Abstract
This article presents a new Ruhrstahl–Heraeus top slag modifier with an enhanced modification effect at an enterprise producing O5 automobile sheets. To compare the modification effect of the original and new modifiers, the thermodynamic and dynamic conditions of the top slag modification were studied by thermodynamic calculations and dynamic analysis. The results showed that the utilization of both modifiers reduced the total iron (TFe) content in the top slag. The new modifier controlled the CaO/Al2O3 (C/A) and TFe content of the top slag to approximately 1.22–1.30 and 6.07–7.97%, respectively. The adsorption capacity of top slag for inclusions was stronger than that of the top slag modified with the original modifier. The concentrations of Al2O3-type inclusions, Ti x O·Al2O3 composite inclusions, and oxide inclusions were reduced by 13.4, 21.2, and 3.4 per cm2 with the new modifier compared with the original modifier. The total oxygen content in molten steel was 2.8 ppm lower than that of the original when the new modifier was used. Moreover, the new modifier exhibited a better dynamic condition on the surface of top slag, as determined by R-G-B numerical analysis. Overall, the new modifier improved the dynamic conditions of top slag modification, reduced top slag oxidation, and effectively improved the cleanliness of the molten steel.
1 Introduction
O5 automobile sheet is an ultra-low carbon (ULC) steel product widely used in the automobile industry due to their excellent deep drawing performance [1]. The quantity, size distribution, composition, and morphology of non-metallic inclusions in steel can adversely affect the quality of automobile sheets. Particularly, inclusions in molten steel can cause surface defects in cold-rolled automobile sheet products. Thus, the control of inclusions in ULC steel is extremely strict [2,3,4]. The oxidation of slag strongly affects the control of inclusions in steel, and the content of total iron (TFe) in slag affects the slag oxidation [5,6]. Studies have shown that a linear relationship exists between the content of TFe in the top slag and the content of inclusions in molten steel after Ruhrstahl–Heraeus (RH) smelting O5 automobile sheets. When the content of TFe in slag is low, the number of inclusions in the molten steel is relatively low. However, an excessively low TFe content in top slag can reduce the [O] content in molten steel, resulting in oxygen-blown decarbonization [7,8]. When the TFe content in top slag is high, oxygen transfer from the top slag to the molten steel occurs, resulting in the generation of inclusions in the molten steel. This affects the smooth casting of the tundish, which in turn seriously affects the quality of the ULC steel [9,10].
Therefore, top slag needs to be modified during the production process of O5 automobile sheets. The purpose of this modification is to reduce the content of TFe in the top slag, reduce the transfer of oxygen from the top slag to the molten steel, and reduce the aluminum loss rate in the molten steel. This reduces the secondary pollution in molten steel caused by oxide inclusions generated by the RH refining process [11,12]. At the same time, the slag system is also modified to promote the adsorption of inclusions by the top slag. The stability and rationality of this modification effect significantly affect the cleanliness of the molten steel and the final product quality. At present, the most widely used modifiers are mainly Al + CaO, Al + CaO + Al2O3, and Al + CaO + CaF. Different modifiers have different modification effects. The most commonly used modifier, Al + CaO + Al2O3, adds a certain amount of CaF to promote the flow of the top slag [13,14].
Currently, most research on the modification of top slag focuses on improving the slag retaining efficiency of the converter, improving the Al content in the modifier, which reduces the TFe content in the top slag [15,16]. However, no studies have focused on improving the dynamic conditions of the reaction with regard to the modifiers and top slag. Meanwhile, little research has been attempted to solve the instability factors of modification from dynamics points of view. In this article, a new RH top slag modifier was developed based on the thermodynamic and dynamic conditions of the modifier-slag-inclusion reaction. Furthermore, a new top slag system was discovered to facilitate inclusion adsorption. The modification effects of the original and new modifiers were compared through industrial experiments. Thermodynamic analysis was performed with FactSage software to compare the inclusion adsorption capacity of the top slag with the two modifiers. Furthermore, a novel mechanism for the comparison of the dynamic conditions was proposed by using the Python programming language. By improving the thermodynamic and dynamic conditions of the top slag modification, the secondary oxidation of molten steel by the top slag in the RH smelting process and the content of inclusions in the O5 automobile sheets were both reduced. This composition optimization improves the adsorption capacity of RH top slag for inclusions in molten steel and is of great significance for enhancing the cleanliness of O5 automobile sheets in the RH smelting process.
2 Industrial experiment
Highly oxidized top slag significantly affects the quality of molten steel. Oxidized top slag increases the number of inclusions in steel, especially large Al2O3 inclusions. This seriously affects the surface quality of products, leads to ladle corrosion, and increases the consumption of refractory materials [17,18]. In RH refining process, highly oxidized top slag can increase alloy consumption and reduce the deoxidation effect. Therefore, improving the oxidative stability of top slag is of great significance [19]. In this article, based on the RH smelting of O5 automobile sheets in a factory, a new RH top slag modifier was developed to enhance the stability and effectiveness of top slag modification by improving the thermodynamic and dynamic conditions of the modifier-slag-inclusion reaction. The effect of the new modifier on the quality of O5 automobile sheets was studied in comparison to the original modifier.
Typically, O5 automobile sheets were produced via a basic oxygen furnace (BOF) → RH degassing → continuous casting (CC) process. The modifiers were added on the top of the ladle after the converter. Digital photos and surface scanning images of the two modifiers are shown in Figure 1 and their compositions are reported in Table 1. The melting characteristics of the two modifiers were measured by a CQKJ-II slag melting temperature characteristic tester. A schematic diagram of the melting characteristic measurement and silhouette photographs of typical melting characteristics are shown in Figure 2. The melting temperature of the new modifier was 1,291°C, while that of the original modifier was 1,306°C. Furthermore, “shattering” and “bubbling” phenomena were observed during the melting process of the new modifier. This was mainly caused by CaCO3.
