Abstract
The influence of iron sulfide on the rate of cathodic and anodic processes of steels with ferrite, perlite and ferrite perlite structure was shown. It was established that the hydrogenation and corrosion rate of steels with different structures depends not only its structure, but also the nature of sulfide-containing products of corrosion. The well-known scheme of the influence of hydrogen sulfide on the processes of corrosion and hydrogenation of steel was supplemented by reactions of formation iron sulfides. They can affect the rate of recombination of hydrogen atoms and, respectively, its molation and absorption by metals.
1 Introduction
A large percentage of oil and gas, which are extracted in the world, contains hydrogen sulfide (Hernández-Espejel et al. 2010; Smith and Joosten 2006; Smith et al. 2011; Yavorskiy et al. 2016) and sulfur-containing organic compounds which can generate it (Benavides et al. 2007; Jacob et al. 2008). Hydrogen sulfide accelerates corrosion (Ren et al. 2005; Sun and Nesic 2007; Tang et al. 2010; Zhou et al. 2013) and causes hydrogen embrittlement (Khoma 2010; Marcus 2011; Radkevych and Pokhmurs’ kyi 2001; Woodtli and Kieselbach 2000) of steels according to steady views. Therefore, decreases exploitation resource of equipment and pipelines in gas-exploration and processing industries (Haibin and Zhenling 2010). It causes annual losses due to metal losses and accidents involving personnel accidents (NACE International 2016). The medium containing hydrogen sulfide is catalyzing the formation of hydrogen atoms on the steel surface. Its absorption is depended on chemical potential at the interface and diffusion rate, which is determined by the chemical composition and steel structure, temperature and mechanical factors, etc.
In hydrogen sulfide media corrosion promotes formation on steels of mackinawite, the crystalline lattice of which contains numerous defects (Vedage et al. 1993). It can be converted into more stable sulfides later. On Armco iron with increasing concentration of hydrogen sulfide and pH media in corrosion products prevail pyrite (before 2.0 mg/L, pH ∼ 3.0), troilite (2–20 mg/L, pH = 7.0) and kansite (more than 20 mg/L, pH = 11.0) (Sardisco and Pitts 1965).
The increase of temperature contributes the formation of sulfides more enriched by Sulfur in the following sequence: mackinawite – cubic FeS – troilite - pyrrhotite – pyrite (Shi et al. 2016). The protective properties of sulfides are increasing with increased sulfur content. Investigations of the sulfides composition in 5% NaCl solution at different pH and concentration of hydrogen sulphide 0.2…20 mM on ferrite steel X65 showed that the sulfide film is consisted of mackinawite and crystalline FeS. As the concentration of hydrogen sulfide and pH are increasing, the compactness and film thickness increases (Huang et al. 2017). Investigation of the sulfides composition on low carbon steel in chloride- and acetate-containing media with 1.0 MPa H2S showed that the corrosion products mainly consist of mackinawite and troilite. The blocking effect of sulfide films on hydrogen absorption is established (Zheng et al. 2013). Consequently, the composition of iron sulfides and their protective properties are depended on conditions: the structure and chemical composition of steel, the concentration of hydrogen sulfide, pH, temperature, pressure, etc. However, the effect of iron sulfides with different composition on the allocation and absorption of hydrogen by steels of different structures is not studied sufficiently.
The thickness and density of corrosion products can affect the diffusion of oxidants to the surface of the metal, and, consequently, the corrosion rate. Films presence can slow down the cathodic and anodic processes. So the formation of iron sulfides on the steels surface make effect on localization, corrosion, evolution and absorption of hydrogen, and determine the further their corrosion-mechanical destruction.
Thus, the determination of the influence of iron sulfides on the corrosion rate and hydrogenation of steels different structures is an extremely important issue in predicting behavior of metal in specific hydrogen sulfide environments.
2 Materials and methods
Armco iron, 0.8% C and 0.45% C steels were investigated. In order to obtain structures of equilibrium perlite (0.8% C steel) and ferrite-perlite (0.45% C steel) steels were annealed at T = 800 °С during 0.5 h. The chemical composition of samples is presented in Table 1 and microstructure of Armco iron and steels presented in Figure 1. Cylindrical samples were pressed into fluoroplastic bushes, then wet-ground with P1200 emery paper.
Chemical composition of samples.
| Sample | Element (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Ni | Cr | Cu | S | P | |
| Armco iron | ≤0.03 | ≤0.05 | ≤0.03 | – | – | – | 0.03 | 0.02 |
| 0.8% C steel | 0.80 | 0.19 | 0.2 | 0.15 | 0.18 | 0.20 | 0.02 | 0.02 |
| 0.45% C steel | 0.50 | 0.25 | 0.6 | 0.20 | 0.21 | 0.20 | 0.03 | 0.03 |

Microstructure of Armco iron (a), 0.8% C steel (b), and 0.45% C steel (c).
Electrochemical studies of the samples were carried out in the chloride-acetate solution, which is the base of the NACE solution (NACE Standard 1996), at a temperature of 18 ± 2 °C. All solutions were prepared based on distilled water. Electrode potentials were measured as difference between working electrode and silver chloride reference electrode and recalculated to the potential of the hydrogen electrode. Platinum was used as an auxiliary electrode. The rate of the cathodic and anodic processes was calculated based on voltage–current dependences and Tafel slopes.
