women with intra-amniotic inflammation using a minimally invasive approach, since preterm contractions are often the only symptom of suspicion. Nonetheless, before implementation as a point-of-care tool, external validation is needed.
Thus, the objective of this study was to prospectively validate the diagnostic performance of the Rapid IAI System to predict intra-amniotic inflammation in a Spanish cohort of women admitted with PTL and intact membranes and to compare its diagnostic accuracy with other cervicovaginal point-of-care tests previously reported.
Materials and methods
Study design
This was a prospective observational study performed at the Hospital Clinic and Hospital Sant Joan de Déu, Barcelona during the period from June 2017 to July 2022.
We included singleton pregnancies admitted with a diagnosis of PTL and intact membranes between 23+0 and 33+6 weeks which did not meet the exclusion criteria, and with an amniocentesis to rule-in/out intra-amniotic infection/inflammation.
Preterm labor was defined as labor prior to 37 weeks in patients with intact membranes defining labor as the presence of uterine contractions with cervical changes. Cervical changes were evaluated measuring cervical length by transvaginal ultrasound using different cutoff of risk according to gestational age [24].
Gestational age was established according to crown-rump length at the first-trimester ultrasound scan [25].
Our main outcome was the occurrence of intra-amniotic inflammation, defined by the presence of amniotic fluid IL-6 concentrations ≥11.3 ng/mL measured using the Rapid IAI System provided by Hologic Inc. This cut-off was previously referenced by other authors [26], 27].
We defined microbial invasion of amniotic cavity (MIAC) as the presence of a positive amniotic fluid culture for aerobic and anaerobic bacteria and yeasts (aerobic chocolate agar, anaerobic Schaedler agar and thioglycollate broth), genital mycoplasma (Mycoplasma IST 2, bioMérieux for Ureaplasma spp. or Mycoplasma hominis), and/or by specific (PCR) amplification of the 16S ribosomal RNA gene.
Sterile inflammation was defined as the presence of amniotic fluid IL-6 levels amniotic fluid IL-6 concentrations ≥11.3 ng/mL with a negative amniotic fluid culture and a negative PCR amplification of the 16S ribosomal RNA gene.
Intra-amniotic infection was defined when both, MIAC and intra-amniotic inflammation, were present.
We excluded women presenting PPROM, maternal age <18 years, multiple gestations, clinical chorioamnionitis, defined by the presence of fever ≥38 °C, fetal tachycardia (>160 heart beat per minute >10 min) and maternal white blood cell count >15,000/mm3 (not justified by the administration of antenatal corticosteroids) [28], cervical dilatation >5 cm, major structural malformations of fetal complications, women with an indication for preterm delivery (e.g., pre-eclampsia) and women unable to provide written informed consent.
Written informed consent was obtained from all subjects. Patient selection and sampling procedures were performed in accordance with the Declaration of Helsinki and applicable local regulatory requirements after approval from the Institutional Review Boards (HCB/2016/0523; PIC-98-16).
Vaginal fluid collection
Vaginal fluid was collected using swabs submerged in 5.0 mL of sodium chloride (NaCl) and kept at 4 °C until processing. Vaginal fluid was centrifuged, ranging between 2,000 and 3,000×g at 4 °C for 10 min. Supernatants and pellets were stored separately at −80 °C.
Device description
The Rapid IAI System is an in vitro diagnostic device used to measure IL-6 and AFP values, on a single cassette utilizing vaginal fluid and is comprised of the Rapid IAI Specimen Collection Kit, Rapid IAI Cassette, Rapid IAI Analyzer and Printer, Rapid IAI QCette and the Rapid IAI Control Kit.
The Rapid IAI System is composed of a plastic housing that contains a lateral flow test strip. The specimen is applied to the test strip through the sample application well of the IAI Cassette. The sample flows from an absorbent pad across a nitrocellulose membrane, via capillary action, through a reaction zone containing two antibody-blue microsphere conjugates; one conjugate contains anti-IL-6 antibody and the other conjugate contains anti-AFP antibody. The antibody conjugates embedded in the membrane are mobilized by the flow of the sample through two reaction zones: one zone contains immobilized anti-IL-6 antibody and the other zone contains immobilized anti-AFP antibody. In the presence of IL-6, the conjugate-IL-6 complex binds at the IL-6 reaction zone at an amount that is proportional to the amount of IL-6 present in the specimen. In the presence of AFP, the conjugate-AFP complex binds at the AFP reaction zone at an amount that is proportional to the amount of AFP present in the specimen. The remaining sample flows through a control zone(s) that capture the unbound conjugates.
The Rapid IAI Analyzer is an electronic optical reflectance device that converts the colorimetric reaction from the Rapid IAI Cassette into a digitized format and reports a single diagnostic result based on the quantitative levels of IL-6 and/or AFP contained in the specimen (Figure 1).