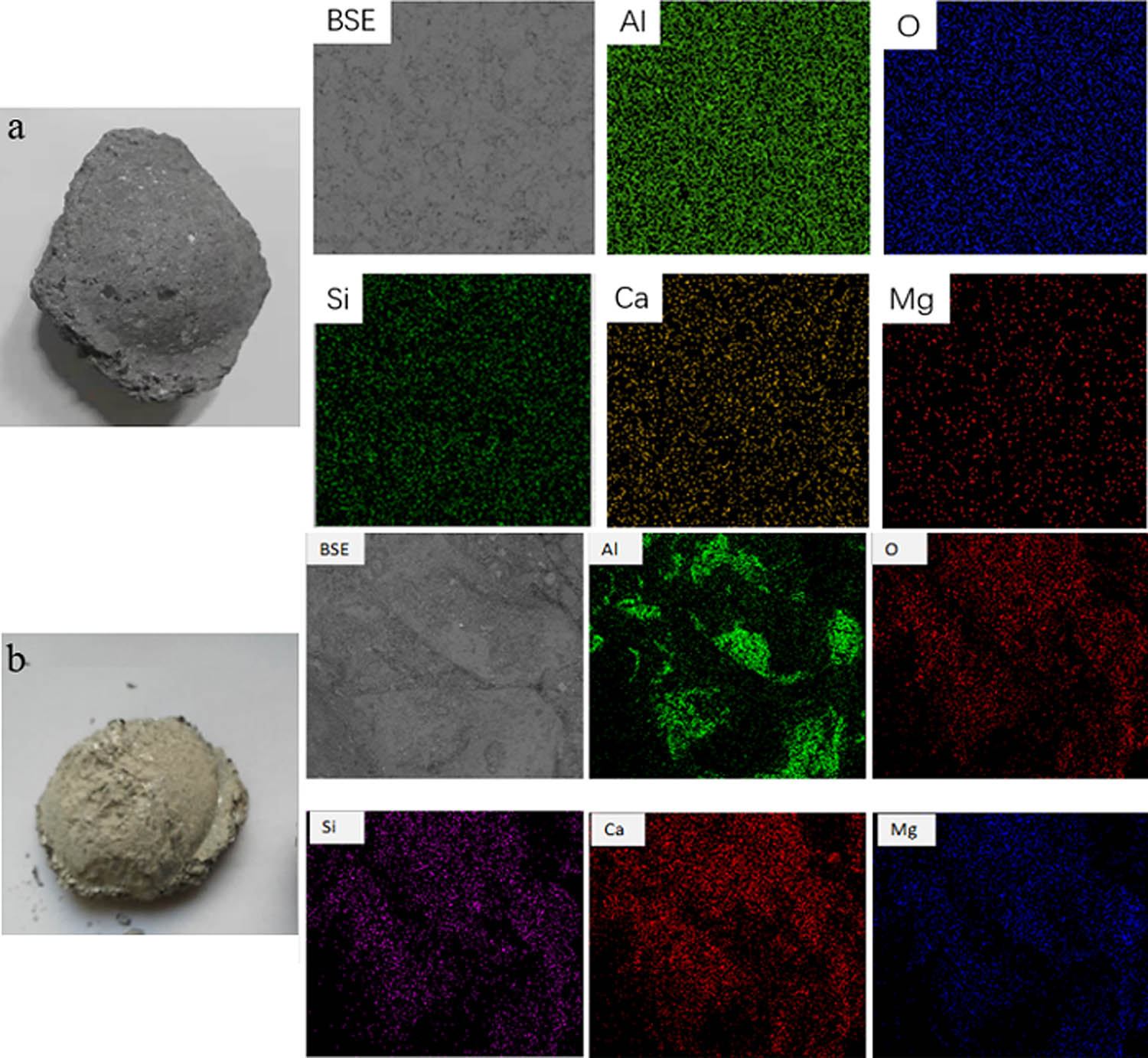
Digital photos and surface scanning of the two modifiers: (a) original modifier and (b) new modifier.
Compositions of the original and new modifiers (%)
| Sample | Al | CaO | CaCO3 | Al2O3 | SiO2 | Promoter | H2O | Size (mm) |
|---|---|---|---|---|---|---|---|---|
| Original | 43–48 | 20–27 | — | 5–15 | ≤1 | 5 | ≤0.5 | 10–50 |
| New | 40–45 | 25–30 | 5 | 5–15 | ≤1 | 5 | ≤0.5 | 10–50 |

(a) Schematic diagram of the melting characteristic equipment type of CQKJ-II, (b–d) silhouette photographs of typical melting characteristics of original modifier, and (e–g) silhouette photographs of typical melting characteristics of new modifier.
The composition specifications of the O5 automobile sheets are shown in Table 2. After BOF drawing, 10 furnace slag samples were taken and numbered 1–10. After steel drawing, 100 kg original modifier and 100 kg new modifier were added to the furnaces 1–5 and 6–10, respectively. The spreading effect of the modifiers on the top slag was observed with a top slag surface monitoring method. The slag samples were obtained after BOF drawing and at the end of RH refining, the slag compositions were analyzed by a Panaco Epsilon 3x EDXRF spectrometer. Racket and pail samples were obtained at the end of RH refining, and the molten steel compositions were analyzed by a direct reading spectrometer. 25 mm × 25 mm × 20 mm steel samples were cut from the pail samples. These steel samples were then polished, and the number, composition, and distribution of their inclusions were analyzed by an ASPEX Explorer automatic scanning electron microscope. The nozzle conditions of the tundish were observed during the casting process under both the modifiers, and the nodules in the inner walls of the nozzles were analyzed by X-ray diffraction (XRD).
Composition requirements of O5 automotive sheets (%)
| C | Si | Mn | S | P | Als | Ti | N |
|---|---|---|---|---|---|---|---|
| ≤0.0020 | ≤0.02 | 0.07–0.15 | ≤0.010 | ≤0.012 | 0.028–0.050 | 0.06–0.072 | ≤0.0030 |
3 Results and discussion
3.1 Results
The compositions of the slag samples at the converter and at the end of the RH process are reported in Table 3. The TFe content in these slag samples significantly decreased after adding the original and new modifiers, while Al2O3 content significantly increased. The CaO/Al2O3 (C/A) ratios of the top slags were controlled in different ranges after adding the different modifiers. The original modifier exhibited a C/A ratio between 1.07 and 1.15, while the new modifier had a C/A ratio between 1.22 and 1.30. The compositions of the molten steel obtained at the end of the RH process are shown in Table 4. This result indicated that the molten steel compositions could meet the O5 automobile sheets’ requirements after modification with either of the modifier. Moreover, the content of Als in groups 6–10 in molten steel is higher than that in groups 1–5 in Table 4. The consumption of aluminum in molten steel probably resulted from the utilization of the new modifier.
