Abstract
The practice of reuse of treated wastewater (TWW) is seen as a strategy for water conservation in regions where water scarcity is a natural reality and in those where population growth and/or climate change foresee this scarcity. In situations of lower water scarcity, reuse is practiced by imperatives of environmental protection of the receiving media, reducing the discharge of effiuents from wastewater treatment plants. The artificial recharge of aquifers (RAQ) with TWW is a very common practice at the international level, but little considered in Portugal. However, residual waste from TWW (e.g heavy metals), when deposited in soil or water, can cause significant environmental impacts on its uses, and cause serious health problems in several animal species due to their bioaccumulation in food chains. The present study intends to show that the granitic residual soils of the Quinta de Gonçalo Martins (Guarda), in the Beira Interior region of Portugal, present physical-chemical and mineralogical characteristics favorable to the infiltration of TWW into RAQ. The results of the batch sorption tests indicate that the soil has a reactive capacity to remove the Cu and Zn residual load at TWW at high efficiencies by adsorption and ion exchange mechanisms. The pseudo-first order model explained the reaction kinetics for the three heavy metals removal and when the sorption equilibrium state was reached, the removal of these metals was explained by the Freundlich isotherm.
1 Introduction
Liquid effiuents produced in wastewater treatment plants (WWTP) can be discharged into water bodies, if they meet the discharge limits for various quality parameters, as defined in legislation [1, 2]. There is thus a residual pollutant load that is allowed to enter into natural water bodies, if there is no risk of negative and significant environmental impacts for the water resources and its uses. However, climate change has been affecting the hydrological regime in several regions of the world, which has been manifested by periods of longer and more frequent droughts, which are interspersed with periods of floods, and which have led to the reduction of volumes of water for recharging surface and underground water bodies [3]. Therefore, the discharge of treated urban effiuents into low flow water masses can produce negative and significant environmental impacts, especially in periods of low or no rainfall.
It should be noted that international and national strategies for integrated water management include reuse of treated wastewater (TWW) as part of the solution to meet water needs and to ensure the sustainability of water services. The reuse of TWW is a well-studied practice for uses such as agricultural irrigation, landscape irrigation, industry, artificial recharge of aquifers (RAQ), recreational and environmental uses and non-potable urban uses. The RAQ with TWW is a practice that is already very common at international level, with examples of success in Spain [4], Israel [5], USA [6], Finland [7] and Cyprus [8], but little considered in Portugal. It can contribute to the replenishment of volumes of water in the soil, which can be very advantageous in areas with water deficit or over-exploitation of groundwater. The Soil Aquifer Treatment (SAT) has been shown to be a technically and economically viable alternative for refining secondary treatment effluents prior to their inclusion in aquifers, as demonstrated by some studies [9, 10, 11, 12]. However, if the soil does not present favorable conditions for the infiltration of TWW, the residual loads of these waters (e.g heavy metals) can be a disadvantage to groundwater quality. It should be noted that the soil can act as a reactive filter capable of removing the residual load of the TWW.
Clay minerals, such as a kaolinite, illite, montmorillonite, among others, have reactive properties that allow the removal of metallic cations, as well as inorganic cations, essentially by sorption mechanisms, as proved in [13, 14, 15, 16, 17, 18, 19, 20]. However, it is difficult to assess the efficiency and mode of removal of heavy metals in situ, and it is more practical to use laboratory experiments (e.g batch tests). Thus, the objective of this work was to evaluate the ability to remove three heavy metals, namely chromium (Cr), copper (Cu), and zinc (Zn), using the fine component of residual soils of Quinta de Gonçalo Martins (Guarda, Portugal), using batch tests, and to verify the sorption mechanisms responsible for the removal of these pollutants.
2 Methodology
2.1 Characterization of the granitic residual soil
The Quinta de Gonçalo Martins (Guarda), in the Beira Interior region of Portugal, was selected for TWW infiltration from the WWTP of Vila Fernando [21, 22] (Figure 1), using for example infiltration, retention or detention basins, like suggested by [19]. From a soil sample (Figure 2(a)) a fraction with less than 0.075 mm (fine soil) was extracted (Figure 2(b)) for the sorption experiments, as it is the most reactive fractions of the soil, namely silt and clay.
For understanding the importance of soil properties in the removal of heavy metals, some physical, chemical and mineralogical properties were determined. The differential and cumulative volumes of the fine soil as a function of the particle size, as well as the specific surface, were determined by laser diffraction using the Coulter LS200. The density of the solid particles was determined by the pycnometer method [23] and the porosity using the procedures described in [19]. The clay mineral fraction (< 2 μm) was determined by X-ray Diffraction (XRD) using a Philips Analytical X-Ray BV diffractometer, consisting of a 3710 mpd/00 PW controller and a PW high voltage generator 1830, P, operating at 40 KV and 30 mA with a copper ampoule (Cu Kα radiation). The chemical composition (oxides analysis) was determined with the Dispersive Energy Spectrometer (EDS) coupled to the Scanning Electron Microscope (SEM), Hitachi S-2700, USA. The cation exchange capacity was determined by the method of ammonium acetate buffered at pH 7, described in [24]. The organic matter by the Walkley-Black method, described in [25] and the soil pH, determined in H2O and KCl by potentiometric method, suspended (soil: water, 1: 2.5), described in [26].