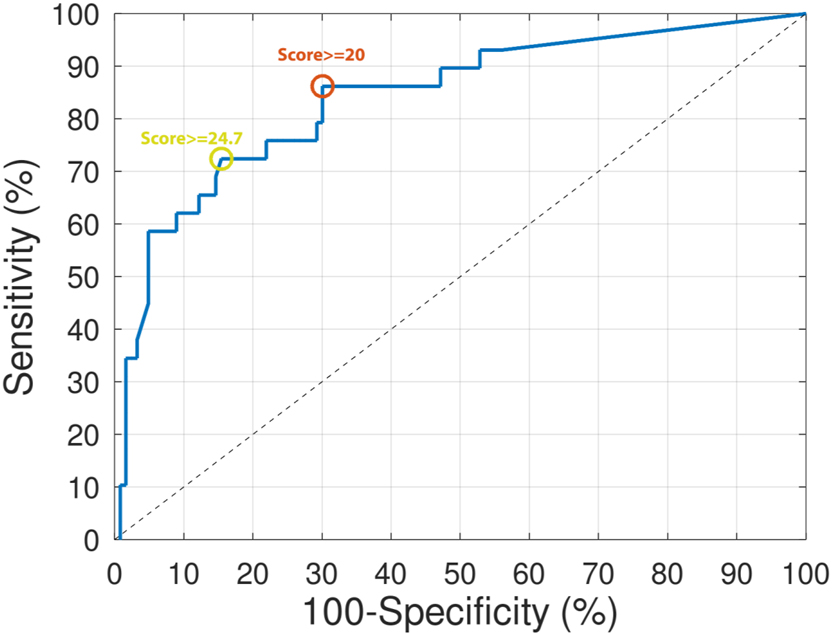
Receiver Operating Curve (ROC) of the algorithm to predict intra-amniotic inflammation in preterm labor.
Vaginal fluid IL-6 concentration ranges from 0 to 600 pg/mL. The minimal detection level is 18.9 pg/mL with a coefficient of variation ≤20 % and an accuracy ≥85 %.
Vaginal fluid AFP concentration ranges from 0 to 60 ng/mL. The minimal detection level is 4.5 ng/mL with a coefficient of variation ≤20 % and an accuracy ≥85 %.
Finally, the Rapid IAI AF Inflammation test quantitatively measures IL-6 in amniotic fluid. Amniotic fluid IL-6 concentration ranges from 0 to 600 pg/mL. The detection level is 37.9 pg/mL with a coefficient of variation ≤20 % and an accuracy ≥85 %. The total analysis takes approximately 30 min from sample addition.
Algorithm developed by Hologic Inc.
In the original study, Hologic Inc included 196 patients with PTL and intact membranes between 22+0 and 36+6 weeks. Intra-amniotic inflammation was defined when amniotic fluid IL-6 concentrations were ≥11.3 ng/mL. The prevalence of intra-amniotic inflammation was 18 % (35/196). Latency from amniocentesis to delivery was shorter in women with intra-amniotic inflammation. Using this data, Hologic Inc. developed an algorithm based on vaginal fluid IL-6 and AFP to classify patients with intra-amniotic inflammation vs. those without (unpublished data).
First, concentrations of both vaginal proteins were transformed to the natural log scale. Then, logistic regression modeling was used to determine the linear combination of protein concentrations that best separated the group with intra-amniotic inflammation from the rest. The resulting algorithm score was a linear combination (i.e., weighted sum) of the vaginal measurements. The equation was the following:
When this score was above a certain threshold, the test was deemed positive, otherwise negative. In the original study, they deemed a score ≥20.0 as the optimal cut-off threshold value. With this formula, they assessed the operating characteristics of the algorithm, and it exceeded the pre-specified minimally acceptable criteria – 73 % sensitivity, 78 % specificity – required by their sample size computations. The algorithm was locked down at this point before examining the validation set.
The performance characteristics of the non-invasive vaginal test (vaginal fluid IL-6, vaginal fluid AFP, and gestational age) were evaluated. They anticipated that the vaginal test would correctly identify women without intra-amniotic inflammation 96 % of the time and correctly identify women with inflammation 56 % of the time.
Independent clinical validation
In the external validation phase, the original algorithm was locked down; it was not altered based on data collected during the clinical validation. We measured the two vaginal proteins and amniotic fluid IL-6, applied the algorithm, and assessed its classification performance in our cohort of patients. The diagnostic indices obtained were compared to their a priori selected minimally acceptable criteria. Clinicians were blinded to the Rapid IAI System results.
Clinical management
As previously reported, and as part of our clinical protocol, women with PTL and intact membranes before 34+0 weeks were offered amniocentesis to rule-in/out intra-amniotic infection and/or inflammation. A complete course of antenatal steroids, betamethasone 12 mg intramuscular injection with two doses given 24 h apart, was administered until 34 completed weeks for fetal lung maturation. Tocolysis (nifedipine, atosiban) was administered during fetal maturation with steroids and magnesium sulfate if imminent delivery was suspected. Broad-spectrum antibiotics were initiated on high suspicion of intra-amniotic infection, based on the presence of a low amniotic fluid glucose concentration (<5 mg/dL) and/or the presence of microorganisms identified by amniotic fluid Gram staining. From 2018 to 2019, patients with high suspicion of microbial invasion of the amniotic cavity received parenteral ampicillin 1 g/6 h and gentamycin 80 mg/8 h and a single dose of oral azithromycin 1 g. Beyond 2019, our local protocol substituted this antibiotic combination to parenteral ceftriaxone 1 g/12 h and ampicillin 2 g/6 h and oral clarithromycin 500 mg/8 h. Antibiotic treatment was discontinued if amniotic fluid cultures were negative. In women diagnosed with subclinical intra-amniotic infection who remained pregnant after microbiological results, we individualized the antibiotic treatment according to the microorganism isolated until 7–10 days or until spontaneous onset of labor. Labor induction was considered only if clinical chorioamnionitis occurred.