Slag sample compositions at the converter and at the end of the RH process (%)
| Sam. | Converter slag sample composition (%) | Top slag composition at the end of the RH process (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFe | CaO | MgO | SiO2 | Al2O3 | TFe | CaO | MgO | SiO2 | Al2O3 | C/A | |
| 1 | 17.9 | 48.04 | 9.09 | 12.92 | 8.25 | 7.22 | 37.97 | 7.17 | 4.66 | 33.38 | 1.14 |
| 2 | 17.88 | 47.48 | 8.98 | 13.66 | 9.23 | 5.89 | 40.13 | 7.12 | 5.02 | 34.91 | 1.15 |
| 3 | 18.04 | 45.95 | 9.61 | 13.83 | 8.71 | 5.94 | 39.3 | 7.18 | 4.99 | 35.84 | 1.10 |
| 4 | 19.32 | 44.96 | 9.34 | 13.49 | 6.52 | 6.87 | 38.71 | 7.03 | 4.94 | 35.18 | 1.10 |
| 5 | 18.14 | 47.43 | 8.20 | 13.7 | 6.94 | 5.95 | 38.19 | 7.25 | 4.83 | 35.69 | 1.07 |
| 6 | 19.76 | 44.93 | 9.47 | 13.94 | 6.67 | 7.92 | 38 | 7.33 | 5.17 | 31.04 | 1.22 |
| 7 | 19.51 | 44.36 | 8.61 | 13.09 | 7.57 | 6.07 | 40.09 | 7.03 | 5.03 | 32.21 | 1.24 |
| 8 | 17.24 | 47.48 | 8.25 | 13.89 | 6.95 | 7.97 | 38.58 | 7.35 | 5.19 | 31.41 | 1.23 |
| 9 | 18.37 | 45.8 | 8.74 | 13.58 | 7.41 | 7.08 | 41.14 | 7.15 | 4.86 | 31.76 | 1.30 |
| 10 | 18.34 | 45.45 | 9.00 | 14.47 | 8.12 | 7.13 | 38.35 | 7.53 | 4.99 | 31.07 | 1.23 |
Molten steel compositions at the end of the RH process (%)
| Sample | C | Mn | S | P | Si | Als | N | Ti |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.0010 | 0.105 | 0.005 | 0.009 | 0.004 | 0.0411 | 0.0014 | 0.069 |
| 2 | 0.0013 | 0.106 | 0.004 | 0.011 | 0.006 | 0.0428 | 0.0017 | 0.068 |
| 3 | 0.0013 | 0.106 | 0.005 | 0.010 | 0.004 | 0.0444 | 0.0014 | 0.068 |
| 4 | 0.0016 | 0.122 | 0.005 | 0.009 | 0.004 | 0.043 | 0.0019 | 0.069 |
| 5 | 0.0013 | 0.119 | 0.004 | 0.009 | 0.005 | 0.042 | 0.0021 | 0.068 |
| 6 | 0.0016 | 0.110 | 0.006 | 0.008 | 0.004 | 0.0428 | 0.0017 | 0.068 |
| 7 | 0.0009 | 0.113 | 0.006 | 0.007 | 0.003 | 0.0451 | 0.0014 | 0.068 |
| 8 | 0.0011 | 0.124 | 0.004 | 0.009 | 0.004 | 0.0489 | 0.0013 | 0.070 |
| 9 | 0.0015 | 0.099 | 0.006 | 0.010 | 0.003 | 0.0459 | 0.0015 | 0.068 |
| 10 | 0.0019 | 0.109 | 0.006 | 0.010 | 0.003 | 0.0468 | 0.0019 | 0.068 |
Ten pail samples were taken at the end of the RH process, and the inclusion content of these pail samples was analyzed by transmission electron microscopy, as shown in Figure 3. After modification with either of the modifier, the inclusions in the molten steel samples were mainly spherical Al2O3 inclusions containing a small amount of CaO and MgO, Ti x O·Al2O3 composite inclusions, and oxide inclusions. The number of inclusions is shown in Table 5. The average content of Al2O3-type inclusions at the end of the RH process with the original modifier was 146.2 per cm2. In contrast, the average Al2O3-type inclusion content with the new modifier was 132.8 per cm2, demonstrating a reduction of 13.4 per cm2. Moreover, the concentration of Ti x O·Al2O3 composite inclusions and oxide inclusions in the molten steel, respectively, decreased by 21.2 and 3.4 per cm2 due to the use of the new modifier compared with the original modifier. The size distributions of the inclusions in the molten steel are shown in Figure 4. As demonstrated by the inclusion size distributions, the molten steel modified with the new modifier exhibited a narrow inclusion distribution compared with the molten steel modified with the original modifier, especially for sizes larger than 10 µm. The new modifier had a more favorable effect on inclusion removal from the molten steel.

The morphology and compositions of typical inclusions: (a–c) with original modifier and (d–f) with new modifier.
Overall inclusion quantity in molten steel samples modified by the original modifier (samples 1–5) and the new modifier (samples 6–10)
| Sample | Detection area (mm2) | Overall inclusion density per cm2 | |||
|---|---|---|---|---|---|
| Al2O3-type | Ti x O·Al2O3 | Oxide | Total | ||
| 1 | 1,392 | 164 | 92 | 33 | 289 |
| 2 | 1,453 | 136 | 99 | 31 | 266 |
| 3 | 1,401 | 126 | 109 | 20 | 255 |
| 4 | 1,438 | 149 | 121 | 29 | 299 |
| 5 | 1,476 | 156 | 115 | 26 | 297 |
| 6 | 1,399 | 143 | 98 | 25 | 266 |
| 7 | 1,427 | 123 | 101 | 21 | 245 |
| 8 | 1,422 | 122 | 79 | 18 | 219 |
| 9 | 1,458 | 135 | 84 | 28 | 247 |
| 10 | 1,439 | 141 | 68 | 30 | 239 |

The size distribution of inclusions.
The total oxygen content of the molten steel was analyzed at the end of the RH process, as shown in Figure 5. With the new modifier, the total oxygen content in the molten steel was reduced by an average of 2.8 ppm compared with that in the molten steel with the original modifier. From what has been discussed above, the development of the new modifier was beneficial for promoting the top slag inclusion adsorption capacity and improving the cleanliness of the molten steel.

Total oxygen content of molten steel at the end of RH process.
The nozzle casting conditions of the tundishes are shown in Figure 6. Nodulation and side hole bonding were more serious when the original modifier was used compared with the new modifier. The nodules of both nozzles were mainly composed of Al2O3 and Al2O3·MgO, as determined by the XRD patterns reported in Figure 6c and f. Moreover, the presence of Ti3O5 was observed in the XRD pattern obtained after using the original modifier.

Comparison of nozzle conditions of two modifiers and the XRD patterns of the nodules. (a) Upper nozzle with the original modifier, (b) lower nozzle side hole with the original modifier, (c) XRD patterns of nodular at the nozzle with the original modifier, (d) upper nozzle with the new modifier, (e) lower nozzle side hole with the new modifier, and (f) XRD patterns of nodular at the nozzle with the new modifier.