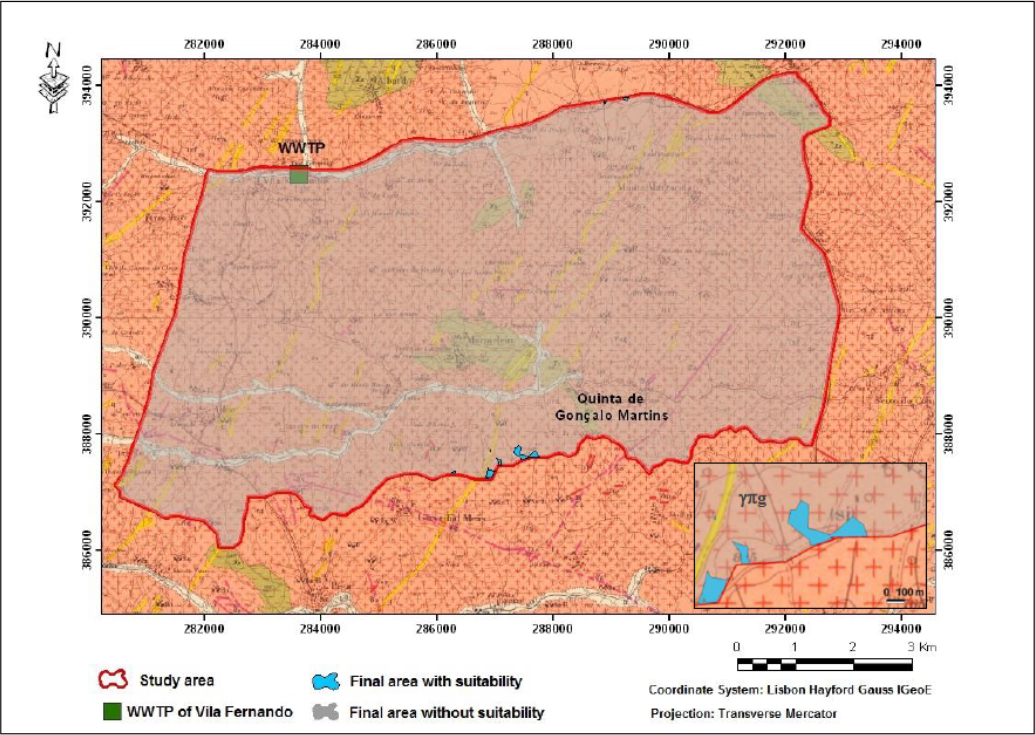
Location of the soil sampling area for infiltration of treated wastewater (blue spots) in an extract map from the Geological Chart of Guarda: Sheet 18-C

Pickling process for collecting the soil sample, in the selected site for the infiltration of treated wastewater (Quinta Gonçalo Martins, Guarda, coordinates M = 287699 m and P = 387657 m): colleting raw samples (a); sample of fine soil (b)
2.2 Sorption experiments with Cr, Cu and Zn
In the batch sorption tests, concentrated solutions of 1.0 g/L of potassium chromate (K2CrO4), copper sulphate (CuSO4) and zinc chloride (ZnCl2) were used to study the removal of Cr, Cu and Zn metal ions, respectively. For the reaction kinetics, were used aqueous solutions of K2CrO4, CuSO4 and ZnCl2 with the following theoretical concentrations (Ct): 0; 1; 2.5; 5; 7.5 and 10 mg/L. Samples of 0.5 g of fine soil were placed in six 500 mL glass vessels. To each vessel were added 200 mL of aqueous solution of ions at the mentioned concentrations. The vessels were shaken for 24 hours, and 5 mL of liquid sample was withdrawn at the following times: 0, 0.25; 0.75; 2; 5; 11 and 24 hours and the pH and temperature values were measures. The experimental equipment used consisted of a Flask Shaker SF1 mechanical stirrer from Stuart Scientific (England), which was calibrated to promote a constant rotation and equal to 120 oscillations per minute, as used by [27]. For measurement of pH and temperature a SenTix 41 probe attached to a Multi 340i meter from WTW, Germany, was used. The determination of metals was carried out by means of an atomic absorption spectrophotometer GBC-906 (Australia), according to standard [28]. For the study of sorption isotherms, the same aqueous solutions were used, with the following theoretical concentrations (Ct): 0; 1; 5 and 10 mg/L and for three soil masses: 0.1, 0.5 and 1.0 g.
3 Analysis and discussion of results
3.1 Characteristics of the granitic residual soil
The residual soil has about 4.94% of clay (particle size less than 2 μm). According to [29], in order to avoid soil sealing and to ensure the treatment of residual water, the soil should have a low fraction of clay, namely less than 10%. The sample had a density of 2.65, porosity of 48.0% and specific surface of 0.29 m2/g (Figure 3). It contains mainly silica (60.44%) and alumina (31.76%), with lower levels of iron (3.99%) and potassium (3.81%). Cation exchange capacity (at pH = 7) it considered a median value (11.68 cmolc/kg) according to [30], the organic matter content is very low (0.45%) and the soil is very acidic (pH = 4.44). The study of the clay fraction (< 2 μm) by X-ray diffraction (XRD) (Figure 4) revealed the presence of kaolinite (K), illite (I) and smectite (S), with kaolinite accounting for about 60% of the clayey material present in the soil. Other clay minerals similar proportions, that is, illite (21.41%) and smectite (18.73%). The clay-colloidal complex of this soil allow considered that has reactive properties that allow it to remove pollutants by sorption mechanisms, as well as a specific surface suitable for the development of the biofilm with the capacity to remove pollutants and pathogens through biodegradation mechanisms, as demonstrated in [19].
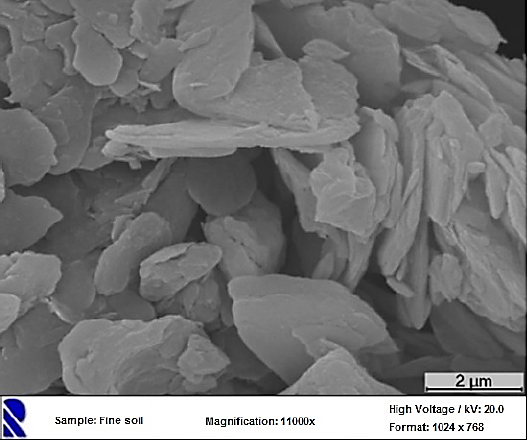
SEM image of the fine soil, magnification of 11000x
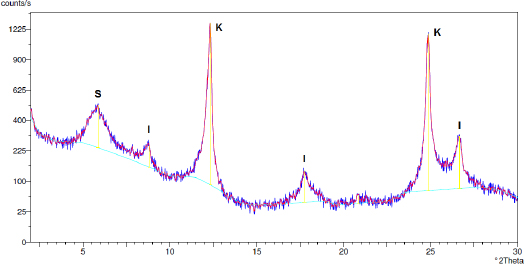
X-ray diffraction of the soil sample (< 2 μm)
3.2 Sorption experiments with Cr, Cu and Zn
Tables 1 to 3 show the results of the tests performed for the study of reaction kinetics with Cr, Cu and Zn and 0.5 g of fine soil. The results show that the equilibrium concentrations (Ce) were reached between 2h and 5h of contact, for Cr (Table 1), between 5h and 11h for Cu (Table 2) and at 2h for Zn (Table 3). In general, a decrease in pH was observed shortly after 15 minutes of contact, continuing to decrease over time to the 24 hour duration of the assays. pH values for the Cr tests ranged from 6.95 (beginning) to 5.60 (final), although it was almost always above 6.00), for Cu assays changed from 6.09 (beginning ) to 4.89 (final) and for the Zn assays ranged from 6.10 (beginning) to 5.00 (final). Temperature values were recorded between 18.8∘C (minimum value) and 24.1∘C (maximum value).