Information of 16s ribosomal RNA gene sequencing and amniotic fluid IL-6 was not available for clinical decision-making.
Sample size
Hologic Inc. anticipated that the test would have similar operating characteristics to those observed in earlier studies (sensitivity=82 % and specificity 85 %). Power was set at 80 % and the type 1 error (significance) at 5 %, and both metrics were estimated jointly. The lower value of the 95 % confidence interval for sensitivity was not less than 55 %. The upper value for a false positive was no more than 20 %. Considering the prevalence of the subjects observed in this study (19.2 %), statistical power analysis [29] suggested that a minimum of 124 patients were required to validate results.
Statistical analysis
Statistical analysis was performed using MATLAB (Mathworks, USA). The Shapiro Wilk test was initially used to assess continuous data for normality. We compared maternal characteristics and perinatal outcomes between the derivation and validation cohorts; continuous variables were compared using a non-parametric Mann-Whitney U test presented as median (interquartile range (IQR)). Categorical variables were compared using the Chi-squared or Fisher exact test. Differences were considered statistically significant with a p<0.05 with two-sided alternative hypotheses.
The vaginal fluid protein (IL-6 and AFP) values were used to compute the score using the equation previously described. From the score output, the diagnostic performance to discriminate patients with intra-amniotic inflammation was calculated using the ROC curve. The ROC curve measures sensitivity (a.k.a. true positive rate) and false positive rate (inverse of specificity) at different threshold values of the score output.
First, from the ROC curve the area under the curve (AUC) was computed. Then, the optimal cut-off threshold was selected as that maximizing sensitivity for specificities above 78 % following minimal specification criteria. Sensitivity, specificity, PPV, NPV, LR+, and LR− were computed using this cut-off threshold. Finally, we also reported diagnostic performance metrics for the originally selected threshold (score≥20.0).
Results
During the study period (2017–2022), 151 women diagnosed with PTL and intact membranes and no suspicion of clinical chorioamnionitis agreed and signed the consent form to participate in this study. The prevalence of intra-amniotic inflammation was 19 % (29/151). MIAC was observed in 11.9 % (18/151) of patients.
The maternal characteristics and perinatal outcomes according to the occurrence of intra-amniotic infection and/or inflammation in patients diagnosed with PTL and intact membranes are shown in the Table 1.
Maternal characteristics and perinatal outcomes in patients with preterm labor and intact membranes (n 151).
| Intra-amniotic inflammation (n 29) | Non-intra-amniotic inflammation (n 122) | p-Value | |
|---|---|---|---|
| Maternal age at admission, years | 31.7 (26.0; 36–7) | 31.1 (25.6; 34.6) | 0.65 |
| Body mass index | 23.5 (21.8; 26.6) | 23.1 (20.6; 28.7) | 0.99 |
| Ethnicity | 0.68 | ||
| Caucasian | 16 | 68 | |
| Hispanic | 3 | 19 | |
| Southern Asia | 3 | 4 | |
| Magreb | 3 | 10 | |
| Black | 0 | 1 | |
| Others | 4 | 20 | |
| Nulliparity | 15 | 81 | 0.14 |
| Smoking | 7 | 16 | 0.14 |
| Gestational age at admission, weeks | 27.3 (25.6; 30.4) | 29.2 (27.1; 31.2) | 0.02 |
| Gestational age at amniocentesis, weeks | 27.3 (25.6; 30.4) | 29.2 (27.1; 31.2) | 0.03 |
| Ultrasound cervical length, mm | 15 (7.5; 20) | 13.5 (10; 18) | 0.58 |
| Maternal C-reactive protein, mg/dL | 2.26 (1.27; 3.9) | 0.52 (0.27; 1.13) | <0.0001 |
| Maternal white blood cells (109) | 14,200 (12,440; 17,755) | 12,050 (10,100; 14,300) | 0.0005 |
| Amniotic fluid glucose, mg/dL | 17 (2; 26) | 27 (19; 39) | 0.0001 |
| Amniotic fluid IL-6, ng/L | 44 (29; 60) | 6 (2; 8) | <0.0001 |
| Intra-amniotic infection | 29 | 3 | <0.0001 |
| Vaginal IL-6, pg/mL | 42 (9; 68) | 1 (1; 40) | 0.0029 |
| Vaginal AFP, pg/mL | 5 (2; 9) | 2 (2; 8) | 0.65 |
| Gestational age at delivery, weeks | 27.9 (26.5; 30.9) | 35.8 (32.3; 38.7) | <0.0001 |
| Latency from admission to delivery, days | 3 (1; 5.5) | 40 (12;65) | <0.0001 |
| Neonatal weight, g | 1,000 (930; 1,625) | 2,518 (1,810; 3,046) | <0.0001 |
| 1 min Apgar<7 | 10/24 | 14/114 | 0.001 |
| 5 min Apgar<7 | 4/24 | 3/114 | 0.004 |
-
Continuous variables were compared using a nonparametric Mann Whitney U test presented as medians (25th percentile; 75 % percentile). Categorical variables were compared using Chi-square or Fisher exact tests and presented as number (%).