The modification effect of the top slag in the RH smelting process depended on the composition of the modifier as well as the reaction area between the modifier and the top slag in the O5 automobile sheets, that is, the dynamic conditions of the reaction between the modifier and top slag. In this article, the spreading effect of the modifier on the top slag interface was used to directly represent the reaction area between the modifier and the top slag. When the spreading effect of the modifier was poor or clumping was observed, the modification effect of the top slag was poor and the final composition of the top slag did not meet the modification requirements. This negatively affected the cleanliness of the molten steel. As shown in Figure 7, the new modifier with a small amount of CaCO3 exhibited a strong spreading effect on the surface of the top slag with no clumping or other undesirable phenomena. In contrast, caking occurred on the surface of the top slag with the original modifier, as shown by the dashed blue circles in Figure 7a.

Digital photos of top slag surface with two modifiers: (a) original modifier and (b) new modifier.
In order to describe the spreading effect of the modifiers, an automatic recognition system for the top slag was implemented. This automatic recognition system innovatively used the Python programming language’s image processing techniques to capture R, G, and B images (where R stands for the red channel, G stands for the green channel, and B stands for the blue channel). An R-G-B value of 0 represents black and an R-G-B value of 255 represents white. A higher corresponding R-G-B value means the corresponding component is heavier. A lower R-G-B value means that the liquid level of the top slag is darker and the spreading effect of modifier is better. Therefore, this automatic recognition system was useful for quantifying the spreading effect of the new and original modifiers.
The R-G-B values were obtained by processing photographs taken after the modifiers were added to the top slag, as shown in Figure 8. The R-G-B values of the top slag were generally lower after adding the new modifier to the ladle compared with adding the original modifier. Figure 8 shows that the R-G-B values of top slag samples 6–10 were lower than those of samples 1–5. This indicated that the dynamic conditions of the reaction between the new modifier and the top slag were improved compared with that of the original modifier due to the addition of CaCO3. This result was consistent with the utilization effect shown in Figure 7, the new modifier had a better spreading effect than the original modifier.

R-G-B numerical analysis of two modifiers.
4 Discussion
During the RH smelting process, the molten steel circulates in the vacuum tank and regularly contacts with the top slag. Therefore, the elemental transfer rate of the steel/slag interface is the limiting factor for the secondary oxidation of molten steel by the top slag. Ti is used to fix C and N in O5 automobile sheets molten steel. The Ti and Al present in the molten steel can react with FeO in the top slag to form corresponding oxides. These chemical reactions are shown in reactions (1) and (2). The main limiting factor of the reaction is the transfer rate of FeO from the top slag to molten steel. However, due to the presence of a large amount of FeO in the top slag, FeO can decompose at the steel/slag interface during the RH smelting process. This results in a large amount of dissolved oxygen at the steel/slag interface. Due to the continuous contact of molten steel and top slag, the dissolved oxygen at the steel/slag interface reacts with Al and Ti in the molten steel, generating a large number of oxides that become inclusions in the molten steel. The main reactions are reported as reactions (3)–(6). Therefore, reducing the FeO content in the top slag can reduce the dissolved oxygen content at the steel/slag interface. This reduces the transfer of oxide inclusions to the molten steel, which leads to a higher number of inclusions in the O5 automobile sheets [20]. The Al in the modifier reacts with FeO in the top slag, which reduces the FeO content. This chemical reaction is shown in reaction (7). A schematic diagram of this modifier-slag-inclusion mechanism is shown in Figure 9.
The top slag was modified to reduce the TFe content, to avoid top slag pollution in molten steel, and to adjust the top slag composition to enhance its ability to remove inclusions in the molten steel. Inclusions floated up through the molten steel to the steel/slag interface, which were then absorbed by the top slag, entered the slag phase, and finally dissolved in the slag. The top slag composition was mainly FeO, CaO, SiO2, MgO, and Al2O3. The MgO content and SiO2 content in the top slag were, respectively, controlled at 7.03–7.53 and 4.66–5.19% in this work. The changes in MgO and SiO2 content were much lower than the changes in the content of the other three oxides in the O5 automobile sheets. Therefore, in this work, the slag system of the O5 automobile sheets was simplified to a FeO–CaO–Al2O3–SiO2–MgO five-element slag system in which the MgO content was 7% and the SiO2 content was 5%. The influence of top slag composition on the cleanliness of the O5 automobile sheets after slag modification was studied by using FactSage thermodynamic software.

Schematic diagram of reaction mechanism of modifier-slag-inclusion.
Top slag modification takes the oxidation of top slag to molten steel into account as well as the ability of the top slag to dissolve and adsorb inclusions in the O5 automobile sheets. Because the O5 automobile sheets were Al-killed steel, the inclusions in the molten steel were mainly Al2O3 [20]. The dissolution rate of these Al2O3 inclusions in the slag phase was proportional to the dissolution driving force of Al2O3 in the slag phase (ΔC) but inversely proportional to the slag viscosity (η) [21]. Therefore, the parameter ΔC/η was used to measure the ability of slag to dissolve Al2O3 inclusions, where ΔC was the difference in mass concentration between the saturation concentration of Al2O3 and the concentration of Al2O3 in the slag system [22]. The capacity of the RH top slag to dissolve Al2O3 inclusions is expressed in equation (8):
where
The position of the liquid phase in the FeO–CaO–Al2O3–5% SiO2–7% MgO five-element slag system at 1,600°C was also calculated by FactSage thermodynamics software. The ΔC and η values of each component in the five-element slag system were calculated by using the viscosity module, and the corresponding ΔC/η values were then obtained. The contour lines of ΔC/η in the phase diagram shown in Figure 10 were obtained. The value of ΔC/η increased with the increase in the C/A. In other words, when the slag system components moved toward the CaO saturation point, the inclusion dissolution ability of the top slag was enhanced. Therefore, increasing the content of CaO in top slag could improve the Al2O3 inclusion dissolving performance. 5% CaCO3 was added to the new modifier, this enhanced the CaO content in the top slag, which subsequently improved the inclusion adsorption capacity of the modified top slag.

The ΔC/η isoline diagram of FeO–CaO–Al2O3–5% SiO2–7% MgO five-element slag system at 1,600°C.
As shown in Figure 10, the C/A value of the intersection between the liquidus and the CaO–Al2O3 slag system was about 1.3 at 1,600°C. When the C/A value of the top slag was higher than 1.3, the top slag components were located in the solid phase region. Thus, the dynamic conditions were not ideal for inclusion removal. The melting point of the slag system was not significantly reduced even for slags with higher FeO content. Studies have shown that under a certain FeO content, increasing the value of C/A can promote the dissolution of Al2O3; however, this promoting effect becomes less obvious with higher C/A ratios [11]. Figure 10 shows the composition positions of slag samples 1–10 after the RH process. As can be seen, the inclusion adsorption capacity of the top slag with the new modifier was enhanced compared with the top slag with the original modifier. The utilization of the new modifier was beneficial for promoting the adsorption of inclusions by the top slag in O5 automobile sheets, consistent with the results shown in Figure 4 and Table 5.