Results for experiments with Cr
| Parameters | Theoretical | Sampling time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| concentration (Ct) (mg/L) | ||||||||
| 0 | 0.25 | 0.75 | 2 | 5 | 11 | 24 | ||
| C 1) (mg/L) | 1.0 | 0.94 | 0.89 | 0.88 | 0.852) | 0.88 | 0.86 | 0.85 |
| pH | 6.20 | 6.00 | 5.99 | 6.12 | 6.07 | 6.01 | 6.34 | |
| Temp. (∘C) | 19.90 | 20.30 | 20.60 | 20.70 | 21.10 | 20.30 | 19.90 | |
| C 1) (mg/L) | 2.5 | 2.33 | 2.27 | 2.30 | 2.25 | 2.222) | 2.24 | 2.20 |
| pH | 6.61 | 6.14 | 6.30 | 6.08 | 5.61 | 6.03 | 5.60 | |
| Temp. (∘C) | 19.90 | 20.30 | 20.70 | 20.90 | 21.30 | 20.60 | 20.20 | |
| C 1) (mg/L) | 5.0 | 5.62 | 5.18 | 5.22 | 5.082) | 5.16 | 5.23 | 5.21 |
| pH | 6.71 | 6.15 | 6.10 | 6.14 | 6.08 | 6.11 | 6.19 | |
| Temp. (∘C) | 19.90 | 20.40 | 20.70 | 20.90 | 21.20 | 20.60 | 21.10 | |
| C 1) (mg/L) | 7.5 | 6.88 | 6.73 | 6.30 | 6.35 | 6.312) | 6.36 | 6.35 |
| pH | 6.79 | 6.30 | 6.25 | 6.31 | 6.22 | 6.18 | 6.16 | |
| Temp. (∘C) | 20.00 | 20.50 | 20.70 | 21,10 | 21.40 | 20.40 | 20.10 | |
| C 1) (mg/L) | 10.0 | 9.66 | 8.98 | 8.74 | 8.72 | 8.682) | 8.70 | 8.73 |
| pH | 6.95 | 6.41 | 6.35 | 6.34 | 6.33 | 6.29 | 6.30 | |
| Temp. (∘C) | 20.00 | 20.40 | 20.60 | 20.90 | 21.10 | 20.40 | 19.90 | |
1) Concentration after contact with the soil
2) Equilibrium concentration
Results for experiments with Cu
| Parameters | Theoretical | Sampling time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| concentration (Ct) (mg/L) | ||||||||
| 0 | 0.25 | 0.75 | 2 | 5 | 11 | 24 | ||
| C 1) (mg/L) | 1.0 | 0.92 | 0.63 | 0.51 | 0.48 | 0.45 | 0.442) | 0.46 |
| pH | 6.09 | 5.87 | 5.66 | 5.66 | 5.63 | 5.67 | 5.46 | |
| Temp. (∘C) | 19.80 | 19.70 | 19.90 | 19.80 | 19.60 | 19.20 | 19.60 | |
| C 1) (mg/L) | 2.5 | 2.24 | 1.14 | 0.75 | 0.71 | 0.78 | 0.712) | 0.70 |
| pH | 5.60 | 5.40 | 5.32 | 5.41 | 5.38 | 5.42 | 5.35 | |
| Temp. (∘C) | 19.80 | 19.80 | 19.90 | 19.90 | 19.60 | 19.10 | 19.80 | |
| C 1) (mg/L) | 5.0 | 5.35 | 4.21 | 4.27 | 4.33 | 4.312) | 4.36 | 4.38 |
| pH | 5.21 | 5.10 | 5.01 | 5.05 | 5.04 | 5.01 | 5.01 | |
| Temp. (∘C) | 19.80 | 19.90 | 20.10 | 20.30 | 19.70 | 19.40 | 19.80 | |
| C 1) (mg/L) | 7.5 | 7.27 | 6.05 | 5.91 | 5.88 | 5.842) | 5.88 | 5.90 |
| pH | 5.04 | 4.91 | 4.82 | 4.90 | 4.87 | 4.83 | 4.89 | |
| Temp. (∘C) | 19.00 | 19.90 | 20.10 | 20.30 | 19.60 | 19.40 | 19.60 | |
| C 1) (mg/L) | 10.0 | 10.13 | 8.52 | 8.18 | 8.07 | 8.12 | 8.052) | 8.13 |
| pH | 5.10 | 5.01 | 4.94 | 4.97 | 5.01 | 4.96 | 4.95 | |
| Temp. (∘C) | 19.90 | 19.90 | 20.00 | 19.80 | 19.60 | 19.10 | 19.60 | |
1) Concentration after contact with the soil
2) Equilibrium concentration
Results for experiments with Zn
| Parameters | Theoretical | Sampling time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| concentration (Ct) (mg/L) | ||||||||
| 0 | 0.25 | 0.75 | 2 | 5 | 11 | 24 | ||
| C 1) (mg/L) | 1.0 | 1.12 | 0.37 | 0.27 | 0.232) | 0.22 | 0.25 | 0.21 |
| pH | 6.10 | 5.60 | 5.50 | 5.37 | 5.71 | 5.16 | 5.64 | |
| Temp. (∘C) | 19.10 | 19.00 | 23.50 | 24.10 | 20.90 | 18.80 | 21.80 | |
| C 1) (mg/L) | 2.5 | 2.55 | 1.52 | 1.24 | 1.212) | 1.22 | 1.24 | 1.22 |
| pH | 6.07 | 5.32 | 5.31 | 5.32 | 5.28 | 5.39 | 5.42 | |
| Temp. (∘C) | 18.90 | 19.00 | 22.60 | 23.70 | 20.60 | 19.30 | 22.10 | |
| C 1) (mg/L) | 5.0 | 5.18 | 3.12 | 3.03 | 3.082) | 3.06 | 3.10 | 3.07 |
| pH | 5.90 | 5.44 | 5.20 | 5.12 | 5.10 | 5.06 | 5.11 | |
| Temp. (∘C) | 19.00 | 19.10 | 22.70 | 23.00 | 20.70 | 19.00 | 22.30 | |
| C 1) (mg/L) | 7.5 | 7.44 | 5.21 | 5.08 | 5.032) | 5.05 | 5.03 | 5.09 |
| pH | 5.83 | 5.28 | 5.03 | 5.04 | 5.05 | 5.05 | 5.00 | |
| Temp. (∘C) | 19.00 | 19.30 | 23.10 | 22.80 | 20.60 | 19.20 | 22.30 | |
| C 1) (mg/L) | 10.0 | 10.33 | 7.16 | 7.07 | 6.932) | 6.91 | 6.97 | 7.00 |
| pH | 5.91 | 5.33 | 5.15 | 5.07 | 4.98 | 5.07 | 5.05 | |
| Temp. (∘C) | 19.00 | 19.70 | 22.80 | 22.80 | 20.60 | 19.60 | 22.50 | |
1) Concentration after contact with the soil
2) Equilibrium concentration
The removal efficiency (RE), in percentage, of the metals over time, was calculated through Eq. (1)
where Ci and Cn are the initial concentration and in the time n of solute in solution (mg/L), respectively.
There were observed RE of Cr of 10.1%, of Cu of 68.3% and of Zn of 79.5% for the Ce of 8.68 mg Cr/ L, 0.71 mg Cu/ L e 0.23 mg Zn/ L, respectively.