We did not observe any complication related to the invasive procedure.
Among the entire population, 94 % (143/151) were treated with antenatal steroids. As expected, we observed an earlier gestational age at admission, at delivery, a shorter latency from amniocentesis to delivery and a higher prevalence of clinical chorioamnionitis at labor in patients with intra-amniotic inflammation. Based on the different phenotypes of infection and inflammation proposed by some authors [1], 26], the latency from admission to delivery in the group with intra-amniotic infection (MIAC with intra-amniotic inflammation) was (median (IQR)) 2 (0;3) days, being 5 (2;44) days in the group with MIAC alone, 5.5 (3;12) days in the sterile intra-amniotic inflammation group, and 46 (15;66) days in the non-infection/non-inflammation group.
Figure 2 shows the full ROC curve. The algorithm including vaginal IL-6 and AFP showed an overall AUC of 80.3 % (95 % Confidence interval CI of 75; 85.6 %) to predict intra-amniotic inflammation. Following the above-mentioned criteria, an optimal cut-off threshold was found at a score ≥24.7. Using this threshold, the diagnostic performance to predict intra-amniotic inflammation showed a sensitivity of 72.4 % (21/29), a specificity of 84.6 % (104/123), a PPV of 52.5 % (21/40), a NPV of 92.9 % (104/112), a LR+ of 4.7 and a LR− of 0.33.
Picture of the instrument used for analysis.
The diagnostic performance using the original cut-off threshold (score≥20) showed an accuracy of 72.4 % (95 % CI 71.6; 73.2 %), a sensitivity of 82.8 % (24/29), a specificity of 69.9 % (86/123), a PPV of 39.3 % (24/61), a NPV of 94.5 % (86/91), a LR+ of 2.8 and a LR− of 0.25.
Comparison of the algorithm including vaginal IL-6 and AFP with other cervicovaginal point-of-care tests for the prediction of intra-amniotic inflammation in patients with preterm labor and intact membranes is summarized in Table 2.
Comparison of the algorithm including vaginal IL-6 and AFP with other rapid non-invasive tests for the prediction of intra-amniotic inflammation in patients with preterm labor and intact membranes.
| Definition of intra-amniotic inflammation | Cut-off | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|---|
| Hologic Inc. algorithm including IL-6 + AFP | AF IL-6≥11.3 ng/mL | 72.4 | 84.6 | 52.5 | 92.9 | 80.3 | 4.7 | 0.33 | |
| fFN13 | AF MMP-8>23 ng/mL | ≥50 ng/mL | 89.7 | 59.8 | 51.5 | 92.4 | 69.4 | 2.23 | 0.17 |
| ≥150 ng/mL | 86.2 | 71.3 | 58.8 | 91.6 | 76.1 | 3.0 | 0.19 | ||
| IL-612 | AF WBC≥50 cells/mm3 | 85.7 | 64.1 | 32.7 | 95.7 | 67.7 | 2.4 | 0.2 | |
| MMP-812 | 85.7 | 72.8 | 39.1 | 96.2 | 75 | 3.2 | 0.2 | ||
| fFN+maternal CRP+ cervical dilatation + gestational age17 | AF MMP-8>23 ng/mL | Score≥4 | 94.9 | 90.9 | 80.4 | 97.8 | 92 | 10.4 | 0.06 |
-
IL-6, Interleukin-6; AFP, alpha-fetoprotein; fFN, fetal fibronectin; MMP-8, Metalloproteinase-8; CRP, C-reactive protein; WBC, white blood cells; AF, amniotic fluid; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
Principal findings
This external validation of a non-invasive rapid point-of-care tool, including vaginal fluid AFP and IL-6 values (Rapid IAI System), showed good diagnostic performance for predicting the absence of intra-amniotic inflammation in patients with PTL and intact membranes.
Discussion
It is widely known that intra-amniotic inflammation, with or without infection, is the most frequent origin of spontaneous preterm delivery at early gestational ages [3]. The indiscriminate antibiotic treatment of patients with PTL and intact membranes has not shown benefit in the short-term [30] and has shown a higher risk of neurodevelopmental handicaps in long-term outcomes [31] such as functional impairment; cerebral palsy or neonatal death. However, these trials lack to demonstrate whether the targeted antibiotic treatment in the right population (the group with intra-amniotic infection and/or inflammation) improves neonatal outcome. In this regard, in the last years, there has been growing evidence suggesting that intra-amniotic inflammation and even infection might be eradicated in some cases of cervical incompetence (59 % of patients) [32], PTL with intact membranes (32 %) [7] and might ameliorate the inflammatory response in PPROM [33] using a broad-spectrum antibiotic treatment including clarithromycin that has also shown an anti-inflammatory effect. These findings, despite the observational design of the studies, open a window of opportunity, in tertiary centers such as ours, to target patients at high-risk of delivery in the following days who might most benefit from antenatal antibiotic treatment.