Iron sulfides FeS2, FeS, and Fe9S8 were formed during 1 h after anodic polarization at E = −600 mV and concentrations of hydrogen sulfide 1, 10 and 100 mg/L in solutions with pH = 3.2; 7.2; 11.2, respectively. The H2S content was determined by iodometric titration method. The value of the hydrogen index of the working solutions was controlled by pH-meter pH-673.M with a glass electrode ESL-63-07. X-ray phase analysis of samples was used to identify iron sulfides on DRON 3.0 M diffractometer, as shown in Figure 2.

XDR diffractogram of iron sulfide samples obtained during 1 h after anodic polarization at E = −600 mV and concentrations of hydrogen sulfide 1: (a), 10 (b), and 100 (c) mg/L in solutions with pH = 3.2; 7.2; 11.2, respectively.

SEM images of systems «steel-sulfide»: Armco iron + FeS2 (a), Armco iron + FeS (b), Armco iron + Fe9S8 (c), 0.8% C steel + FeS2 (d), 0.8% C steel + FeS (e), 0.8% C steel + Fe9S8 (f), 0.45% C steel + FeS2 (g), 0.45% C steel + FeS (h), 0.45% C steel + Fe9S8 (i).
Electrolytic hydrogenation of the samples was performed at the same overvoltage E = −350 mV for 1 h duration in a solution of 0.5% CH3COOH + 5% NaCl, in which the composition of the formed sulfide films did not change. Due to the much higher rate of hydrogen depolarization against oxygen, it was assumed that all amount of electricity which was passed through the electrochemical cell corresponded to the process of hydrogen evolution. Amount of hydrogen calculated according to the Faraday’s law.
The concentration of absorbed hydrogen was determined by vacuum extraction at elevated temperatures (Chuchman 2014). Hydrogen desorbed at 200 °С could be release from the metal over time at room temperature, while the hydrogen that desorbed at 800 °С is characterized by higher metal-binding energy (Takai and Watanuki 2003). The error of determination on Armco iron, 0.8% C and 0.45% C steels of СН200 was ±0.14; ±0.10; ±0.16 ppm, and СН800 was ±0.16; ±0.20; ±0.25 ppm, respectively. The total concentration of hydrogen in steels and Armco iron was equal to the sum of hydrogen concentrations determined at different temperatures and after 1 h in СНΣ equal to СН200 + СН800 solutions.
3 Results and discussion
Corrosion of Armco iron and 0.8% C steel which at the absence of the iron sulfides in the freely aired 0.5% CH3COOH + 5% NaCl solution was run under mixed cathodic anodic control with rates of ∼29.18 and 37.85 mpy respectively, and 0.45% C steel – run under anodic control with rate of ∼0.218 mA/cm2. On armco iron pyrite (FeS2) decreases, and kansite (Fe9S8) increases the rate of cathodic and anodic reactions. Troilite (FeS) increases the cathodic, but reduces the anodic reaction. The FeS2 and FeS formation on Armco iron reduced the corrosion rate (determined by the anodic reactions) by ∼10 and 64%, respectively. And Fe9S8 increased in ∼2.4 times and run under mixed control (Table 2).
Rate of cathodic and anodic processes on Armco iron, 0.8% C and 0.45% C steels with iron sulfides on their surfaces.
| Electrode | Rate of cathodic and anodic processes (mpy) | |||||
|---|---|---|---|---|---|---|
| Armco iron | 0.8% C steel | 0.45% C steel | ||||
| Cathod. | Anod. | Cathod. | Anod. | Cathod. | Anod. | |
| Without iron sulfides | 29.18 | 32.83 | 38.30 | 40.13 | 153.22 | 99.41 |
| FeS2 | 19.15 | 10.03 | 46.05 | 9.58 | 150.48 | 423.17 |
| FeS | 41.04 | 25.54 | 44.23 | 5.02 | 133.61 | 179.66 |
| Fe9S8 | 69.31 | 66.57 | 43.78 | 16.87 | 354.77 | 112.63 |
The presence of FeS2, FeS, and Fe9S8 on 0.8% C steel led to decreasing of the corrosion rate in ∼4.0; 7.5; and 2.2 times, respectively. Sulfides contribute deceleration of the anodic rate and insignificant increasing of the cathodic reactions, resulted in corrosion run under anodic control. FeS2 and FeS on ferrite–perlite practically do not change the rate of cathodic reactions and increase the anodic in ∼4.0 and 1.8 times, respectively. The presence of Fe9S8 on steel increased the rate of cathodic processes in ∼2.3 times and did not change the rate of anodic. Corrosion of 0.45% C steel at the formation of FeS2 and FeS was run under cathodic, and Fe9S8 – under anodic control.
Electrochemical investigations of Armco iron with iron sulfides showed that in case of cathodic polarization (50 mV) of samples with FeS2 currents are decreasing in ∼1.5 times, and with FeS and Fe9S8 – increasing in ∼1.6 and ∼3.0 times, respectively (Figure 4a).