The sorption rates (qs), in (mg/g), were compiled through Eq. (2) which means, for each instant, the mass of metal retained in 0.5 g of fine soil.
where, Ci is the initial concentration of solute in solution (mg/L), Cf is the final concentration or the equilibrium concentration of solute in solution (mg/L), V is the volume of the assays (L) and ms is the mass of fine soil (g).
The sorption kinetics for the three heavy metals were explained by Lagergren’s pseudo-first order kinetic model [31] according to Eq. (3)
where qe and qt in (mg/g) are the sorption rate in the equilibrium and in the time t, respectively, and k1 is the pseudo-first order kinetic constant (min−1).
The mean square error (MSE) was used as the fitting measure [32, 33,], using Eq. (4) This methodology allowed to fit the values of the variables characteristic of the model by minimizing the sum of the square of the difference between the values calculated by the model and the experimental values.
where i is the number of values, j the maximum number of values, Xexp the experimental values and X sim the simulated values with the model.
The sorption rate (qe) were 0.392 mg Cr/g, 0.833 mg Cu/g and 1.360 mg Zn/g for the initial higher concentrations (Ci) (namely 9.66 mg Cr/L, 10.13 mg Cu/L and 10.33
mg Zn/L). For all metal the sorption rates were higher theoretical concentrations (10mg/L). The sequence for the kinetics of sorption of metals is Cr < Cu < Zn.
For the study of sorption isotherms with Cr, Cu and Zn and 0.1 g, 0.5 g and 1.0 g of fine soil, Freundlich model [34] (Eq. (5)) better explains the equilibrium isotherm, and the results are presented in Figures 5 to 7.
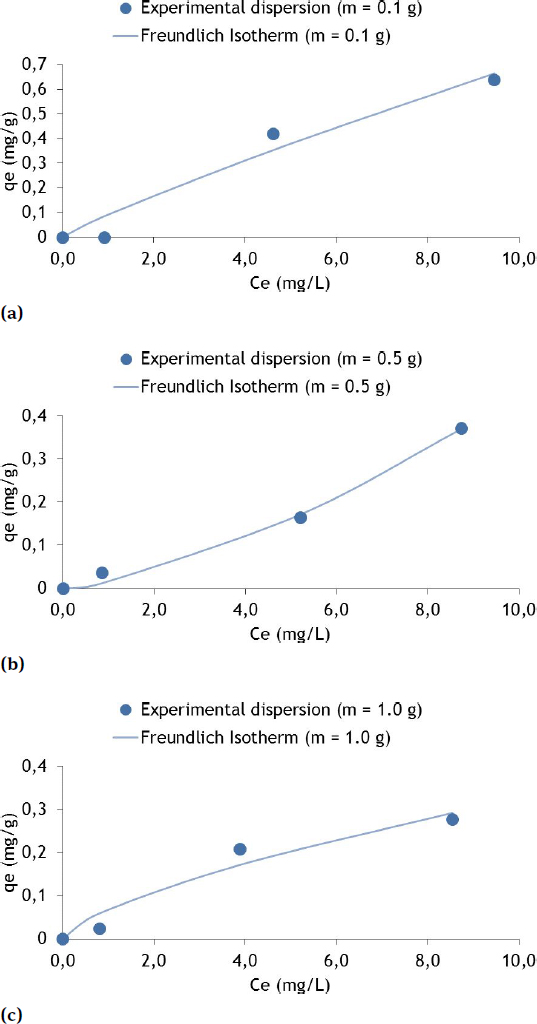
Sorption isotherms for the Freundlich model with Cr and soil masses of 0.1 g (a), 0.5 (b) and 1.0 g (c)
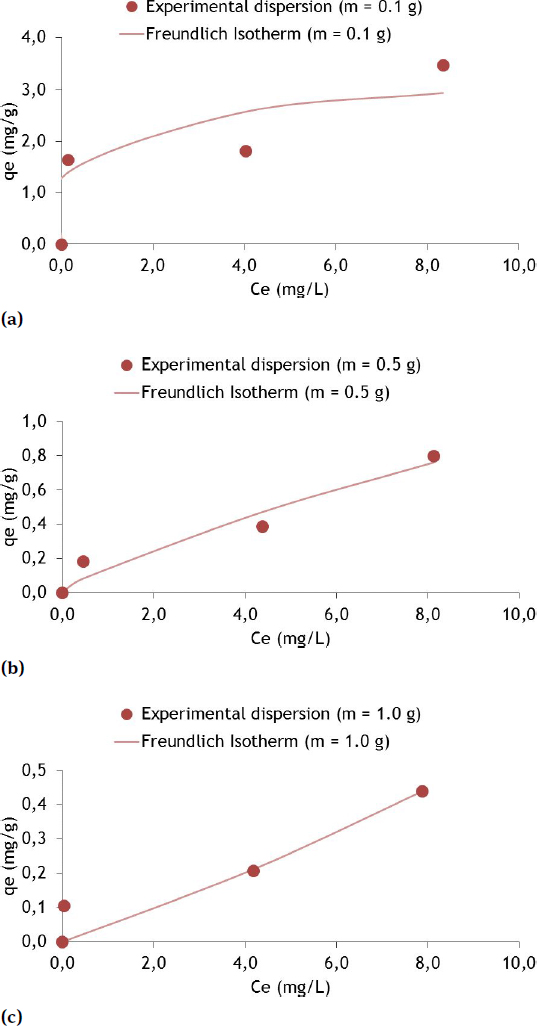
Sorption isotherms for the Freundlich model with Cu and soil masses of 0.1 g (a), 0.5 (b) and 1.0 g (c)
where qe (mg/g) is the sorption rate at the equilibrium, kf is the Freundlich coefficient (L/g), Ce the equilibrium concentration of solute in solution (mg/L) and 1/n is a coefficient that depends on the solute, porous media and environmental conditions.
It is possible to observe that the qe for Cr, Cu and Zn were higher for the higher values of Ct (10 mg/L) and also higher for lower values of mass (m = 0.1 g).
From the analysis of Figure 5(a) and 0.1 g of fine soil the qe of Cr was 0.664 mg/g for the Ce of 9.46mg/L. The pH ranged from 6.98 (beginning) to 6.59 (final). The temperature varied from 21.1∘C (beginning) to 23.1∘C (final).