In addition, although future studies are necessary to determine how to use this information clinically in cases with a positive result, management strategies for women with negative results have already been developed. In the present study, the latency to delivery in the non-infection/inflammation group was of a median (IQR) of 40 days (15; 66) with a gestational age at delivery of 35.9 (32.3; 38.7) weeks. According to the results of recent literature describing worse neurodevelopmental outcomes in infants antenatally exposed to steroids delivering near term [34], perhaps the need for antenatal steroid treatment in this group of patients should be questioned.
Clinical implications
We believe that a non-invasive screening tool able to discriminate high and low-risk groups of intra-amniotic inflammation might encourage more clinicians to perform amniocentesis in the high-risk group. Since all women with MIAC had intra-amniotic inflammation, this test also identify the risk to have MIAC. With a negative non-invasive test, clinicians can identify pregnancies in which inflammation is highly unlikely and in which amniocentesis is not justified.
Although other authors have proposed other non-invasive prediction models, alone (fetal fibronectin, IL-6, MMP-8) or in combination, to predict intra-amniotic inflammation [12], 13], [15], [16], [17] with similar diagnostic accuracy, external validation is lacking. In addition, in some cases, definition of intra-amniotic inflammation was based on amniotic fluid MMP-8 concentrations. Contrary to IL-6, this limits clinical decision making since results of MMP-8 are not available for patient management. This is the first study to validate a non-invasive algorithm constructed by Hologic Inc. in a Spanish cohort of patients with PTL and intact membranes, ratifying the good performance of the tool found in the initial cohort of American patients, mainly to rule out intra-amniotic inflammation.
Research implications
Future studies are required to prospectively evaluate the influence of a non-invasive point-of-care tool, such as the Rapid IAI System, in improving clinical management and the potential benefits of early antibiotic treatment in patients with a high-predicted risk of intra-amniotic infection and/or inflammation.
Strengths and limitations
One of the main strengths of this study was the validation of the two models proposed in an independent cohort. In addition, the diagnosis of intra-amniotic infection/inflammation was based on microbial cultures as well as PCR amplification targeting the 16S ribosomal RNA gene sequence. Finally, other strengths were the prospective design of the study, the use of fresh amniotic fluid samples and the fact that clinicians were blinded to the Rapid IAI System results.
A limitation of this study was that it was not designed to evaluate whether our prediction models improve perinatal outcomes, and this is of great relevance in cases of a positive test. The prevalence of intra-amniotic inflammation was lower than expected, probably because we selected an amniotic fluid IL-6 cutoff that identified the more severe cases (≥11.3 ng/mL). Combs et al. [26] categorized groups of patients with PTL with intact membranes according to amniotic fluid IL6 concentrations being those with levels ≥11.3 ng/mL considered patients with severe inflammation. If we have selected those with amniotic fluid IL-6>2.6 ng/mL (mild inflammation), our prevalence should have been like previously reported. We also consider that external validation was carried out in a single Spanish medical center. It would have been of interest to verify our results in other centers before considering the possibility of generalizing the proposed diagnostic tool. Finally, to our knowledge, the Rapid IAI System provided by Hologic Inc. is not commercially available.
Conclusions
A non-invasive point-of-care tool including vaginal fluid AFP and IL-6 values showed very good diagnostic performance for predicting the absence of intra-amniotic inflammation in patients with PTL and intact membranes.
Funding source: Instituto de Salud Carlos III
Award Identifier / Grant number: PI22/00663
Funding source: Hologic Inc
Acknowledgments
The authors want to acknowledge Hologic Inc. for the financial support and to Jerome Lapointe for his enormous enthusiasm to make this project possible.
-
Research ethics: Patient selection and sampling procedures were performed in accordance with the Declaration of Helsinki and applicable local regulatory requirements after approval from the Institutional Review Boards (HCB/2016/0523; PIC-98-16).
-
Informed consent: Written informed consent was obtained from all subjects.
-
Author contributions: Teresa Cobo, Marian Kacerovsky, Bo Jacobsson and Montse Palacio contributed to the concept. Teresa Cobo, Marian Kacerovsky, Bo Jacobsson, Montse Palacio and Xavier P. Burgos-Artizzu contributed to the study design. Silvia Ferrero, Ana B. Sánchez-García, Jordi Bosch, Amadeu Gené, Clara Murillo, Claudia Rueda, David Boada, Maria Teresa Sánchez-Antón and Teresa Cobo contributed to the inclusion of participants, methodology and data collection. Teresa Cobo, Bo Jacobsson, Marian Kacerovsky, Montse Palacio and Xavier P. Burgos-Artizzu contributed to data analysis and interpretation. Teresa Cobo and Xavier P. Burgos-Artizzu wrote the original draft. All authors contributed to the drafting and critical revision of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Hologic Inc. has funded the study but has not contributed to the analysis or interpretation of findings. The authors state no other conflict of interest.