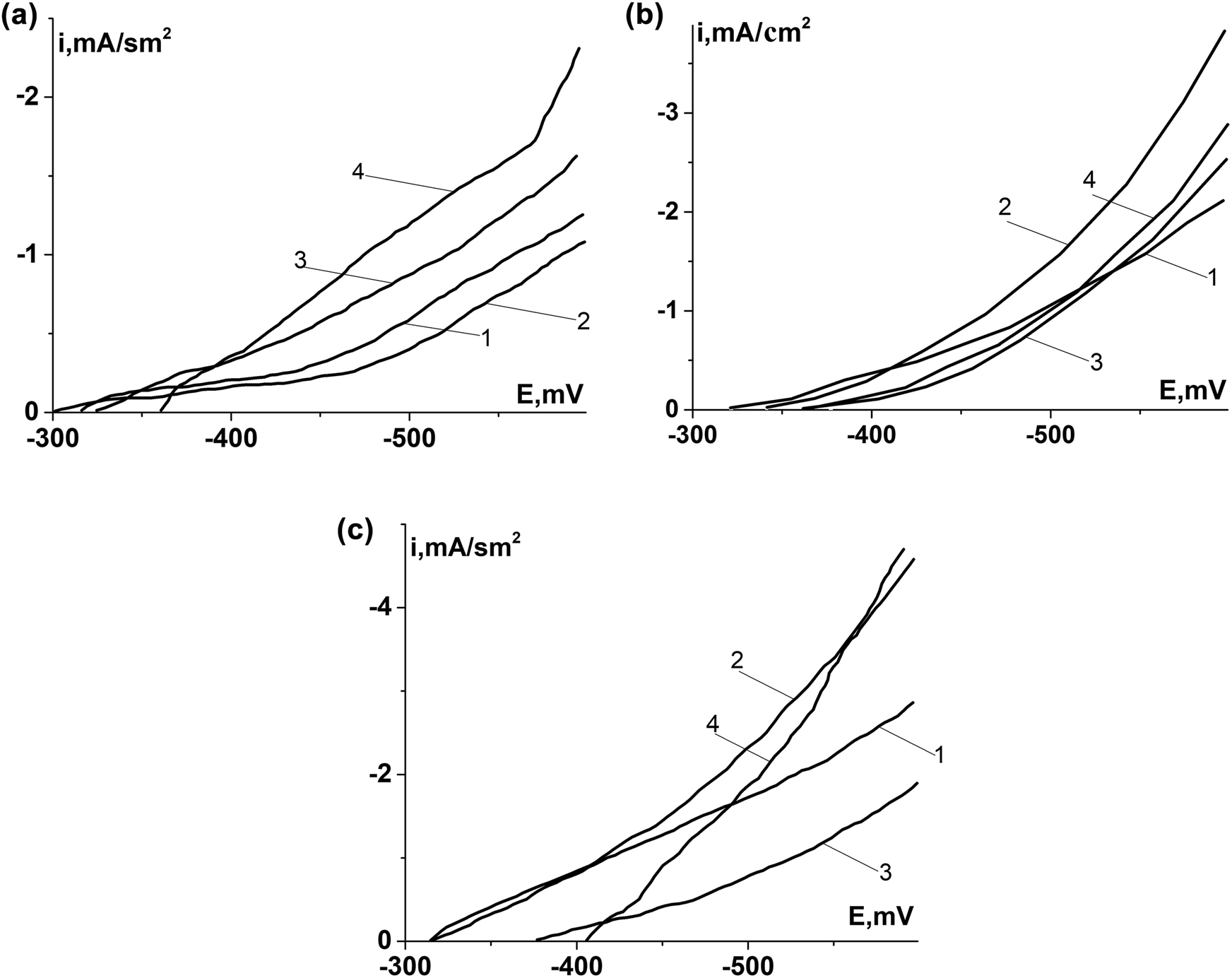
Cathodic polarization curves of Armco iron (a), 0.8% C steel (b), and 0.45% C steel (c) without sulfides (1), with iron sulfides on their surfaces (2 – FeS2; 3 – FeS; 4 – Fe9S8).
Cathodic currents in case of the same polarization on 0.8% C steel without sulfides were higher in ∼2.0 times than on Armco iron. The formation of Fe9S8 did not change the values, FeS – reduced in ∼1.2 times, and FeS2 – increased in ∼1.3 times (Figure 4b). Consequently, the influence of sulfides on cathodic processes was depends on steel structure. The presence of two phases in the 0.45% C steel – ferrite and perlite led to increasing of the cathodic currents: compared with Armco iron in ∼6.0 and 0.8% C steel in ∼3.0 times. This is caused by interaction of the two phases and the peculiarities of the formation of grain boundaries between them. The formation of FeS2 on 0.45% C steel did not affect cathodic polarization currents, FeS – reduced them in ∼2.1 times, and Fe9S8 – increased in ∼1.8 times (Figure 4c). In chloride-acetate solutions under cathodic polarization, Hydrogen evolution was a prevented reaction. The results indicate that its rate depends from structure of the steel and the chemical composition of iron sulfides on its surface. It can also be influenced by their adhesion and density (porosity).
The formation of FeS2 on Armco iron didn’t affect rate of anodic reactions. FeS and Fe9S8 at anodic polarization of 50 mV increased its rate in ∼2.4 and ∼2.6 times respectively (Figure 5a). At the absence of sulfides, the currents of anodic reactions on 0.8% C steel compared with Armco iron higher in 1.4 times. FeS2 and FeS led to decreasing of currents of the anodic processes in ∼6.7 and ∼3.2 times respectively, while Fe9S8 did not affect the rate (Figure 5b). Anodic reactions on 0.45% C steel over the same polarization run at maximum rate of 0.72 mA/cm2. FeS2 and Fe9S8 increased the rate of anodic reactions in ∼3.2 and ∼1.9 times, respectively, while the FeS reduced it in ∼3.0 times (Figure 5c). Consequently the influence of sulfides on rate of anodic processes was ambiguous and determined by the structure of steels and the nature of the sulfides on their surfaces.