For 0.1 g of fine soil (Figure 6(a)) the qe of Cu was 2.939 mg/g for the Ce of 8.34 mg/L. The pH ranged from 5.54 (beginning) to 5.22 (final). The temperature varied from 21.1∘C (beginning) to 23.2∘C (final). The results are superior to those presented by [13], which used a clay rock in the El Hamma area of Tunisia, obtaining a qe value of 0.021 mg Cu/g with a heavy metal Ci of 0.5 mg/L and using clay masses of 0.5 and 5.5 g. Although the clay has a high specific surface (about 490 m2/g), the high pH of the solution (between 8 and 10) would not favor the adsorption of Cu, which was practically removed by complexation and precipitation in the form of hydroxides and carbonates. This seems to indicate that pH has much more influence on the removal of metals than the specific surface of the material. On the other hand, in a study with crude Egyptian kaolin and bentonites treated with calcium and sodium (Ca-B and Na-B) for the removal of heavy metals from polluted waters, [20] showed that qe increased with increasing Ce, as observed in the present study, with higher values of removed mass and Cu mass in the aqueous solution, from about 12 mg Cu/g to Ce of 8 mg/L for the soil with Ca-B and 4 mg Cu/g for the case of crude kaolin. The Freundlich model was also the one that best fit the results obtained.
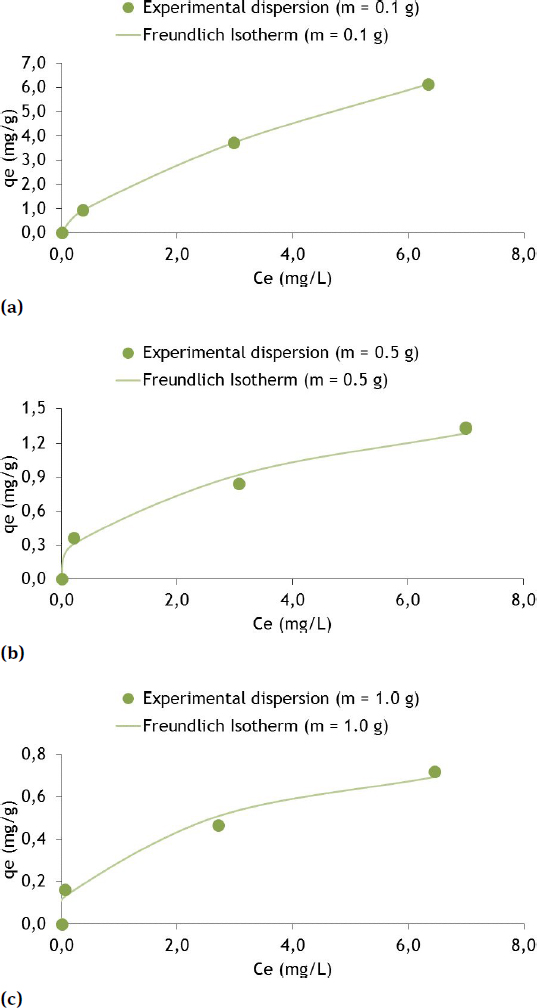
Sorption isotherms for the Freundlich model with Zn and soil masses of 0.1 g (a), 0.5 (b) and 1.0 g (c)
For 0.1 g of fine soil (Figure 7(a)) the qe of Zn was 6.138 mg/g for the Ce of 6.34 mg/L. The pH ranged from 5.59 (beginning) to 5.37 (final). The temperature varied from 22.3∘C (beginning) to 20.3∘C (final). In the study by [20] qe increased with increasing Ce, as in the present study, but presented generally higher values of removed mass and mass of Zn in the aqueous solution, with maximum values of 12 mg Zn/g for Ce of 8 mg/L (for Ca-B and Na-B soils) and from 6 mg Zn/g to 8 mg/L in the case of crude kaolin.
In general, in tests performed with Cr (where its removal was lower) although the pH dropped, it was almost always above 6. This decrease in pH would have been associated with the dissociation of H+ ions from the clay-colloidal complex of the soil to the aqueous medium due to the hydrolysis of tetrahedral and octahedral structures [14, 35,], as well as to possible hydration of aluminium and iron oxides, which may have reduced the OH− concentration in the solution. The removal of Cr ion was low because the ideal pH for its electrostatic adsorption and ion exchange was between 2 and 4 [36]. On the other hand, removal by complexation and precipitation in the form of hydroxides only occurs significantly at pH values above 6 [13, 15, 37,], which in general occurred with this ion.
In the Cu and Zn experiments, in addition to mentioned dissociation, ion exchange will also occur between the H+ ions and Cu and Zn ions (hence the pH values are lower in the tests with these four ions, with values close to 5), although there also appears to have been ion exchange with the Al3+ and Fe3+ ions present in the clay-colloidal complex, and OH− reduction due to the hydration of iron and aluminium oxides. The ion exchange of H+, Al3+ and Fe3+ by Cu and Zn is favored at pH values below 5.5 as referred in [15]. The removal of Cu and Zn would not have occurred by complexation and precipitation in the form of hydroxides, since it only has significance for values above 6 [13, 15, 37,].
4 Conclusions
The fine component of the granitic residual soil of Quinta de Gonçalo Martins (Guarda, Portugal), has reactive properties that give it a good capacity for removing Cu and Zn by sorption mechanisms, which allows it to act as a barrier to water contamination during the artificial recharge of aquifers with treated wastewater. With respect to the batch tests, Cu and Zn removal was observed to be high and occurred mainly by adsorption and ion exchange mechanisms, according to the pseudo-first order kinetics model. Sorption rates (qe) of 0.833 mg Cu/g and 1.360 mg Zn/g were found for the highest theoretical concentrations (Ct) (10 mg/L) and for a fine soil mass of 0.5 g. The removal of Cr in the soil was low. When the sorption equilibrium state was reached, the removal of these pollutants was explained by the Freundlich isotherm. Sorption rates (qe) were highest for the lowest fine mass value (m = 0.1 g), namely 2.939 mg Cu/g and 6.138 mg Zn/g. The use of this soil as a means of filling infiltration infrastructures can still become economically competitive when compared to the reactive materials currently used.