-
Research funding: Hologic Inc. has funded the study. Linguistic revision was partially supported by the Institute of Health Carlos III (Proyectos de investigación en Salud, PI22/00663).
-
Data availability: Data is available if required. Email contact: tcobo@clinic.cat.
References
1. Romero, R, Miranda, J, Chaiworapongsa, T, Korzeniewski, SJ, Chaemsaithong, P, Gotsch, F, et al.. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74. https://doi.org/10.1111/aji.12296.Search in Google Scholar PubMed PubMed Central
2. Rodríguez-Trujillo, A, Cobo, T, Vives, I, Bosch, J, Kacerovsky, M, Posadas, DE, et al.. Gestational age is more important for short-term neonatal outcome than microbial invasion of the amniotic cavity or intra-amniotic inflammation in preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand 2016;95:926–33. https://doi.org/10.1111/aogs.12905.Search in Google Scholar PubMed
3. Cobo, T, Vives, I, Rodríguez-Trujillo, A, Murillo, C, Ángeles, MA, Bosch, J, et al.. Impact of microbial invasion of amniotic cavity and the type of microorganisms on short-term neonatal outcome in women with preterm labor and intact membranes. Acta Obstet Gynecol Scand 2017;96:570–9. https://doi.org/10.1111/aogs.13095.Search in Google Scholar PubMed
4. Yoon, BH, Romero, R, Park, JS, Kim, CJ, Kim, SH, Choi, JH, et al.. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81. https://doi.org/10.1067/mob.2000.104207.Search in Google Scholar PubMed
5. Rodríguez-Trujillo, A, Ríos, J, Ángeles, MA, Posadas, DE, Murillo, C, Rueda, C, et al.. Influence of perinatal inflammation on the neurodevelopmental outcome of premature infants. J Matern Fetal Neonatal Med 2019;32:1069–77. https://doi.org/10.1080/14767058.2017.1399118.Search in Google Scholar PubMed
6. Cobo, T, Aldecoa, V, Figueras, F, Herranz, A, Ferrero, S, Izquierdo, M, et al.. Development and validation of a multivariable prediction model of spontaneous preterm delivery and microbial invasion of the amniotic cavity in women with preterm labor. Am J Obstet Gynecol 2020;223:421.e1–14. https://doi.org/10.1016/j.ajog.2020.02.049.Search in Google Scholar PubMed
7. Yoon, BH, Romero, R, Park, JY, Oh, KJ, Lee, J, Conde-Agudelo, A, et al.. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol 2019;221:142.e1–22. https://doi.org/10.1016/j.ajog.2019.03.018.Search in Google Scholar PubMed PubMed Central
8. Roberts, D, Brown, J, Medley, N, Dalziel, SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. In: Pregnancy, C, Group, C, editors. Cochrane Database Syst Rev; 2017. https://doi.wiley.com/10.1002/14651858.CD004454.pub3 [Accessed 3 Dec 2023].10.1002/14651858.CD004454.pub3Search in Google Scholar PubMed PubMed Central
9. Huusom, LD, Wolf, HT. Antenatal magnesium sulfate treatment for women at risk of preterm birth is safe and might decrease the risk of cerebral palsy. BMJ Evid Based Med 2018;23:195–6. https://doi.org/10.1136/bmjebm-2018-110897.Search in Google Scholar PubMed
10. Ramirez-Montiel, ML, Casillas-Barrera, M, Morales-Morales, MP, Ortiz, MI, Lopez De Lara-Diaz De Leon, O, Carrasco-Blancas, ER, et al.. Complications associated with amniocentesis in the third trimester of pregnancy. J Clin Gynecol Obstet 2017;6:34–6. https://doi.org/10.14740/jcgo452w.Search in Google Scholar
11. Musilova, I, Bestvina, T, Stranik, J, Stepan, M, Jacobsson, B, Kacerovsky, M. Transabdominal amniocentesis is a feasible and safe procedure in preterm prelabor rupture of membranes. Fetal Diagn Ther 2017;42:257–61. https://doi.org/10.1159/000457951.Search in Google Scholar PubMed
12. Chaemsaithong, P, Romero, R, Docheva, N, Chaiyasit, N, Bhatti, G, Pacora, P, et al.. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2018;31:228–44. https://doi.org/10.1080/14767058.2017.1281904.Search in Google Scholar PubMed PubMed Central
13. Oh, KJ, Romero, R, Park, JY, Kang, J, Hong, JS, Yoon, BH. A high concentration of fetal fibronectin in cervical secretions increases the risk of intra-amniotic infection and inflammation in patients with preterm labor and intact membranes. J Perinat Med 2019;47:288–303. https://doi.org/10.1515/jpm-2018-0351.Search in Google Scholar PubMed PubMed Central
14. Hitti, J, Lapidus, JA, Lu, X, Reddy, AP, Jacob, T, Dasari, S, et al.. Noninvasive diagnosis of intraamniotic infection: proteomic biomarkers in vaginal fluid. Am J Obstet Gynecol 2010;203:32.e1–8. https://doi.org/10.1016/j.ajog.2010.03.037.Search in Google Scholar PubMed PubMed Central
15. Combs, CA, Garite, TJ, Lapidus, JA, Lapointe, JP, Gravett, M, Rael, J, et al.. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol 2015;212:482.e1–e12. https://doi.org/10.1016/j.ajog.2015.02.007.Search in Google Scholar PubMed