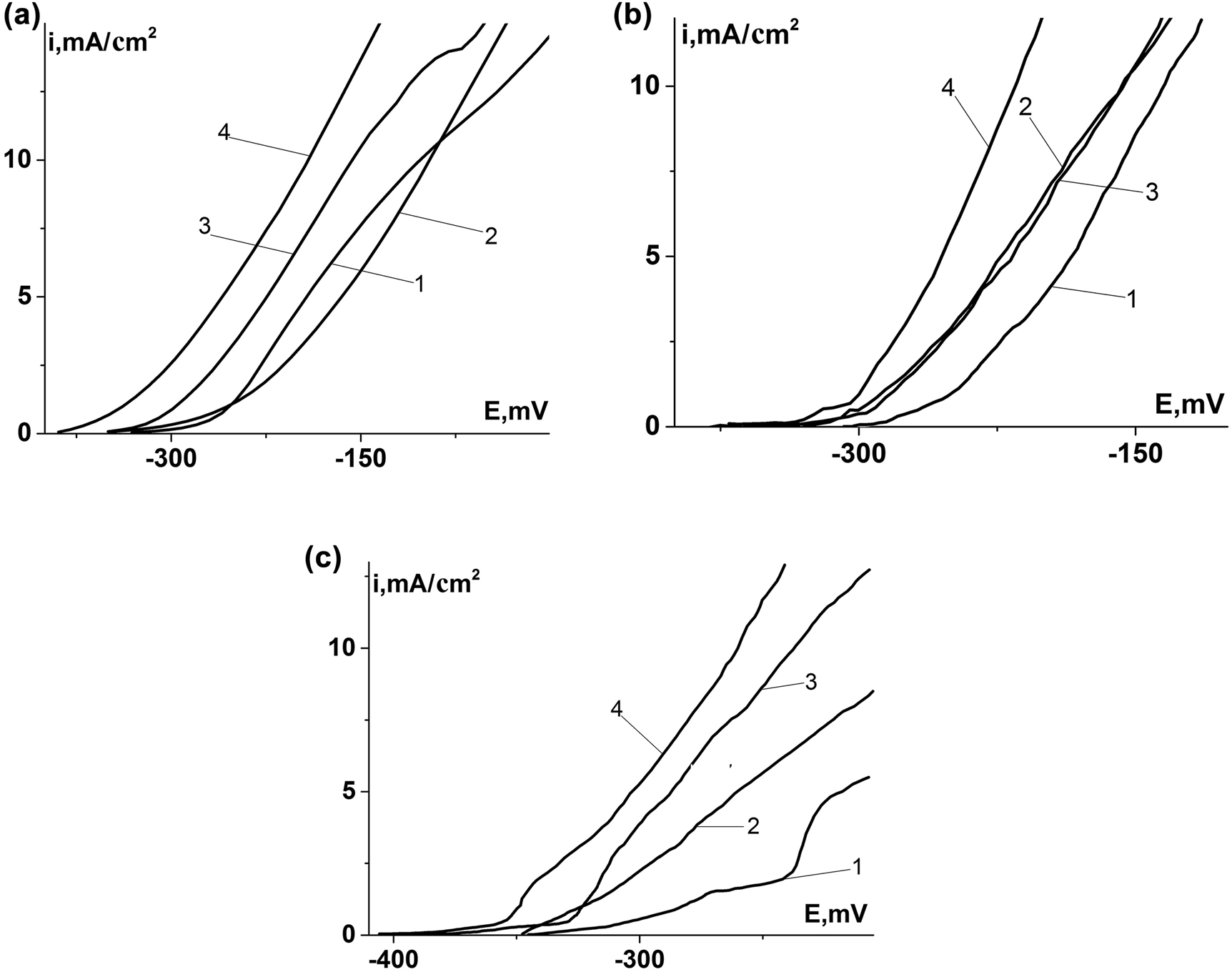
Anodic polarization curves of Armco iron (a), 0.8% C steel (b), and 0.45% C steel (c) without sulfides (1), with iron sulfides on their surfaces (2 – FeS2; 3 – FeS; 4 – Fe9S8).
It was established that is due to electrolytic hydrogenation of the investigated metals iron sulfides on their surface increased the volume of generated hydrogen (Figure 6). But FeS on the 0.8% C steel did not affect these processes. The most significant increase of hydrogen amount was observed in case of formation Fe9S8 on steels. At the same time, the volume of absorbed hydrogen also increased, excepted of pyrite and troilite on Armco iron and pyrite on 0.8% C steel (Figure 6b). At the presence of Fe9S8 on Armco iron hydrogen is reduced more than in ∼7.1 times, and hydrogenation increased only in ∼2.1 times. On 0.8% C and 0.45% C steels, where the volume of released hydrogen increases at presence of Fe9S8 in ∼3.8, volume of adsorbed hydrogen higher in ∼1.4 times.