References
[1] Decree law no. 152/97 of 19 June, Portuguese Law, 1997, Lisbon, Portugal. (In Portuguese)Search in Google Scholar
[2] Decree law no. 236/98 of 1 August, Portuguese Law, 1998, Lisbon, Portugal. (In Portuguese)Search in Google Scholar
[3] IPCC, Climate change 2014 - Impacts, Adaptation, and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the IPCC Fifth Assessment Report, Intergovernmental Panel on Climate Change, 2014, Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO978110741537910.1017/CBO9781107415379Search in Google Scholar
[4] Díaz J., Gomez J., Armayor J., Castano S., Recarga artificial de acuíferos. Síntesis metodológica. Estudios y actuaciones realizadas en la provincia de Alicante, Geta, J., Hernández L. (Edt), 2000, Geological and Mining Institute of Spain.Search in Google Scholar
[5] Bensabat J., Artificial recharge in Israel, AQUA2006, Water Science and Technology Integrated Management of Water Resources, Athens, Hellas, 23-26 November 2006.Search in Google Scholar
[6] Lluria M., Successful application of managed aquifer recharge in the improvement of the water resources management of semi-arid regions: Examples from Arizona and the Southwestern USA, Boletín Geológico y Minero, 2009, 120, 111-120.Search in Google Scholar
[7] Nojd P., Lindroos A., Smolander A., Derome J., Lumme I., Helmisaari H., Artificial recharge of groundwater through sprinkling infiltration: Impacts on forest soil and the nutrient status and growth of Scots pine, Science of the Total Environment, 2009, 407, 3365-3371. https://doi.org/10.1016/j.scitotenv.2009.01.06210.1016/j.scitotenv.2009.01.062Search in Google Scholar
[8] Voudouris K., Artificial recharge via boreholes using treated wastewater: Possibilities and prospects, Water, 2011, 3, 964-975. https://doi.org/10.3390/w304096410.3390/w3040964Search in Google Scholar
[9] Bdour A., Hamdi M., Tarawneh Z., Perspectives on sustainable wastewater treatment technologies and reuse options in the urban areas of the Mediterranean region, Desalination, 2009, 237, 162-174. https://doi.org/10.1016/j.desal.2007.12.03010.1061/40927(243)565Search in Google Scholar
[10] Essandoh H., Tizaoui C., Mohamed M., Amy G., Brdjanovic D., Soil aquifer treatment of artificial wastewater under saturated conditions, Water Research, 2011, 45, 4211-4226. https://doi.org/10.1016/j.watres.2011.05.01710.1016/j.watres.2011.05.017Search in Google Scholar PubMed
[11] Grunheid S., Amy G., Jekel M., Removal of bulk dissolved organic carbon (DOC) and trace organic compounds by bank filtration and artificial recharge, Water Research, 2005, 39, 3219-3228. https://doi.org/10.1016/j.watres.2005.05.03010.1016/j.watres.2005.05.030Search in Google Scholar PubMed
[12] Pescod M., Wastewater treatment and use in agriculture, FAO irrigation and drainage paper, 47, FAO, 1992, Rome, Italy.Search in Google Scholar
[13] Chaari I., Medhioub M., Jamoussi F., Use of clay to remove heavy metals from Jebel Chakir landfill leachate, Journal of Applied Sciences in Environmental Sanitation, 2011, 6, 143-148.Search in Google Scholar
[14] Costa, J., Caracterização e constituição do solo (8th ed.), 2011, Lisbon: Fundação Calouste Gulbenkian. (In Portuguese)Search in Google Scholar
[15] Fike W., Sorption of cadmium, copper, lead, and zinc as influenced by pH, ionic strength and selected soil components (PhD thesis), 2001, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA.Search in Google Scholar
[16] Lukman S., Essa M., Mu’azu N., Bukhari A., Basheer C., Adsorption and desorption of heavy metals onto natural clay material: lnfluence of initial pH, Journal of Environmental Science and Technology, 2013, 6, 1-15. DOI: 10.3923/jest.2013.1.1510.3923/jest.2013.1.15Search in Google Scholar
[17] Meurer E., Rheinheimer D., Bissani C., Fenômenos de sorção em solos, In: Meurer E. (Ed.), (3rd ed.), Fundamentos de química do solo, 2006, Porto Alegre: Evangraf.Search in Google Scholar
[18] Ramísio P., Retenção de metais pesados de escorrências rodoviárias por filtração reactiva (PhD thesis), 2007, Braga: University of Minho. (In Portuguese)Search in Google Scholar
[19] Silva F., Avaliação da Capacidade Reativa de Solos Residuais destinados à Infiltração de Águas Residuais Tratadas (PhD thesis), 2015, Covilhã: University of Beira Interior. (In Portuguese)Search in Google Scholar
[20] Talaat H., El Defrawy N., Abulnour A., Tawfik A., Evaluation of heavy metals removal using some Egyptian clays, In Proceedings of the 2nd International Conference on Environmental Science and Technology, IPCBEE, Singapore, 26-28 February 2011.Search in Google Scholar
[21] Silva F., Estudo do Potencial de Recarga de Aquíferos com Águas Residuais Tratadas utilizando Sistemas de Informação Geográfica (MSc. thesis), 2011, Covilhã: University of Beira Interior. (In Portuguese)Search in Google Scholar
[22] Silva F., Scalize P., Cruvinel K., Albuquerque A., Caracterização de solos residuais para infiltração de efluente de estação de tratamento de esgoto [Characterization of residual soils for infiltration of reclaimed water], Engenharia Sanitária e Ambiental, 2017, 22, 95-102. http://dx.doi.org/10.1590/s1413-4152201614167710.1590/s1413-41522016141677Search in Google Scholar
[23] NP 83, Solos. Determinação da densidade das partículas, 1965, Lisbon: IGPAI. (In Portuguese)Search in Google Scholar
[24] Houba V., Van der Lee J., Novozamsky I., Soil Analysis Procedures, Department of Soil Science and Plant Nutrition, 1995,Wageningen: Wageningen Agricultural University.Search in Google Scholar
[25] Nelson D., Sommers L., Total carbon, organic carbon and organicmatter: In Methods of soil analysis, Part 3, Chemical Methods - SSSA, vol. Book series 5, 1996, 961-1010.10.2136/sssabookser5.3.c34Search in Google Scholar
[26] Van Reeuwijk, Procedures for soil analysis (6th ed.), 2002, ISRIC, FAO.Search in Google Scholar
[27] Ruan H., Gilkes R., Kinetics of phosphate sorption and desorption by synthetic aluminous goethite before and after thermal transformation to hematite, Clay Minerals, 1996, 31, 63-74.10.1180/claymin.1996.031.1.06Search in Google Scholar
[28] ISO 15586, Water quality. Determination of trace elements using atomic absorption spectrometry with graphite furnace, 2003, Genebra: International Organization for Standardization.Search in Google Scholar
[29] Kallali H., Anane M., Jellali S., Tarhouni J., GIS-Based multicriteria analysis for potential wastewater aquifer recharge sites, Desalination, 2007, 215, 111-119. https://doi.org/10.1016/j.desal.2006.11.01610.1016/j.desal.2006.11.016Search in Google Scholar
[30] LQARS, Manual de Fertilização das Culturas, 2006, Lisbon: INIAP - Laboratório Químico Agrícola Rebelo da Silva. (In Portuguese)Search in Google Scholar
[31] Lagergren S., About the theory of so called adsorption of soluble substances. Ksver Veterskapsakad Handling, 1898, 24, 1-39.Search in Google Scholar
[32] Albuquerque A., Contribuição para o estudo da remoção de residuais de carbono em filtros biológicos de leito imerso e fluxo descendente (PhD thesis), 2003, Covilhã: University of Beira Interior. (In Portuguese)Search in Google Scholar
[33] Silva I., Desenvolvimento de Agregados Artificiais por Ativação Alcalina de Lamas Residuais para Utilização no Tratamento de Águas Residuais (PhD thesis), 2013, Covilhã: University of Beira Interior. (In Portuguese)Search in Google Scholar
[34] Freundlich H., Over the Adsorption in Solution, Journal of Physical Chemistry, 1906, 57, 385-470.10.1515/zpch-1907-5723Search in Google Scholar
[35] Koppelman M., Emerson A., Dillard J., Adsorbed Cr (III) on Chlorite, Illite and Kaolinite: on X-Ray photoelectron spectroscopic study, Clays and Clay Minerals, 1980, 28, 119-124. DOI: 10.1346/CCMN.1980.028020710.1346/CCMN.1980.0280207Search in Google Scholar
[36] Wu Y., Zhang S., Guo X., Huang H., Adsorption of chromium (III) on lignin. Bioresource Technology, 2008, 99, 7709-7715. https://doi.org/10.1016/j.biortech.2008.01.06910.1016/j.biortech.2008.01.069Search in Google Scholar
[37] Csobán K., Párkányi-Berka M., Joó P., Behra Ph., Sorption experiments of Cr (III) onto silica, Colloids and Surfaces A: Physico-chemical and Engineering Aspects, 1998, 141, 347-364. https://doi.org/10.1016/S0927-7757(98)00244-110.1016/S0927-7757(98)00244-1Search in Google Scholar
© 2018 F. Silva et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 License.