16. Cobo, T, Burgos-Artizzu, XP, Collado, MC, Andreu-Fernández, V, Sanchez-Garcia, AB, Filella, X, et al.. Noninvasive prediction models of intra-amniotic infection in women with preterm labor. Am J Obstet Gynecol 2023;228:78.e1–e13. https://doi.org/10.1016/j.ajog.2022.07.027.Search in Google Scholar PubMed
17. Oh, KJ, Romero, R, Kim, HJ, Lee, J, Hong, JS, Yoon, BH. Preterm labor with intact membranes: a simple noninvasive method to identify patients at risk for intra-amniotic infection and/or inflammation. J Matern Fetal Neonatal Med 2022;35:10514–29. https://doi.org/10.1080/14767058.2022.2131388.Search in Google Scholar PubMed PubMed Central
18. Mizejewski, GJ. Alpha-fetoprotein (AFP) and inflammation: is AFP an acute and/or chronic phase reactant? J Hematol Thromboembolic Dis 2015;3:1–9. https://doi.org/10.4172/2329-8790.1000191.Search in Google Scholar
19. Martinon, F, Burns, K, Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–26. https://doi.org/10.1016/s1097-2765(02)00599-3.Search in Google Scholar PubMed
20. Hitti, J, Hillier, SL, Agnew, KJ, Krohn, MA, Reisner, DP, Eschenbach, DA. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol 2001;97:211–9. https://doi.org/10.1097/00006250-200102000-00010.Search in Google Scholar
21. Park, KH, Kim, SN, Oh, KJ, Lee, SY, Jeong, EH, Ryu, A. Noninvasive prediction of intra-amniotic infection and/or inflammation in preterm premature rupture of membranes. Reprod Sci 2012;19:658–65. https://doi.org/10.1177/1933719111432869.Search in Google Scholar PubMed
22. Holst, RM, Mattsby-Baltzer, I, Wennerholm, UB, Hagberg, H, Jacobsson, B. Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand 2005;84:551–7. https://doi.org/10.1080/j.0001-6349.2005.00708.x.Search in Google Scholar
23. E Inman Laderman, TH Grove. Detection of intraamniotic infection [Internet]. Hologic Inc, 35 Crosby drive, Bedford, MA 01730 (US); WO 2011/065976 A1, 2011. p. 78. Available from: https://patents.google.com/patent/WO2011065976A1/en.Search in Google Scholar
24. Palacio, M, Cobo, T, Bosch, J, Filella, X, Navarro-Sastre, A, Ribes, A, et al.. Cervical length and gestational age at admission as predictors of intra-amniotic inflammation in preterm labor with intact membranes. Ultrasound Obstet Gynecol 2009;34:441–7. https://doi.org/10.1002/uog.6437.Search in Google Scholar PubMed
25. Robinson, HP. Sonar measurement of fetal crown-rump length as means of assessing maturity in first trimester of pregnancy. BMJ 1973;4:28–31. https://doi.org/10.1136/bmj.4.5883.28.Search in Google Scholar PubMed PubMed Central
26. Combs, CA, Gravett, M, Garite, TJ, Hickok, DE, Lapidus, J, Porreco, R, et al.. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125.e1–15. https://doi.org/10.1016/j.ajog.2013.11.032.Search in Google Scholar PubMed
27. Romero, R, Yoon, BH, Mazor, M, Gomez, R, Diamond, MP, Kenney, JS, et al.. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and Gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1993;169:805–16. https://doi.org/10.1016/0002-9378(93)90009-8.Search in Google Scholar PubMed
28. Peng, CC, Chang, JH, Lin, HY, Cheng, PJ, Su, BH. Intrauterine inflammation, infection, or both (Triple I): a new concept for chorioamnionitis. Pediatr Neonatol 2018;59:231–7. https://doi.org/10.1016/j.pedneo.2017.09.001.Search in Google Scholar PubMed
29. Buderer, NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 1996;3:895–900. https://doi.org/10.1111/j.1553-2712.1996.tb03538.x.Search in Google Scholar PubMed
30. Kenyon, S, Taylor, D, Tarnow-Mordi, W. Broad-spectrum antibiotics for spontaneous preterm labour: the ORACLE II randomised trial. Lancet 2001;357:989–94. https://doi.org/10.1016/s0140-6736(00)04234-3.Search in Google Scholar PubMed
31. Kenyon, S, Pike, K, Jones, D, Brocklehurst, P, Marlow, N, Salt, A, et al.. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 2008;372:1319–27. https://doi.org/10.1016/s0140-6736(08)61203-9.Search in Google Scholar PubMed
32. Oh, KJ, Romero, R, Park, JY, Lee, J, Conde-Agudelo, A, Hong, JS, et al.. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol 2019;221:140.e1–e18. https://doi.org/10.1016/j.ajog.2019.03.017.Search in Google Scholar PubMed PubMed Central
33. Kacerovsky, M, Romero, R, Stepan, M, Stranik, J, Maly, J, Pliskova, L, et al.. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol 2020;223:114.e1–e20. https://doi.org/10.1016/j.ajog.2020.01.043.Search in Google Scholar PubMed PubMed Central
34. Räikkönen, K, Gissler, M, Kajantie, E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA 2020;323:1924. https://doi.org/10.1001/jama.2020.3937.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Sex differences in lung function of adolescents or young adults born prematurely or of very low birth weight: a systematic review
- Original Articles – Obstetrics