The utilization of the new modifier improved the performance of the top slag for adsorbing inclusions from the molten steel. Thus, the inclusion content of the molten steel was reduced compared to the process using the original modifier. Furthermore, the inclusion content of Ti x O·Al2O3 decreased most significantly. After Ti alloy was added to the molten steel, Ti oxides were mainly formed. Al reacted with these Ti oxides as follows [23,24]:
As the temperature of the RH smelting process decreased, reactions (9)–(12) moved in the direction of Al2O3 inclusion formation, resulting in a reduction in the Ti x O·Al2O3 composite inclusion content. The equilibrium phase diagram of the Al–Ti–O system is shown in Figure 11. In the deoxidizing alloying and pure cycle stage, the inclusions in the molten steel changed with higher total oxygen content, the formation of Al2O3·TiO2 and Ti3O5 were easier as shown in Figure 11, which led to higher Ti x O·Al2O3 composite inclusion and Ti3O5 inclusion. However, both the Ti x O·Al2O3 and Ti3O5 inclusions reduced as the total oxygen content decreased in the molten steel. This result was consistent with the results displayed in Table 5. Ti x O·Al2O3 inclusions caused serious nozzle clogging [25]. As demonstrated by Figure 6 and Table 5, Ti x O·Al2O3 and Ti3O5 inclusions led to more serious nozzle clogging with the original modifier, but the disappearance of Ti3O5 inclusions was probably dependent on the lower total oxygen content in the molten steel.

Equilibrium phase diagram of the Al–Ti–O system.
In addition, during the reaction between the modifier and the top slag, Al in the modifier reacted with MgO in the slag. Thus, a large amount of dissolved Mg formed at the steel/slag interface. Al2O3 inclusions in the molten steel reacted with dissolved Mg, and the inclusions were transformed from Al2O3 to Al2O3·MgO composite inclusions. These Al2O3·MgO composite inclusions accumulated in the nozzle and led to nozzle blockage during the casting process. This was consistent with the XRD patterns shown in Figure 6c and f, in which Al2O3·MgO composite inclusion phases were observed in the nodules with the two modifiers. The chemical reactions are shown as Reactions (14) and (15).
In addition to the thermodynamic conditions, the dynamic conditions of the reaction between the modifier and top slag significantly influenced the cleanliness of the molten steel. The reaction between modifier and top slag is the main factor restricting the modification of top slag, which not only affects the modification rate, but also affects the modification effect.
The new modifier used in this work contains 5% CaCO3. The decomposition of CaCO3 during stirring leads to the generation of CO2 gas, which induces crushing of the modifier, so the initial radius of reaction between the modifier and top slag was reduced. This significantly increases the dynamic conditions of the modification. Figure 12 shows a schematic diagram of how modifier crushing can improve the dynamic modification conditions. The CO2 gas generated by CaCO3 decomposition results in modifier “crushing” or “cracking.” This promotes the reaction between the modifier and the top slag, enhancing the dynamic conditions. Moreover, the generated CO2 gas on the surface of the top slag acts as a protector gas to prevent the secondary oxidation of the molten steel by preventing it from contacting the air.
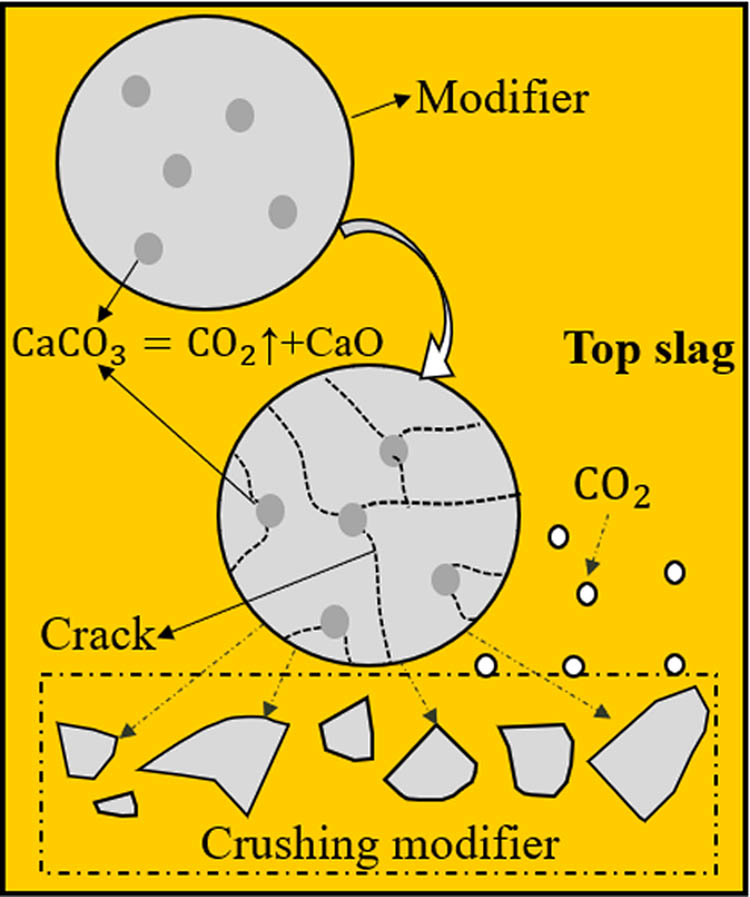
Schematic diagram of the crushing of modifier.
5 Conclusion
The thermodynamic and dynamic conditions of top slag modification were studied in O5 automobile sheets using the original modifier and a new modifier. Through thermodynamic and dynamic analysis, a comprehensive comparison was performed and the following conclusions were obtained:
The utilization of the new modifier controlled the C/A value of the top slag to 1.22–1.30 and the TFe content to 6.07–7.97%. The inclusion adsorption capacity of top slag with the new modifier was stronger than that of the top slag with the original modifier by thermodynamic calculations.
The Al2O3-type inclusion content, Ti x O·Al2O3 composite inclusion content, and oxide inclusion content in the molten steel were reduced by 13.4, 21.2, and 3.4 per cm2 by using the new modifier instead of the original modifier. Using the new modifier reduced the total oxygen by 2.8 ppm compared with using the original modifier. This improved the cleanliness of the molten steel and reduced the nozzle nodulation.