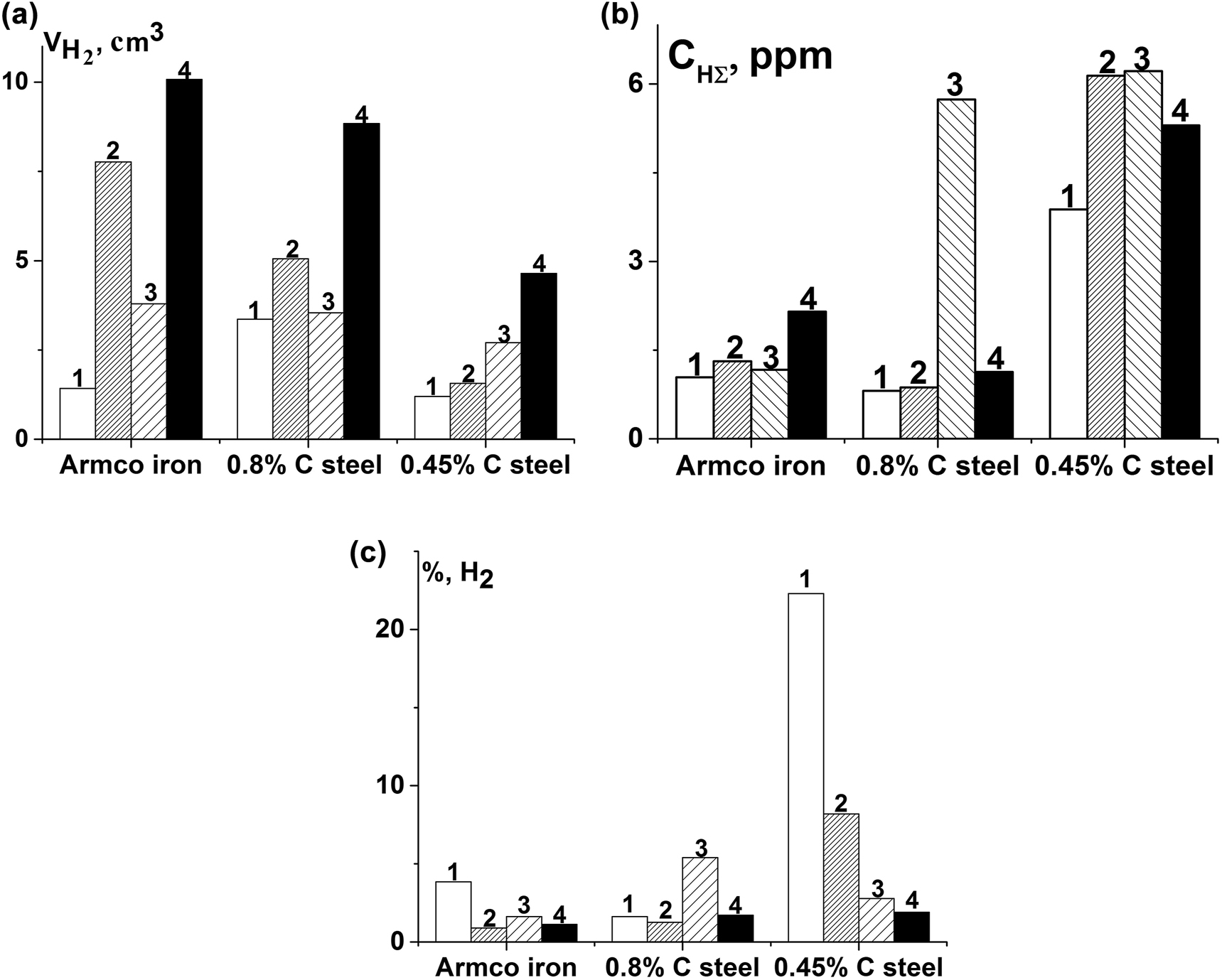
The volume of generated hydrogen in the process of electrolytic hydrogenation (a) and absorbed hydrogen (b), part of absorbed hydrogen to its total amount, released under corrosion (c) without sulfides (1), and their presence on Armco iron, 0.8% C steel and 0.45% C steel (2 – FeS2; 3 – FeS; 4 – Fe9S8).
The part of absorbed hydrogen on Armco iron, at the presence of sulfides, reduces in ∼2.4…4.3, while on 0.45% C steel in ∼2.7…11.8 times (Figure 6c). On 0.8% C steel their influence was different: FeS2 and Fe9S8 slightly reduced the proportion of absorbed hydrogen, and FeS increased it in ∼3.3 times. However, this is not reduced the hydrogenation of the investigated metals. Iron sulfides differently affect the rate of individual stages of hydrogen release and can inhibit the absorption of hydrogen. Its evolution on steels consists of several stages. The first stage occurs without inhibition – the delivery of participants of the electro-chemical reaction to the metals surface. After that they are discharging and adsorbed atoms are forming. Such atoms can form molecular hydrogen or diffuse into metal. Sulfides preferentially increase the volume of the generated and absorbed hydrogen. It could be assumed that they slow down the reaction of catalytic recombination of hydrogen atoms, which occurs simultaneously with its desorption. The catalyst of this process was sulfide formed on the surface.
The mechanism of the influence of hydrogen sulfide on hydrogen absorption remains controversial. This is due to the large number of oxidation–reduction reactions, which can run on the surface of steels and the nature of transition complexes; corrosion products. The influence of hydrogen sulfide on the rate of anodic reactions based on literature data could be presented as follows: at the beginning of the interaction of steel with a hydrogen sulfide medium, a surface catalytically-active complex formed (Hansson et al. 2006; Sherar et al. 2011). Future, its oxidation run into
However, in case of steel corrosion in hydrogen sulfide media a number of sulfides can form, which solubility equilibrium is SPFeS = 3.8·10−20, SPFe2S3 ∼ 10−88 (Wypych 2019). Consequently, when concentrations of sulfide ions more than ∼2.58 10−53 mol/L can be formed sulfides on steel, which also affect the corrosion rate and reaction of hydrogen evolution, which is very sensitive to the surface state on which this process happened.