- Shifts in peak month of births and socio-economic factors: a study of divided and reunified Germany 1950–2022
- The predictive role of serial transperineal sonography during the first stage of labor for cesarean section
- Gestational weight gain and obstetric outcomes in women with obesity in an inner-city population
- Placental growth factor as a predictive marker of preeclampsia in twin pregnancy
- Learning curve for the perinatal outcomes of radiofrequency ablation for selective fetal reduction: a single-center, 10-year experience from 2013 to 2023
- External validation of a non-invasive vaginal tool to assess the risk of intra-amniotic inflammation in pregnant women with preterm labor and intact membranes
- Placental fetal vascular malperfusion in maternal diabetes mellitus
- The importance of the cerebro-placental ratio at term for predicting adverse perinatal outcomes in appropriate for gestational age fetuses
- Comparing achievability and reproducibility of pulsed wave Doppler and tissue Doppler myocardial performance index and spatiotemporal image correlation annular plane systolic excursion in the cardiac function assessment of normal pregnancies
- Characteristics of the pregnancy and labour course in women who underwent COVID-19 during pregnancy
- Original Articles – Fetus
- Sonographic visualization and measurement of the fetal optic chiasm and optic tract and association with the cavum septum pellucidum
- The association among fetal head position, fetal head rotation and descent during the progress of labor: a clinical study of an ultrasound-based longitudinal cohort study in nulliparous women
- Fetal hypoplastic left heart syndrome: key factors shaping prognosis
- The value of ultrasound spectra of middle cerebral artery and umbilical artery blood flow in adverse pregnancy outcomes
- Original Articles – Neonates
- A family-centric, comprehensive nurse-led home oxygen programme for neonatal chronic lung disease: home oxygen policy evaluation (HOPE) study
- Effects of a respiratory function indicator light on visual attention and ventilation quality during neonatal resuscitation: a randomised controlled crossover simulation trial
- Short Communication
- Incidence and awareness of dysphoric milk ejection reflex (DMER)
Articles in the same Issue
- Frontmatter
- Review
- Sex differences in lung function of adolescents or young adults born prematurely or of very low birth weight: a systematic review
- Original Articles – Obstetrics
- Shifts in peak month of births and socio-economic factors: a study of divided and reunified Germany 1950–2022
- The predictive role of serial transperineal sonography during the first stage of labor for cesarean section
- Gestational weight gain and obstetric outcomes in women with obesity in an inner-city population
- Placental growth factor as a predictive marker of preeclampsia in twin pregnancy
- Learning curve for the perinatal outcomes of radiofrequency ablation for selective fetal reduction: a single-center, 10-year experience from 2013 to 2023
- External validation of a non-invasive vaginal tool to assess the risk of intra-amniotic inflammation in pregnant women with preterm labor and intact membranes
- Placental fetal vascular malperfusion in maternal diabetes mellitus
- The importance of the cerebro-placental ratio at term for predicting adverse perinatal outcomes in appropriate for gestational age fetuses
- Comparing achievability and reproducibility of pulsed wave Doppler and tissue Doppler myocardial performance index and spatiotemporal image correlation annular plane systolic excursion in the cardiac function assessment of normal pregnancies
- Characteristics of the pregnancy and labour course in women who underwent COVID-19 during pregnancy
- Original Articles – Fetus
- Sonographic visualization and measurement of the fetal optic chiasm and optic tract and association with the cavum septum pellucidum
- The association among fetal head position, fetal head rotation and descent during the progress of labor: a clinical study of an ultrasound-based longitudinal cohort study in nulliparous women
- Fetal hypoplastic left heart syndrome: key factors shaping prognosis
- The value of ultrasound spectra of middle cerebral artery and umbilical artery blood flow in adverse pregnancy outcomes
- Original Articles – Neonates
- A family-centric, comprehensive nurse-led home oxygen programme for neonatal chronic lung disease: home oxygen policy evaluation (HOPE) study
- Effects of a respiratory function indicator light on visual attention and ventilation quality during neonatal resuscitation: a randomised controlled crossover simulation trial
- Short Communication
- Incidence and awareness of dysphoric milk ejection reflex (DMER)