Articles in the same Issue
- Regular Article
- Real-scale comparison between simple and composite raw sewage sampling
- 10.1515/eng-2018-0017
- The risks associated with falling parts of glazed facades in case of fire
- Implementation of high speed machining in thin-walled aircraft integral elements
- Evaluating structural crashworthiness and progressive failure of double hull tanker under accidental grounding: bottom raking case
- Influence of Silica (SiO2) Loading on the Thermal and Swelling Properties of Hydrogenated-Nitrile-Butadiene-Rubber/Silica (HNBR/Silica) Composites
- Statistical Variations and New Correlation Models to Predict the Mechanical Behavior and Ultimate Shear Strength of Gypsum Rock
- Analytic approximate solutions to the chemically reactive solute transfer problem with partial slip in the flow of a viscous fluid over an exponentially stretching sheet with suction/blowing
- Thermo-mechanical behavior simulation coupled with the hydrostatic-pressure-dependent grain-scale fission gas swelling calculation for a monolithic UMo fuel plate under heterogeneous neutron irradiation
- Optimal Auxiliary Functions Method for viscous flow due to a stretching surface with partial slip
- Vibrations Analysis of Rectangular Plates with Clamped Corners
- Evaluating Lean Performance of Indian Small and Medium Sized Enterprises in Automotive Sector
- FPGA–implementation of PID-controller by differential evolution optimization
- Thermal properties and morphology of polypropylene based on exfoliated graphite nanoplatelets/nanomagnesium oxide
- A computer-based renewable resource management system for a construction company
- Hygrothermal Aging of Amine Epoxy: Reversible Static and Fatigue Properties
- The selected roof covering technologies in the aspect of their life cycle costs
- Influence of insulated glass units thickness and weight reduction on their functional properties
- Structural analysis of conditions determining the selection of construction technology for structures in the centres of urban agglomerations
- Selection of the optimal solution of acoustic screens in a graphical interpretation of biplot and radar charts method
- Subsidy Risk Related to Construction Projects: Seeking Causes
- Multidimensional sensitivity study of the fuzzy risk assessment module in the life cycle of building objects
- Planning repetitive construction projects considering technological constraints
- Identification of risk investment using the risk matrix on railway facilities
- Comparison of energy parameters of a centrifugal pump with a multi-piped impeller in cooperation either with an annular channel and a spiral channel
- Influence of the contractor’s payment method on the economic effectiveness of the construction project from the contractor’s point of view
- Special Issue Automation in Finland
- Diagnostics and Identification of Injection Duration of Common Rail Diesel Injectors
- An advanced teaching scheme for integrating problem-based learning in control education
- A survey of telerobotic surface finishing
- Wireless Light-Weight IEC 61850 Based Loss of Mains Protection for Smart Grid
- Smart Adaptive Big Data Analysis with Advanced Deep Learning
- Topical Issue Desktop Grids for High Performance Computing
- A Bitslice Implementation of Anderson’s Attack on A5/1
- Efficient Redundancy Techniques in Cloud and Desktop Grid Systems using MAP/G/c-type Queues
- Templet Web: the use of volunteer computing approach in PaaS-style cloud
- Using virtualization to protect the proprietary material science applications in volunteer computing
- Parallel Processing of Images in Mobile Devices using BOINC
- “XANSONS for COD”: a new small BOINC project in crystallography
- Special Issue on Sustainable Energy, Engineering, Materials and Environment
- An experimental study on premixed CNG/H2/CO2 mixture flames
- Tidal current energy potential of Nalón river estuary assessment using a high precision flow model
- Special Spring Issue 2017
- Context Analysis of Customer Requests using a Hybrid Adaptive Neuro Fuzzy Inference System and Hidden Markov Models in the Natural Language Call Routing Problem
- Special Issue on Non-ferrous metals and minerals
- Study of strength properties of semi-finished products from economically alloyed high-strength aluminium-scandium alloys for application in automobile transport and shipbuilding
- Use of Humic Sorbent from Sapropel for Extraction of Palladium Ions from Chloride Solutions
- Topical Issue on Mathematical Modelling in Applied Sciences, II
- Numerical simulation of two-phase filtration in the near well bore zone
- Calculation of 3D Coordinates of a Point on the Basis of a Stereoscopic System
- The model of encryption algorithm based on non-positional polynomial notations and constructed on an SP-network
- A computational algorithm and the method of determining the temperature field along the length of the rod of variable cross section
- ICEUBI2017 - International Congress on Engineering-A Vision for the Future
- Use of condensed water from air conditioning systems
- Development of a 4 stroke spark ignition opposed piston engine
- Development of a Coreless Permanent Magnet Synchronous Motor for a Battery Electric Shell Eco Marathon Prototype Vehicle
- Removal of Cr, Cu and Zn from liquid effluents using the fine component of granitic residual soils
- A fuzzy reasoning approach to assess innovation risk in ecosystems
- Special Issue SEALCONF 2018
- Brush seal with thermo-regulating bimetal elements
- The CFD simulation of the flow structure in the sewage pump
- The investigation of the cavitation processes in the radial labyrinth pump
- Testing of the gaskets at liquid nitrogen and ambient temperature
- Probabilistic Approach to Determination of Dynamic Characteristics of Automatic Balancing Device
- The design method of rubber-metallic expansion joint
- The Specific Features of High-Velocity Magnetic Fluid Sealing Complexes
- Effect of contact pressure and sliding speed on the friction of polyurethane elastomer (EPUR) during sliding on steel under water wetting conditions
- Special Issue on Advance Material
- Effect of thermo-mechanical parameters on the mechanical properties of Eurofer97 steel for nuclear applications
- Failure prediction of axi-symmetric cup in deep drawing and expansion processes
- Characterization of cement composites based on recycled cellulosic waste paper fibres
- Innovative Soft Magnetic Composite Materials: Evaluation of magnetic and mechanical properties
- Statistical modelling of recrystallization and grain growth phenomena in stainless steels: effect of initial grain size distribution
- Annealing effect on microstructure and mechanical properties of Cu-Al alloy subjected to Cryo-ECAP
- Influence of heat treatment on corrosion resistance of Mg-Al-Zn alloy processed by severe plastic deformation
- The mechanical properties of OFHC copper and CuCrZr alloys after asymmetric rolling at ambient and cryogenic temperatures