The influence of hydrogen sulfide on the rate of cathodic reactions based on its electrochemical reaction according to Shoesmith et al. (1980):
or electrochemical (Bolmer 1965; Kittel et al. 2013):
According to the hypothesis of surface catalysts the formation of adsorbed Hydrogen atoms occurs when the complex
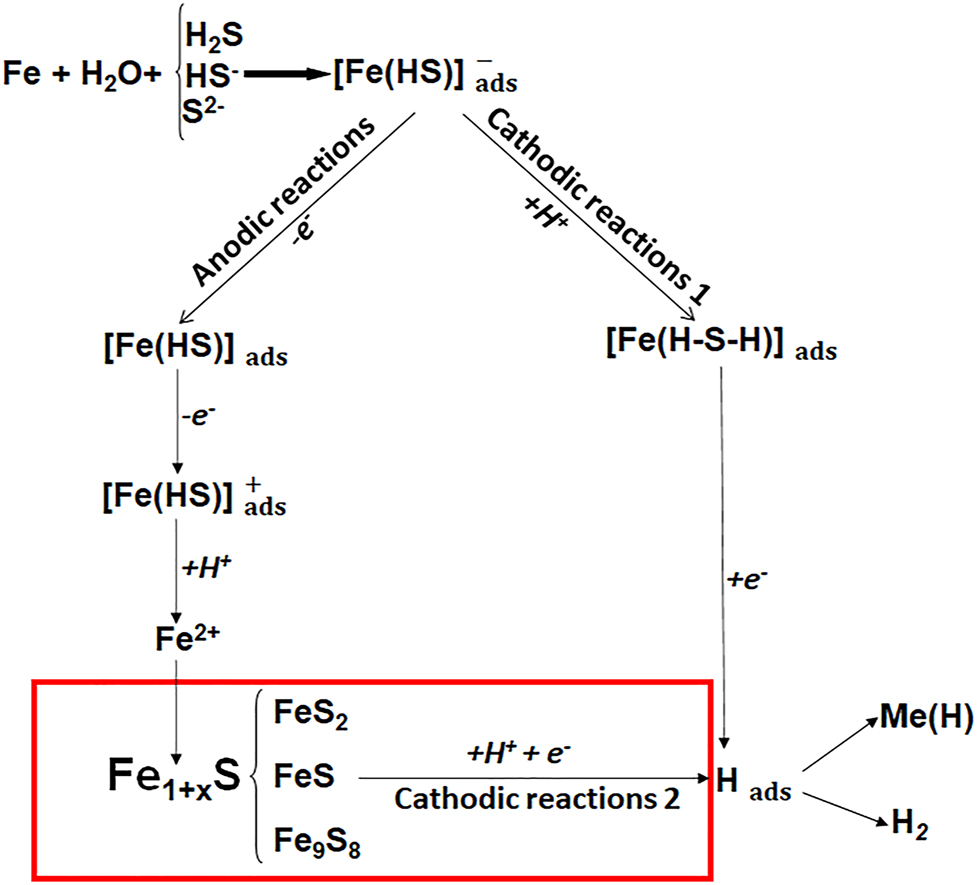
A well-known scheme of electrode processes behind corrosion of steels in hydrogen sulfide media.
According our generalization of the well-known scheme the Fe2+ ions interact with sulfide ions and an unstable mackinawite is formed, which future, depends on the concentration of hydrogen sulfide in the medium, transform into pyrite, troilite, or kansite. Confirm our hypothesis small values of the solubility equilibrium of iron sulfides and the layer formation of sulfide-containing corrosion products on steels used in hydrogen sulfide-containing media. Cathodic reaction with the formation of adsorbed hydrogen atoms, depends on the adhesion and porosity of iron sulfides formed on the steel surface can run on the surfaces (cathodic reaction 2) or on steel (cathodic reaction 1). They can form molecular hydrogen as the result of catalytic recombination or electrochemical desorption or diffuse into metal, causing hydrogen embrittlement of steels. Taking into account that sulfides increase the amount of generated and absorbed hydrogen, it is possible to assume their slowing effect on the formation of molecular hydrogen. As a result, the surface concentration of atomic hydrogen and its chemical potential increases.
4 Conclusions
Armco iron in chloride-acetate solutions corrodes under the cathodic control, 0.8% C steel – under the mixed, and 0.45% C steel – under the anodic. The formation of iron sulfides on its surfaces leads to a change in the nature of limiting stage of corrosion. At the presence of pyrite and troilite on Armco iron it run under the anodic with a lower rate, and the kansite – cathodic–anodic control at a higher rate. On 0.8% C steel iron sulfides, regardless of its chemical composition, increase the efficiency of cathodic processes, but reduce the corrosion rate, which is determined by the anodic reactions. At the presence of pyrite and troilite on the surface 0.45% C steel, corrosion run under the cathodic control, and the kansite – anodic. The formation of sulfides on its surface led to the increase of corrosion rate. Consequently, the influence of sulfides on the rate of oxidation–reduction processes depends on the structure of steels.
It was established that pyrite and troilite does not affect the hydrogenation of Armco iron, and the kansite increases it by ∼50%. 0.8% C steel with kansite and troilite absorbs about 1.4 and ∼7.1 times more hydrogen and its content did not change with pyrite. The sulfides formation on 0.45% C steel increases it hydrogenation by ∼36…60%. Consequently, the hydrogenation of Armco iron and 0.8% C and 0.45% C steels was determined not only by its structure, but also by the nature of sulfide-containing corrosion products on the surface, which can slow down the catalytic recombination of hydrogen atoms.