According to the dynamic analysis and R-G-B values analyzed by an automatic recognition system, it was concluded that the dynamic conditions of the reaction between the new modifier and the top slag were improved compared with that of the original modifier due to the addition of CaCO3.
Acknowledgments
This work was supported by State Key Laboratory of Advanced Processing and Recycling of Non-Ferrous Metals in Lanzhou University of Technology.
-
Funding information: Authors state no funding involved.
-
Author contributions: Shujun Li: formal analysis, methodology, resources, data Curation, and writing – original draft; Xueyan Du: supervision, formal analysis, writing – review & editing, project administration, and funding acquisition.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All authors can confirm that all data used in this article can be published in High Temperature Materials and Process.
References
[1] Minami, H., Y. Funakawa, T. Tsuji, M. Kobayashi, and H. Miura. Effects of excess C on new grain formation and static recrystallization behavior at shear bands in cold-rolled ultra-low carbon steel sheet. ISIJ International, Vol. 105, No. 12, 2019, pp. 1153–1162.10.2355/tetsutohagane.TETSU-2019-019Search in Google Scholar
[2] Dorrer, P., S. K. Michelic, C. Bernhard, A. Penz, and R. Rössler. Study on the influence of FeTi-Addition on the inclusion population in Ti-stabilized ULC steel and its consequences for SEN-clogging. Steel Research International, Vol. 90, 2019, id. 1800635.10.1002/srin.201800635Search in Google Scholar
[3] Wang, M., Y. P. Bao, H. Cui, H. J. Wu, and W. S. Wu. The composition and morphology evolution of oxide inclusions in Ti-bearing ultra-low-carbon steel melt refined in the RH process. ISIJ International, Vol. 50, No. 11, 2010, pp. 1606–1611.10.2355/isijinternational.50.1606Search in Google Scholar
[4] Wang, M. and Y. P. Bao. Source and negative effects of macro-inclusions in Titanium stabilized ultra-low carbon interstitial free (Ti-IF) steel. Metals And Materials International, Vol. 18, No. 1, 2012, pp. 29–35.10.1007/s12540-012-0004-3Search in Google Scholar
[5] Zinngrebe, E., H. C. Van, H. Visser, A. Westendorp, and I. H. Jung. Inclusion population evolution in Ti-alloyed Al-killed steel during secondary steelmaking process. ISIJ International, Vol. 52, No. 1, 2012, pp. 52–61.10.2355/isijinternational.52.52Search in Google Scholar
[6] Li, H. B., P. Yuan, B. Chen, and F. G. Liu. The influence of slag chemistry on the formation of sliver defects. Ironmaking & Steelmaking, Vol. 46, No. 9, 2019, pp. 463–468.10.1080/03019233.2017.1405148Search in Google Scholar
[7] Hu, W., B. Xu, J. Q. Huang, W. R. Zhu, J. Li, R. C. Li, et al. Optimization and production practice of top slag modifier for IF steel DC06 with 260t BOF-RH flowsheet. Special Steel, Vol. 42, No. 3, 2021, pp. 27–30.Search in Google Scholar
[8] Peng, Z. G., J. H. Qi, and C. W. Yang. Influence of slag denaturalization on inclusions in LF steel. Chinese Journal of Engineering, Vol. 40, No. 1, 2018, pp. 174–179.Search in Google Scholar
[9] Guo, J., S. S. Cheng, and H. J. Guo. Thermodynamics and industrial trial on increasing the carbon content at the BOF endpoint to produce ultra-low carbon IF steel by BOF-RH-CSP process. High Temperature Materials and Processes, Vol. 38, 2019, pp. 822–826.10.1515/htmp-2019-0054Search in Google Scholar
[10] Cheng, R. J., R. C. Li, and D. Cheng. Revolution and control of Fe-Al-(Mg, Ti)-O oxide inclusions in IF steel during 260t BOF-RH-CC process. Metals, Vol. 10, No. 4, 2020, id. 528.10.3390/met10040528Search in Google Scholar
[11] Zhou, Y. L., Q. He, Z. Y. Deng, J. F. Zhou, and M. Y. Zhu. Effect of ladle top modification treatment on inclusions in ultra-low carbon stee in 210t BOF-RH-Slab CC flowsheet. Special Steel, Vol. 36, No. 3, 2015, pp. 38–42.Search in Google Scholar
[12] Cui, H., B. Chen, M. Wang, F. G. Liu, L. Qing, and M. W. Jing. Cleanliness control of IF steel during the RH refining process. Journal of University Science Technology Beijing, Vol. 33, No. 1, 2011, pp. 147–150.Search in Google Scholar
[13] Deng, B. R., B. Zhang, J. F. Zhou, L. Liu, and C. X. Li. Effect of 210t RH refining parameters on cleanliness of steelmaking-rolling IF steel and process optimization. Special Steel, Vol. 39, No. 3, 2018, pp. 31–34.Search in Google Scholar
[14] Wang, R. Study on Metallurgical technology and cold sheet defects control of ultralow carbon steel. Doctoral dissertation, University of science and technology Beijing, Beijing, 2017.Search in Google Scholar
[15] Gao, F. B., X. H. Wang, J. S. Liu, Z. J. Wu, and T. Zhang. Experiment on high efficient conditioning treatment of ladle slag in production of ULC automobile sheet steels. Steelmaking, Vol. 37, No. 4, 2021, pp. 5–9.Search in Google Scholar
[16] Qin, Y. M., X. H. Wang, L. P. Li, and F. X. Huang. Effect of oxidizing slag on cleanliness of IF steel during ladle holding process. Steel Research International, Vol. 86, No. 9, 2015, pp. 1037–1045.10.1002/srin.201400349Search in Google Scholar
[17] Yu, H. X., D. X. Yang, M. M. Li, and N. Zhang. Effect of CaO-SiO2-Al2O3-MgO top slag on solute elements and non-metallic inclusions in Fe-xMn (x = 10, 20 mass pct) steel. Metallurgical Research & Technology, Vol. 118, No. 3, 2021, id. 302.10.1051/metal/2021025Search in Google Scholar
[18] Zhu, L. G., Y. N. Jia, Z. G. Liu, C. J. Zhang, X. H. Wang, and P. C. Xiao. Mass-transfer model for steel, slag, and inclusions during ladle-furnace refining. High Temperature Materials and Processes, Vol. 37, No. 7, 2018, pp. 665–674.10.1515/htmp-2017-0011Search in Google Scholar
[19] Jiang, R. B. Study on reducing oxygen transfer from IF ladle slag to molten steel. Steelmaking, Vol. 35, No. 4, 2019, pp. 28–31.Search in Google Scholar
[20] Deng, Z. Y. and M. Y. Zhu. Deoxidation mechanism of Al-Killed steel during industrial refining process. ISIJ International, Vol. 54, No. 7, 2014, pp. 1498–1506.10.2355/isijinternational.54.1498Search in Google Scholar
[21] Guo, Y. T. Study on refining process optimization and sulfide morphology for resulfurized free-cutting structural steel. Doctoral dissertation, College of Materials Science and Engineering of Chongqing University, Chongqing, 2017.Search in Google Scholar
[22] Rocabois, P., J. Lehmann, C. Gatellier, and J. P. Teres. Non-metallic inclusion entrapment by slag: laboratory investigation. Ironmak & Steelmak, Vol. 30, No. 2, 2003, pp. 95–100.10.1179/030192303225001775Search in Google Scholar
[23] Yuan, B. H., J. H. Liu, J. H. Zeng, M. Zhang, J. H. Huang, and X. D. Yang. Evolution of inclusions and cleanliness in Ti-bearing IF steel produced via the BOF–LF–RH–CC process. Metals, Vol. 12, No. 3, 2022, id. 434.10.3390/met12030434Search in Google Scholar
