Abstract
Cadmium (Cd) accumulates in the brain and can damage neurons via complex processes involving oxidative stress induction. In this study we used a homogenous population of neurons which are cerebellar granule neurons (CGNs) to investigate damage induced by Cd and its effects on antioxidant enzyme activity. The exposure of CGNs to increasing concentrations of Cd (2.5 μM-100 μM) during 24 h, 48 h, or 72 h led to the induction of neuronal death in a dose- and exposure time-dependent manner. The necrotic and/or apoptotic pathway involved in the cell death trigged by Cd seems to depend on the concentration of Cd and the exposure time. In addition to its cell damage, Cd was shown to affect the activity of superoxide dismutase (SOD) and catalase (CAT) depending on the concentration of Cd and the exposure time. We also found that the exposure to Cd induces a bigger change in SOD activity than in CAT activity. Taken together, our findings explain, in part, the mechanism of Cd toxicity in a specific type of neuron which can provide information related to neurological pathologies ascribed to Cd toxicity.
1 Introduction
Cadmium (Cd) is a toxic heavy metal that accumulates in the atmosphere, and it is produced by the gradual processes of erosion and abrasion of rocks and soils, caused by events such as forest fires and volcanic eruptions [1]. Currently, this metal is mainly used in the manufacturing of nickel-Cd batteries, for the production of pigments and plastic stabilizers, and in agriculture [2]. Human Cd poisoning is mainly the result of smoking and industrial pollution [2, 3]. The potential health effects of Cd on humans have attracted a lot of attention over the years, since studies showed that Cd accumulates in tissues and has a very long biological half-life [4]. In humans and other mammals, acute Cd intoxication leads to the development of lesions in a number of organs and tissues, such as the liver, kidneys, lung, pancreas, testes, and bones [4]. In the brain, the accumulation of Cd causes very serious toxic effects [5], and leads to the development of several neurological disorders in workers exposed to Cd, such as hyperactivity, memory loss, and learning difficulties [6, 7, 8]. In addition, it was suggested that a cause-effect relationship exists between Cd exposure and amyotrophic lateral sclerosis in workers exposed to Cd in nickel-Cd battery factories [9].
Oxidative stress has been proposed as the most important mechanism underlying the toxic effects of Cd in many organs, including the brain [10, 11]. It is defined as an alteration in the equilibrium state between oxidant and antioxidant agents in cells. Several studies demonstrated that Cd itself is unable to generate free radicals directly, but it is responsible for indirect generation of reactive oxygen species (ROS), including superoxide radical and hydroxyl radical [5, 12, 13]. Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidases (GPx) are involved in the defence against metal oxidative stress [13]. In the central nervous system, the levels of antioxidant enzymes were shown to be increased in astrocytes and cortical neurons treated with Cd [11, 14, 15, 16]. Similarly, Cd administration to rats was demonstrated to lead to an increase in the activities of CAT, SOD, and GPx [17, 18]. However, several other studies demonstrated that the activities of antioxidant enzymes decrease in rat brains treated with Cd [19, 20, 21], indicating that the mechanisms underlying Cd-induced oxidative stress, cellular response, and the putative roles of antioxidant enzymes remain controversial [13]. To date, in vitro studies examining the effects of Cd on neurons were performed exclusively using cortical neurons, a non-homogenous population of cells. Therefore, the use of this cellular model may be the reason for to the variety of responses obtained by Cd treatment, depending on the cell type.
In order to assess the effects of Cd on a homogenous population of neurons, we investigated the cytotoxic effects of Cd on cerebellar granule neurons (CGNs) considering the role of antioxidant enzymes.
2 Experimental Procedures
2.1 Animals
Wistar rats (Pasteur Institute, Tunis) were kept in a temperature controlled room (21± 1°C) under an established photoperiod (12 h/12 h light/dark cycle) with free access to food and water.