A well-known scheme of electrode processes at steel corrosion in hydrogen sulfide media was generalized. It took into account formation of unstable sulfide of mackinawite with its subsequent transformation into pyrite, troilite, and kansite. They will affect kinetic of the cathodic reaction of formation adsorbed hydrogen atoms and its molation due to cathodic reaction or electrochemical desorption. These processes determine the chemical potential of adsorbed hydrogen atoms and its absorption by steels.
-
Author contributions: Conceptualization, Myroslav Khoma; data curation, Vasyl Ivashkiv, Мarian Chuchman, Bogdan Datsko; formal analysis, Svitlana Halaichak, Vasyl Ivashkiv; funding acquisition, Мarian Chuchman; investigation, Svitlana Halaichak, Bogdan Datsko; methodology, Svitlana Halaichak, Vasyl Ivashkiv, Мarian Chuchman; validation, Myroslav Khoma, Bogdan Datsko; writing – original draft, Myroslav Khoma; writing – review & editing, Myroslav Khoma.
-
Research funding: The authors would like to thank the National Academy of Sciences of Ukraine for financial support under project “Study of the processes of hydrogenation of steels of various structures in mineralized hydrogen sulfide media, taking into account the effects of mechanical stresses” (state registration no. 0115U000124).
-
Conflicts of interest: The authors declare that they have no conflicts of interest regarding this article.
References
Benavides, G.A., Squadrito, G.L., Mills, R.W., Patel, H.D., Isbell, T.S., Patel, R.P., Darley-Usmar, V.M., Doeller, J.E., and Kraus, D.W. (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 104: 17977–17982, https://doi.org/10.1073/pnas.0705710104.Search in Google Scholar PubMed PubMed Central
Bolmer, P.W. (1965). Polarization of iron in H2S-NaHS buffers. Corrosion 21: 69–75, https://doi.org/10.5006/0010-9312-21.3.69.Search in Google Scholar
Chuchman, M.R. (2014). Hydrogenation of pipe steels under the action of static and cyclic stresses in hydrogen sulphide environment. Fiz.-Khim. Mekh. Mater. 1: 163–168 (in Ukrainian).Search in Google Scholar
Haibin, L. and Zhenling, L. (2010). Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 54: 1331–1340, https://doi.org/10.1016/j.resconrec.2010.05.005.Search in Google Scholar
Hansson, E.B., Odziemkowski, M.S., and Gillham, R.W. (2006). Formation of poorly crystalline iron monosulfides: surface redox reactions on high purity iron, spectroelectrochemical studies. Corrosion Sci. 48: 3767–3783, https://doi.org/10.1016/j.corsci.2006.03.010.Search in Google Scholar
Hernández-Espejel, A., Dominguez-Crespo, M.A., Cabrera-Sierra, R., Rodríguez-Meneses, C., and Arce-Estrada, E.M. (2010). Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corrosion Sci. 52: 2258–2267, https://doi.org/10.1016/j.corsci.2010.04.003.Search in Google Scholar
Huang, F., Cheng, P., Zhao, X.Y., Liu, J., Hu, Q., and Cheng, Y.F. (2017). Effect of sulfide films formed on X65 steel surface on hydrogen permeation in H2S environments. Int. J. Hydrogen Energy 42: 4561–4570, https://doi.org/10.1016/j.ijhydene.2016.10.130.Search in Google Scholar
Jacob, C., Anwar, A., and Burkholz, T. (2008). Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med. 74: 1580–1592, https://doi.org/10.1055/s-0028-1088299.Search in Google Scholar PubMed
Khoma, M.S. (2010). Problems of fracture of metals in hydrogen-sulfide media. Mater. Sci. 46: 190–200, https://doi.org/10.1007/s11003-010-9277-1.Search in Google Scholar
Kittel, J., Ropital, F., Grosjean, F., Sutter, E.M.M., and Tribollet, B. (2013). Corrosion mechanisms in aqueous solutions containing dissolved H2S. Part 1: characterisation of H2S reduction on a 316L rotating disc electrode. Corrosion Sci. 66: 324–329, https://doi.org/10.1016/j.corsci.2012.09.036.Search in Google Scholar
Marcus, P. (Ed.) (2011). Corrosion mechanisms in theory and practice. Boca Raton: CRC Press.10.1201/b11020Search in Google Scholar
NACE International. (2016). NACE study estimates global cost of corrosion at $2.5 trillion annually, Available at: https://inspectioneering.com/news/2016-03-08/5202/nace-study-estimates-global-cost-ofcorrosion-at-25-trillion-ann.Search in Google Scholar
NACE Standard TM0177-96 (1996). Laboratory testing of metals for resistance to specific forms of environmental cracking in H2S environment (TM0177-96).Search in Google Scholar
Radkevych, O.I. and Pokhmurs’ kyi, V.I. (2001). Influence of hydrogen sulfide on serviceability of materials of gas field equipment. Mater. Sci. 37: 319–332, https://doi.org/10.1023/A:1013275129001.10.1023/A:1013275129001Search in Google Scholar
Ren, C., Liu, D., Bai, Z., and Li, T. (2005). Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. Phys. 93: 305–309, https://doi.org/10.1016/j.matchemphys.2005.03.010.Search in Google Scholar
Sardisco, J.B. and Pitts, R.E. (1965). Corrosion of iron in an H2S–CO2–H2O system composition and protectiveness of the sulfide film as a function of pH. Corrosion 21: 350–354, https://doi.org/10.5006/0010-9312-21.11.350.Search in Google Scholar