Articles in the same Issue
- Regular Article
- Real-scale comparison between simple and composite raw sewage sampling
- 10.1515/eng-2018-0017
- The risks associated with falling parts of glazed facades in case of fire
- Implementation of high speed machining in thin-walled aircraft integral elements
- Evaluating structural crashworthiness and progressive failure of double hull tanker under accidental grounding: bottom raking case
- Influence of Silica (SiO2) Loading on the Thermal and Swelling Properties of Hydrogenated-Nitrile-Butadiene-Rubber/Silica (HNBR/Silica) Composites
- Statistical Variations and New Correlation Models to Predict the Mechanical Behavior and Ultimate Shear Strength of Gypsum Rock
- Analytic approximate solutions to the chemically reactive solute transfer problem with partial slip in the flow of a viscous fluid over an exponentially stretching sheet with suction/blowing
- Thermo-mechanical behavior simulation coupled with the hydrostatic-pressure-dependent grain-scale fission gas swelling calculation for a monolithic UMo fuel plate under heterogeneous neutron irradiation
- Optimal Auxiliary Functions Method for viscous flow due to a stretching surface with partial slip
- Vibrations Analysis of Rectangular Plates with Clamped Corners
- Evaluating Lean Performance of Indian Small and Medium Sized Enterprises in Automotive Sector
- FPGA–implementation of PID-controller by differential evolution optimization
- Thermal properties and morphology of polypropylene based on exfoliated graphite nanoplatelets/nanomagnesium oxide
- A computer-based renewable resource management system for a construction company
- Hygrothermal Aging of Amine Epoxy: Reversible Static and Fatigue Properties
- The selected roof covering technologies in the aspect of their life cycle costs
- Influence of insulated glass units thickness and weight reduction on their functional properties
- Structural analysis of conditions determining the selection of construction technology for structures in the centres of urban agglomerations
- Selection of the optimal solution of acoustic screens in a graphical interpretation of biplot and radar charts method
- Subsidy Risk Related to Construction Projects: Seeking Causes
- Multidimensional sensitivity study of the fuzzy risk assessment module in the life cycle of building objects
- Planning repetitive construction projects considering technological constraints
- Identification of risk investment using the risk matrix on railway facilities
- Comparison of energy parameters of a centrifugal pump with a multi-piped impeller in cooperation either with an annular channel and a spiral channel
- Influence of the contractor’s payment method on the economic effectiveness of the construction project from the contractor’s point of view
- Special Issue Automation in Finland
- Diagnostics and Identification of Injection Duration of Common Rail Diesel Injectors
- An advanced teaching scheme for integrating problem-based learning in control education
- A survey of telerobotic surface finishing
- Wireless Light-Weight IEC 61850 Based Loss of Mains Protection for Smart Grid
- Smart Adaptive Big Data Analysis with Advanced Deep Learning
- Topical Issue Desktop Grids for High Performance Computing
- A Bitslice Implementation of Anderson’s Attack on A5/1
- Efficient Redundancy Techniques in Cloud and Desktop Grid Systems using MAP/G/c-type Queues
- Templet Web: the use of volunteer computing approach in PaaS-style cloud
- Using virtualization to protect the proprietary material science applications in volunteer computing
- Parallel Processing of Images in Mobile Devices using BOINC
- “XANSONS for COD”: a new small BOINC project in crystallography
- Special Issue on Sustainable Energy, Engineering, Materials and Environment
- An experimental study on premixed CNG/H2/CO2 mixture flames
- Tidal current energy potential of Nalón river estuary assessment using a high precision flow model
- Special Spring Issue 2017
- Context Analysis of Customer Requests using a Hybrid Adaptive Neuro Fuzzy Inference System and Hidden Markov Models in the Natural Language Call Routing Problem
- Special Issue on Non-ferrous metals and minerals
- Study of strength properties of semi-finished products from economically alloyed high-strength aluminium-scandium alloys for application in automobile transport and shipbuilding
- Use of Humic Sorbent from Sapropel for Extraction of Palladium Ions from Chloride Solutions
- Topical Issue on Mathematical Modelling in Applied Sciences, II
- Numerical simulation of two-phase filtration in the near well bore zone
- Calculation of 3D Coordinates of a Point on the Basis of a Stereoscopic System
- The model of encryption algorithm based on non-positional polynomial notations and constructed on an SP-network
- A computational algorithm and the method of determining the temperature field along the length of the rod of variable cross section
- ICEUBI2017 - International Congress on Engineering-A Vision for the Future
- Use of condensed water from air conditioning systems
- Development of a 4 stroke spark ignition opposed piston engine
- Development of a Coreless Permanent Magnet Synchronous Motor for a Battery Electric Shell Eco Marathon Prototype Vehicle
- Removal of Cr, Cu and Zn from liquid effluents using the fine component of granitic residual soils
- A fuzzy reasoning approach to assess innovation risk in ecosystems
- Special Issue SEALCONF 2018
- Brush seal with thermo-regulating bimetal elements
- The CFD simulation of the flow structure in the sewage pump
- The investigation of the cavitation processes in the radial labyrinth pump
- Testing of the gaskets at liquid nitrogen and ambient temperature
- Probabilistic Approach to Determination of Dynamic Characteristics of Automatic Balancing Device
- The design method of rubber-metallic expansion joint
- The Specific Features of High-Velocity Magnetic Fluid Sealing Complexes
- Effect of contact pressure and sliding speed on the friction of polyurethane elastomer (EPUR) during sliding on steel under water wetting conditions
- Special Issue on Advance Material
- Effect of thermo-mechanical parameters on the mechanical properties of Eurofer97 steel for nuclear applications
- Failure prediction of axi-symmetric cup in deep drawing and expansion processes
- Characterization of cement composites based on recycled cellulosic waste paper fibres
- Innovative Soft Magnetic Composite Materials: Evaluation of magnetic and mechanical properties
- Statistical modelling of recrystallization and grain growth phenomena in stainless steels: effect of initial grain size distribution
- Annealing effect on microstructure and mechanical properties of Cu-Al alloy subjected to Cryo-ECAP
- Influence of heat treatment on corrosion resistance of Mg-Al-Zn alloy processed by severe plastic deformation
- The mechanical properties of OFHC copper and CuCrZr alloys after asymmetric rolling at ambient and cryogenic temperatures