Ethical approval: The research related to animals use has been complied with all the relevant national regulations and institutional policies for the care and use of animals. All experiments were performed according to the recommendations of the Ethics Committee of Tunis University for Care and Use of Animals, which conformed to the NIH guidelines (approval number: FST/LNFP/ Pro152012).
2.2 Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), Ham’s F-12, insulin, L-glutamine, antibiotic-antimycotic solution, fetal bovine serum (FBS) and trypsin-EDTA buffer were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cytosine-β-D-arabinofuranoside (β- ARAC), trypsin, DNase, trypsin inhibitor, bovine serum albumin (BSA), CdCl2 were purchased from Sigma-Aldrich (St. Louis, MO, USA). 5-6-Chloromethyl-2″, 7″-dichlorodihydrofluorescein diacetate acetyl ester (FDA) was obtained from Molecular Probes (Eugene, Oregon, USA), while lactate dehydrogenase (LDH) assay kit was obtained from Bio-Maghreb (Tunis, Tunisia). Bovine liver catalase and DL-epinephrine were kindly provided by Professor F. Limam (National Science and Technology Park, Borj Cedria, Tunisia).
2.3 Cell culture
CGN suspensions were prepared from the cerebellums of 8-day-old Wistar rats, as described previously [22]. Briefly, freshly dissected cerebellums were dissociated in the presence of trypsin and DNase I and seeded on poly-L-lysine coated dishes. Cells were seeded at a density of 3.5 x106 cells/mL in basal medium. For all experiments, cells were cultured in a chemically defined medium, consisting of 75% DMEM and 25% Ham’s F-12, supplemented with 10% FBS, 2 mM glutamine, 25 mM KCl, and 1% antibiotic solution. Cells were grown at 37°C in a humidified incubator with an atmosphere of 5% CO2/95% air. After 24 h of incubation, β-ARAC was added to the medium.
2.4 Cell survival
Seven-day-old CGNs were incubated at 37°C in the absence or presence of different concentrations of CdCl2 (2.5, 5, 7.5, 10, 15, 20, 50 and 100 μM) for 24 h, 48 h and 72 h. Following this period, the cells were incubated in the dark with FDA (15μg/ mL, 8 min), rinsed once with phosphate-buffered saline (PBS), and lysed with Tris-HCl solution containing 1% sodium dodecyl sulphate (SDS). Fluorescence intensity (l excitation = 485 nm and l emission = 528 nm) was measured with a FL800 TBI fluorescence microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
2.5 Cell cytotoxicity measurements
After the incubation of isolated CGNs for 7 days, they were incubated at 37°C in the fresh serum-free medium, in the absence or presence of various concentrations of CdCl2 (2.5, 5, 7.5, 10, 15, 20, 50 and 100 μM) for 24 h, 48 h and 72 h. Following the incubation, the cytotoxicity of Cd against CGNs was determined by measuring LDH activity in medium using LDH assay kit according to the manufacturer’s instructions. The results were expressed as a percentage of total LDH release after cell lysis with 1% Triton X-100 in PBS (0.1 M, pH 7.4).
2.6 Examination of cell morphology
Seven days after the isolation of CGNs, they were treated as described in the previous subsections for 24 h, 48 h, and 72 h. Following the incubation, culture medium was removed and cells were rinsed twice with the same medium. Cell morphology was observed using an inverted microscope (Axiostar plus, Zeiss, Göttingen, Germany) equipped with a Canon Power Shot A640 photo camera.