[24] Qi, X., X. Wang, H. Di, X. Shen, Z. Liu, P. Huan, et al. Effect of Ti content on the inclusions, microstructure and fracture mechanism of X100 pipeline steel laser-MAG hybrid welds. Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, Vol. 831, 2022, id. 142207.10.1016/j.msea.2021.142207Search in Google Scholar
[25] Basu, S., S. K. Choudhary, and N. U. Girase. Nozzle clogging behaviour of Ti-bearing Al-killed ultra-low carbon steel. ISIJ International, Vol. 44, No. 10, 2004, pp. 1653–1660.10.2355/isijinternational.44.1653Search in Google Scholar
© 2022 Shujun Li and Xueyan Du, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Numerical and experimental research on solidification of T2 copper alloy during the twin-roll casting
- Discrete probability model-based method for recognition of multicomponent combustible gas explosion hazard sources
- Dephosphorization kinetics of high-P-containing reduced iron produced from oolitic hematite ore
- In-phase thermomechanical fatigue studies on P92 steel with different hold time
- Effect of the weld parameter strategy on mechanical properties of double-sided laser-welded 2195 Al–Li alloy joints with filler wire
- The precipitation behavior of second phase in high titanium microalloyed steels and its effect on microstructure and properties of steel
- Development of a huge hybrid 3D-printer based on fused deposition modeling (FDM) incorporated with computer numerical control (CNC) machining for industrial applications
- Effect of different welding procedures on microstructure and mechanical property of TA15 titanium alloy joint
- Single-source-precursor synthesis and characterization of SiAlC(O) ceramics from a hyperbranched polyaluminocarbosilane
- Carbothermal reduction of red mud for iron extraction and sodium removal
- Reduction swelling mechanism of hematite fluxed briquettes
- Effect of in situ observation of cooling rates on acicular ferrite nucleation
- Corrosion behavior of WC–Co coating by plasma transferred arc on EH40 steel in low-temperature
- Study on the thermodynamic stability and evolution of inclusions in Al–Ti deoxidized steel
- Application on oxidation behavior of metallic copper in fire investigation
- Microstructural study of concrete performance after exposure to elevated temperatures via considering C–S–H nanostructure changes
- Prediction model of interfacial heat transfer coefficient changing with time and ingot diameter
- Design, fabrication, and testing of CVI-SiC/SiC turbine blisk under different load spectrums at elevated temperature
- Promoting of metallurgical bonding by ultrasonic insert process in steel–aluminum bimetallic castings
- Pre-reduction of carbon-containing pellets of high chromium vanadium–titanium magnetite at different temperatures
- Optimization of alkali metals discharge performance of blast furnace slag and its extreme value model
- Smelting high purity 55SiCr automobile suspension spring steel with different refractories
- Investigation into the thermal stability of a novel hot-work die steel 5CrNiMoVNb
- Residual stress relaxation considering microstructure evolution in heat treatment of metallic thin-walled part
- Experiments of Ti6Al4V manufactured by low-speed wire cut electrical discharge machining and electrical parameters optimization
- Effect of chloride ion concentration on stress corrosion cracking and electrochemical corrosion of high manganese steel
- Prediction of oxygen-blowing volume in BOF steelmaking process based on BP neural network and incremental learning
- Effect of annealing temperature on the structure and properties of FeCoCrNiMo high-entropy alloy
- Study on physical properties of Al2O3-based slags used for the self-propagating high-temperature synthesis (SHS) – metallurgy method
- Low-temperature corrosion behavior of laser cladding metal-based alloy coatings on EH40 high-strength steel for icebreaker
- Study on thermodynamics and dynamics of top slag modification in O5 automobile sheets
- Structure optimization of continuous casting tundish with channel-type induction heating using mathematical modeling
- Microstructure and mechanical properties of NbC–Ni cermets prepared by microwave sintering
- Spider-based FOPID controller design for temperature control in aluminium extrusion process
- Prediction model of BOF end-point P and O contents based on PCA–GA–BP neural network
- Study on hydrogen-induced stress corrosion of 7N01-T4 aluminum alloy for railway vehicles
- Study on the effect of micro-shrinkage porosity on the ultra-low temperature toughness of ferritic ductile iron
- Characterization of surface decarburization and oxidation behavior of Cr–Mo cold heading steel
- Effect of post-weld heat treatment on the microstructure and mechanical properties of laser-welded joints of SLM-316 L/rolled-316 L
- An investigation on as-cast microstructure and homogenization of nickel base superalloy René 65
- Effect of multiple laser re-melting on microstructure and properties of Fe-based coating
- Experimental study on the preparation of ferrophosphorus alloy using dephosphorization furnace slag by carbothermic reduction
- Research on aging behavior and safe storage life prediction of modified double base propellant
- Evaluation of the calorific value of exothermic sleeve material by the adiabatic calorimeter
- Thermodynamic calculation of phase equilibria in the Al–Fe–Zn–O system
- Effect of rare earth Y on microstructure and texture of oriented silicon steel during hot rolling and cold rolling processes
- Effect of ambient temperature on the jet characteristics of a swirl oxygen lance with mixed injection of CO2 + O2
- Research on the optimisation of the temperature field distribution of a multi microwave source agent system based on group consistency
- The dynamic softening identification and constitutive equation establishment of Ti–6.5Al–2Sn–4Zr–4Mo–1W–0.2Si alloy with initial lamellar microstructure
- Experimental investigation on microstructural characterization and mechanical properties of plasma arc welded Inconel 617 plates
- Numerical simulation and experimental research on cracking mechanism of twin-roll strip casting
- A novel method to control stress distribution and machining-induced deformation for thin-walled metallic parts
- Review Article
- A study on deep reinforcement learning-based crane scheduling model for uncertainty tasks
- Topical Issue on Science and Technology of Solar Energy
- Synthesis of alkaline-earth Zintl phosphides MZn2P2 (M = Ca, Sr, Ba) from Sn solutions
- Dynamics at crystal/melt interface during solidification of multicrystalline silicon
- Boron removal from silicon melt by gas blowing technique
- Removal of SiC and Si3N4 inclusions in solar cell Si scraps through slag refining
- Electrochemical production of silicon