Sherar, B.W.A., Power, I.M., Keech, P.G., Mitlin, S., Southam, G., and Shoesmith, D.W. (2011). Characterizing the effect of carbon steel exposure in sulfide containing solutions to microbially induced corrosion. Corrosion Sci. 53: 955–960, https://doi.org/10.1016/j.corsci.2010.11.027.Search in Google Scholar
Shi, F., Zhang, L., Yang, J., Lu, M., Ding, J., and Li, H. (2016). Polymorphous FeS corrosion products of pipeline steel under highly sour conditions. Corrosion Sci. 102: 103–113, https://doi.org/10.1016/j.corsci.2015.09.024.Search in Google Scholar
Shoesmith, D.W., Taylor, P., Bailey, M.G., and Owen, D.G. (1980). The formation of ferrous monosulfide polymorphs during the corrosion of iron by aqueous hydrogen sulfide at 21 °C. J. Electrochem. Soc. 127: 1007.10.1149/1.2129808Search in Google Scholar
Smith, S.N. and Joosten, M.W. (2006). Corrosion of carbon steel by H2S in CO2 containing oilfield environments, March 2006: corrosion 2006. San Diego, CA: One Petro.10.5006/C2006-06115Search in Google Scholar
Smith, S.N., Brown, B., and Sun, W. (2011). Corrosion at higher H2S concentrations and moderate temperature, March 2011: corrosion 2011. Houston, TX: One Petro.10.5006/C2011-11081Search in Google Scholar
Sun, W. and Nesic, S. (2007). A mechanistic model of H2S corrosion of mild steel, March 2007: corrosion 2007. Nashville, TN: One Petro.10.5006/C2007-07655Search in Google Scholar
Takai, K. and Watanuki, R. (2003). Hydrogen in trapping states innocuous to environmental degradation of high-strength steels. ISIJ Int. 43: 520–526, https://doi.org/10.2355/isijinternational.43.520.Search in Google Scholar
Tang, J., Shao, Y., Guo, J., Zhang, T., Meng, G., and Wang, F. (2010). The effect of H2S concentration on the corrosion behavior of carbon steel at 90 °C. Corrosion Sci. 52: 2050–2058, https://doi.org/10.1016/j.corsci.2010.02.004.Search in Google Scholar
Vedage, H., Ramanarayanan, T.A., Mumford, J.D., and Smith, S.N. (1993). Electrochemical growth of iron sulfide films in H2S-saturated chloride media. Corrosion 49: 114–121, https://doi.org/10.5006/1.3299205.Search in Google Scholar
Woodtli, J. and Kieselbach, R. (2000). Damage due to hydrogen embrittlement and stress corrosion cracking. Eng. Fail. Anal. 7: 427–450, https://doi.org/10.1016/S1350-6307(99)00033-3.Search in Google Scholar
Wypych, G. (Ed.) (2019). Handbook of solvents, 3rd ed. Toronto: ChemTec Publishing.10.1016/B978-1-927885-45-1.50005-8Search in Google Scholar
Yavorskiy, V., Slyuzar, A., and Kalymon, J. (2016). Sulfur gas production in Ukraine. Chem. Chem. Technol. 10: 613–620, https://doi.org/10.23939/chcht10.04si.613.Search in Google Scholar
Zheng, S., Liu, L., Zhou, C., Chen, L., and Chen, C. (2013). Effects of H2S-containing corrosive media on the crystal structures of corrosion product films formed on L360NCS. Int. J. Electrochem. Sci. 8: 1434–1442.10.1016/S1452-3981(23)14109-5Search in Google Scholar
Zhou, C., Zheng, S., Chen, C., and Lu, G. (2013). The effect of the partial pressure of H2S on the permeation of hydrogen in low carbon pipeline steel. Corrosion Sci. 67: 184–192, https://doi.org/10.1016/j.corsci.2012.10.016.Search in Google Scholar
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editor’s Note
- A demonstration of resilience
- Reviews
- Scanning electrochemical microscopy methods (SECM) and ion-selective microelectrodes for corrosion studies
- Corrosion inhibitors for AA6061 and AA6061-SiC composite in aggressive media: a review
- Original Articles
- Oxidation–reduction reactions and hydrogenation of steels of different structures in chloride-acetate solutions in the presence of iron sulfides
- The golden alloy Cu5Zn5Al1Sn: effect of immersion time and anti-corrosion activity of cysteine in 3.5 wt% NaCl solution
- Corrosion inhibition of hydrazine on carbon steel in water solutions with different pH adjusted by ammonia
- Comparison of protective coatings prepared from various trialkoxysilanes and possibilities of spectroelectrochemical approaches for their investigation
- Corrosion behaviour OF HVOF deposited Zn–Ni–Cu and Zn–Ni–Cu–TiB2 coatings on mild steel
Articles in the same Issue
- Frontmatter
- Editor’s Note
- A demonstration of resilience
- Reviews
- Scanning electrochemical microscopy methods (SECM) and ion-selective microelectrodes for corrosion studies
- Corrosion inhibitors for AA6061 and AA6061-SiC composite in aggressive media: a review
- Original Articles
- Oxidation–reduction reactions and hydrogenation of steels of different structures in chloride-acetate solutions in the presence of iron sulfides
- The golden alloy Cu5Zn5Al1Sn: effect of immersion time and anti-corrosion activity of cysteine in 3.5 wt% NaCl solution
- Corrosion inhibition of hydrazine on carbon steel in water solutions with different pH adjusted by ammonia
- Comparison of protective coatings prepared from various trialkoxysilanes and possibilities of spectroelectrochemical approaches for their investigation
- Corrosion behaviour OF HVOF deposited Zn–Ni–Cu and Zn–Ni–Cu–TiB2 coatings on mild steel