2.7 Antioxidant enzyme activity measurements
After incubating 7-day-old CGNs as described, culture medium was removed, and cells were washed twice with PBS and homogenized in the same solution at 4°C. Cells were harvested by centrifugation (350 xg, 4°C, 10 min) and the cell pellets were resuspended in 50 μL of ice-cold lysis buffer, containing 50 mM Tris-HCl (pH 8), 10 mM EDTA, 100 μM phenylmethyl-sulfonylfluoride and 1% Triton X-100. Then, the samples were centrifuged (16,000 xg, 20 min, 4°C) and the collected supernatants were stored at -20°C until the enzyme activity analyses. The activity of SOD was measured using a spectrophotometric assay, in which epinephrine autoxidation induced by superoxide anion is measured. Samples, prepared as described above, were incubated for 3 min with a mixture containing bovine catalase (0.4 U/μL), epinephrine (5 mg/mL), and Na2CO3/NaHCO3 buffer (62.5 mM, pH 10.2). The oxidation of epinephrine was measured at 480 nm with a Bio-Rad spectrophotometer (Bio-Rad Laboratories, Philadelphia, USA). CAT activity was determined by measuring the decrease in H2O2 levels. Samples were mixed with 30 mM H2O2 in PBS. The disappearance of H2O2 was measured at 240 nm for 180 s, at 30 s intervals. CAT activity was calculated using the extinction coefficient of 40 mM-1 cm-1 for H2O2.
2.8 Statistical analysis
Data are expressed as mean ± standard error of mean (SEM) obtained in three independent experiments. ANOVAs, followed by Bonferroni’s test, were used for the analyses. p < 0.05 was considered statistically significant.
3 Results
3.1 The effects of Cd on CGN survival
We examined the effects of different concentrations of CdCl2 on the induction of cell death, and the obtained results demonstrate that Cd shows a significant effect on cell viability. After 24 h of treatment with 10 μM of Cd, the percentage of viable cells was 50% (Fig. 1A), while the incubation of CGNs with different concentrations of Cd for longer time (48 h and 72 h) resulted in a drastic decrease in the percentage of viable cells. After 72 h of incubation, the viability of cells treated with 5 μM of Cd decreased to approximately 6% (Fig. 1C).

Effect of different concentrations of Cd on the survival of CGNs after 24 h (A), 48 h (B), and 72 h (C) of treatment. The results are expressed as the percentage of control cell (0 μM Cd) viability (Ctrl). Each value represents the mean ± SEM of at least four technical replicates obtained in three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Additionally, we have evaluated Cd-cytotoxicity, and the results are presented in Figure 2. LDH release was shown to increase significantly after 24 h and 48 h of the incubation of cells with Cd. This effect was dose-dependent at concentrations ranging from 2.5 to 10 μM, while, at the concentration of 100 μM, LDH release was significantly reduced in comparison with the control (Fig. 2A and 2B). Furthermore, the incubation of cells with different concentrations of Cd for 72 h led to a reduction in LDH release in comparison with the control sample (Fig. 2C).

Effect of Cd treatment on the activity of LDH in CGNs. Cells were treated with Cd for 24 h (A), 48 h (B), and 72 h (C). The values represent mean ± SEM of the measurements obtained from at least 10 technical replicates in three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; NS, no significant difference compared with the control (0 μM Cd) (Ctrl).
3.2 Cd-induced morphological alterations of CGNs
The examination of cells using phase-contrast microscopy revealed regular-shaped untreated, control cells, with granular bodies and long neurites organized in a network (Fig. 3A). In contrast to this, CGNs treated with Cd showed considerable morphological changes that indicated cell death, such as the shrinkage of cell bodies, nuclear condensation, reduction in the neurite network, and decreased number of viable cells (Fig. 3B).

Cd-induced alterations in CGN morphology. (A) Control cells (0 μM Cd). (B) Cells incubated with Cd (50 μM) for 24 h.
3.3 Effects of Cd on SOD and CAT activities in CGNs
In Figure 4, the obtained results, showing a considerable reduction in the activity of SOD after 24 h, 48 h, or 72 h treatment with Cd, are presented. This effect was observed after 24 h of cell incubation with Cd concentrations ranging from 10 to 100 μM, while after 48 h of treatment, the concentrations of Cd as low as 5 μM were shown to affect SOD activity. Overall, SOD activity decreased about 50% compared with its activity in the control samples. Furthermore, our results demonstrate that in the cells incubated with 50 or 100 μM of Cd for 72 h, SOD activity was markedly reduced (≈ 88%) (Fig. 4C). CAT activity was shown to be significantly reduced in CGNs treated with varying concentrations of Cd for different times as well. However, lower concentrations of Cd (2.5 μM and 5 μM) did not have the same effect on CAT activity (Fig. 5).