- Electrical properties of zinc nitride and zinc tin nitride semiconductor thin films toward photovoltaic applications
- Special Issue on The 4th International Conference on Graphene and Novel Nanomaterials (GNN 2022)
- Effect of microstructure on tribocorrosion of FH36 low-temperature steels
Articles in the same Issue
- Research Articles
- Numerical and experimental research on solidification of T2 copper alloy during the twin-roll casting
- Discrete probability model-based method for recognition of multicomponent combustible gas explosion hazard sources
- Dephosphorization kinetics of high-P-containing reduced iron produced from oolitic hematite ore
- In-phase thermomechanical fatigue studies on P92 steel with different hold time
- Effect of the weld parameter strategy on mechanical properties of double-sided laser-welded 2195 Al–Li alloy joints with filler wire
- The precipitation behavior of second phase in high titanium microalloyed steels and its effect on microstructure and properties of steel
- Development of a huge hybrid 3D-printer based on fused deposition modeling (FDM) incorporated with computer numerical control (CNC) machining for industrial applications
- Effect of different welding procedures on microstructure and mechanical property of TA15 titanium alloy joint
- Single-source-precursor synthesis and characterization of SiAlC(O) ceramics from a hyperbranched polyaluminocarbosilane
- Carbothermal reduction of red mud for iron extraction and sodium removal
- Reduction swelling mechanism of hematite fluxed briquettes
- Effect of in situ observation of cooling rates on acicular ferrite nucleation
- Corrosion behavior of WC–Co coating by plasma transferred arc on EH40 steel in low-temperature
- Study on the thermodynamic stability and evolution of inclusions in Al–Ti deoxidized steel
- Application on oxidation behavior of metallic copper in fire investigation
- Microstructural study of concrete performance after exposure to elevated temperatures via considering C–S–H nanostructure changes
- Prediction model of interfacial heat transfer coefficient changing with time and ingot diameter
- Design, fabrication, and testing of CVI-SiC/SiC turbine blisk under different load spectrums at elevated temperature
- Promoting of metallurgical bonding by ultrasonic insert process in steel–aluminum bimetallic castings
- Pre-reduction of carbon-containing pellets of high chromium vanadium–titanium magnetite at different temperatures
- Optimization of alkali metals discharge performance of blast furnace slag and its extreme value model
- Smelting high purity 55SiCr automobile suspension spring steel with different refractories
- Investigation into the thermal stability of a novel hot-work die steel 5CrNiMoVNb
- Residual stress relaxation considering microstructure evolution in heat treatment of metallic thin-walled part
- Experiments of Ti6Al4V manufactured by low-speed wire cut electrical discharge machining and electrical parameters optimization
- Effect of chloride ion concentration on stress corrosion cracking and electrochemical corrosion of high manganese steel
- Prediction of oxygen-blowing volume in BOF steelmaking process based on BP neural network and incremental learning
- Effect of annealing temperature on the structure and properties of FeCoCrNiMo high-entropy alloy
- Study on physical properties of Al2O3-based slags used for the self-propagating high-temperature synthesis (SHS) – metallurgy method
- Low-temperature corrosion behavior of laser cladding metal-based alloy coatings on EH40 high-strength steel for icebreaker
- Study on thermodynamics and dynamics of top slag modification in O5 automobile sheets
- Structure optimization of continuous casting tundish with channel-type induction heating using mathematical modeling
- Microstructure and mechanical properties of NbC–Ni cermets prepared by microwave sintering
- Spider-based FOPID controller design for temperature control in aluminium extrusion process
- Prediction model of BOF end-point P and O contents based on PCA–GA–BP neural network
- Study on hydrogen-induced stress corrosion of 7N01-T4 aluminum alloy for railway vehicles
- Study on the effect of micro-shrinkage porosity on the ultra-low temperature toughness of ferritic ductile iron
- Characterization of surface decarburization and oxidation behavior of Cr–Mo cold heading steel
- Effect of post-weld heat treatment on the microstructure and mechanical properties of laser-welded joints of SLM-316 L/rolled-316 L
- An investigation on as-cast microstructure and homogenization of nickel base superalloy René 65
- Effect of multiple laser re-melting on microstructure and properties of Fe-based coating
- Experimental study on the preparation of ferrophosphorus alloy using dephosphorization furnace slag by carbothermic reduction
- Research on aging behavior and safe storage life prediction of modified double base propellant
- Evaluation of the calorific value of exothermic sleeve material by the adiabatic calorimeter
- Thermodynamic calculation of phase equilibria in the Al–Fe–Zn–O system
- Effect of rare earth Y on microstructure and texture of oriented silicon steel during hot rolling and cold rolling processes
- Effect of ambient temperature on the jet characteristics of a swirl oxygen lance with mixed injection of CO2 + O2
- Research on the optimisation of the temperature field distribution of a multi microwave source agent system based on group consistency
- The dynamic softening identification and constitutive equation establishment of Ti–6.5Al–2Sn–4Zr–4Mo–1W–0.2Si alloy with initial lamellar microstructure
- Experimental investigation on microstructural characterization and mechanical properties of plasma arc welded Inconel 617 plates
- Numerical simulation and experimental research on cracking mechanism of twin-roll strip casting
- A novel method to control stress distribution and machining-induced deformation for thin-walled metallic parts
- Review Article
- A study on deep reinforcement learning-based crane scheduling model for uncertainty tasks
- Topical Issue on Science and Technology of Solar Energy
- Synthesis of alkaline-earth Zintl phosphides MZn2P2 (M = Ca, Sr, Ba) from Sn solutions
- Dynamics at crystal/melt interface during solidification of multicrystalline silicon
- Boron removal from silicon melt by gas blowing technique
- Removal of SiC and Si3N4 inclusions in solar cell Si scraps through slag refining
- Electrochemical production of silicon
- Electrical properties of zinc nitride and zinc tin nitride semiconductor thin films toward photovoltaic applications
- Special Issue on The 4th International Conference on Graphene and Novel Nanomaterials (GNN 2022)
- Effect of microstructure on tribocorrosion of FH36 low-temperature steels