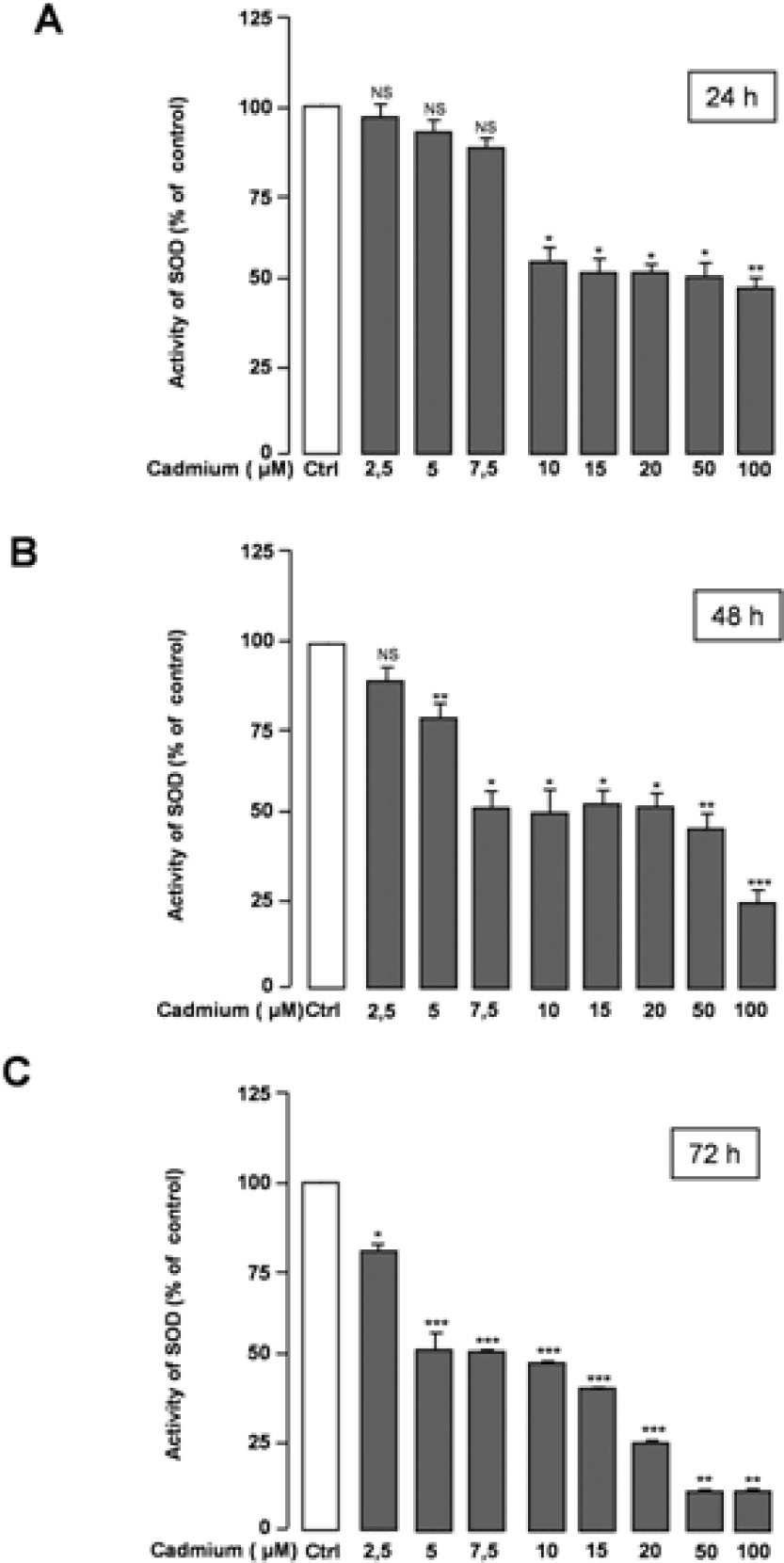
Effects of Cd treatment on SOD activity in CGNs.The obtained results are expressed as the percentage of SOD activity with respect to the control values (Ctrl). The value is presented as mean ± SEM of the measurements obtained from at least four technical replicates in three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; NS, no significant difference compared with the control (0 μM Cd).

Effects of Cd treatment on CAT activity in CGNs. Cells were incubated for 24 h, 48 h, and 72 h with the increasing concentrations of Cd (2.5 μM-100 μM). The obtained results are expressed as the percentage of CAT activity with respect to the control values (Ctrl). The values represent mean ± SEM of the measurements obtained from at least four technical replicates in three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001; NS, no significant difference compared with the control (0 μM Cd)
4 Discussion
Neurotoxic effects of Cd have been observed in a non-homogenous brain cell cultures, such as rat cortical neurons [23], isolated optic nerve preparation [24], anterior pituitary cells [25], and mesencephalic trigeminal neurons [26]. Such models provide controversial data regarding the response process occurring in neurons exposed to Cd toxicity. The present study is focused on the cytotoxic effects of Cd on CGNs in order to evaluate the specific response of neurons to Cd toxicity. We observed considerable morphological alterations in CGNs treated with Cd leading to a reduction in the neurite network and a drastic decrease of viable cells, suggesting that this metal is neurotoxic. Additionally, compared with the controls, Cd treatment led to a significant increase in the release of LDH, a cytosolic enzyme released in the medium following the destabilization of membrane integrity, further demonstrating the cytotoxicity and deleterious effects of Cd in CGNs. However, in the samples incubated with 15 to100 μM Cd for 24h or 48h, the release of LDH was shown to be reduced with increasing Cd concentration (less than control value for 100 μM of Cd). Similarly, after 72 h of incubation with Cd, even when a low concentration (2.5 μM) was used, LDH was considerably inhibited in comparison with the control. Based on these results, we suggest that when low concentrations or short exposure time to Cd are used, Cd induces only necrosis in CGNs, while the application of higher concentrations of Cd or longer exposure time may lead to the induction of apoptosis, and the maintenance of cell membrane integrity. This may explain the reduction in LDH release following the application of high concentrations of Cd or longer incubation times.
Oxidative stress is often involved in Cd-induced toxicity in a variety of cells or in animals [13, 28]. Cd is a heavy metal that can induce potent oxidative stress by enhancing the production of ROS, which is responsible for its toxic effects. Cellular defense system that includes antioxidant molecules (glutathione, vitamins E, C…) and antioxidant enzymes (SOD, CAT, and GPx) plays an important role in cellular defense against oxidative stress [13, 28]. The depletion of glutathione and the inhibition of SOD and CAT activities have been implicated in lipid peroxidation, considered a primary event in Cd-induced cellular toxicity [10, 11, 29]. The effects of the exposure to Cd on SOD and CAT activities have been extensively studied and controversial results have been obtained, depending on the experimental conditions [11, 13]. Our results demonstrated that SOD activity measured in CGNs exposed to low concentrations (2.5-5 μM) of Cd for 24 h or 48 h was similar to that detected in the control cells. However, the activity of this enzyme in CGNs treated with high concentrations of Cd (7.5-100 μM) or incubated for a longer time (72 h) was shown to be significantly decreased. These findings indicate that after 24 h or 48 h of exposure to low concentrations of Cd, most of the treated neurons maintain SOD activity, and the defense against oxidative damage, while the higher concentrations of Cd and longer exposure times affected the normal cellular functions and subsequent inactivation of SOD. These data were supported by our results demonstrating that for high concentrations of Cd or long exposure times the number of viable cells decreased. Similar to Cd effects on SOD activity, in CGNs exposed to the low concentrations of Cd (2.5-5 μM) for 24 h or 48 h, CAT activity was not significantly affected, but, unlike SOD activity, CAT activity did not considerably decrease when CGNs were treated with high concentrations of Cd, or incubated for longer periods, suggesting that CAT are more resistant to Cd toxicity than SOD enzyme. Taken together, the results obtained indicate that SOD, most likely Cu/ZnSOD, known to be expressed in CGNs [30], is very sensitive and is severely inactivated by Cd treatment. Several studies suggest the potential replacement of Zn by Cd, which can consequently lead to the reduction in SOD activity [29, 31, 32]. Additionally, it was suggested that Cd/enzyme interaction may causes a disruption of the topography of the channel localized in SOD that regulates the functioning of this enzyme [30]. A similar mechanism has been suggested to be involved in the interaction between Cd and the catalytic subunit of CAT leading to the reduction of CAT activity [13]. Thus, our findings give information about the behaviour of antioxidant enzymes against Cd cytotoxicity in CGNs. Besides, it was demonstrated in a previous study that Cd also affects on CGNs, the non-enzymatic antioxidant pathway by the depletion of the cellular content of gluthatione [27].
In conclusion, our results show that the necrotic and/ or apoptotic pathway involved in the cell death trigged by Cd seems to depend on the concentration of Cd and the exposure time. Importantly, we found that antioxidant enzymes differ in their response to Cd toxicity. The effects of Cd on SOD activity are greater than on CAT activity. Our findings are essential in order to reveal the exact role of Cd-induced cytotoxicity and alteration of antioxidant capacity in neurons. This may provide clues for future research to ameliorating treatment of neurological disorders ascribed to Cd exposure.
Acknowledgements
The authors thank Mr. Samir Elbahi for his technical assistance.
Conflict of interest: Authors state no conflict of interest
References
[1] Marcano L.B., Carruyo I.M., Montiel X.M., Morales C.B., de Soto P.M., Effect of cadmium on cellular viability in two species of microalgae (Scenedesmus sp. and Dunaliella virdis), Biol. Trace Elem. Res., 2009, 130, 86-93.10.1007/s12011-009-8316-ySuche in Google Scholar PubMed
[2] Jarup L., Hazards of heavy metal contamination, Br. Med. Bull., 2003, 68, 167-182.10.1093/bmb/ldg032Suche in Google Scholar PubMed
[3] Tsutsumi R., Hiroi H., Momoeda M., Hosokawa Y., Nakazawa F., Yano T., Tsutsumi O., Taketani Y., Induction of early decidualization by cadmium, a major contaminant of cigarette smoke, Fertil. Steril., 91, 1614-1617.10.1016/j.fertnstert.2008.12.055Suche in Google Scholar PubMed
[4] Flowler B.A., Monitoring human populations for early markers of cadmium toxicity: a review, Toxicol. Appl. Pharmacol., 2009, 238, 294-300.10.1016/j.taap.2009.05.004Suche in Google Scholar PubMed
[5] Méndez-Armenta M., Rios C., Cadmium neurotoxicity, Environ. Toxicol. Pharmacol., 2007, 23, 350-358.10.1016/B978-0-444-52272-6.00381-0Suche in Google Scholar
[6] Hart R.P., Rose C.S., Hamer R.M., Neuropsychological effects of occupational exposure to cadmium, J. Clin. Exp. Neuropsychol., 1989, 11, 933-948.10.1080/01688638908400946Suche in Google Scholar PubMed
[7] Ciesielski T., Weuve J., Bellinger D.C., Schwartz J., Lanphear B., Wright R.O., Cadmium exposure and neurodevelopment outcomes in U.S. children, Environ. Health Prespect., 2012, 120, 758-763.10.1289/ehp.1104152Suche in Google Scholar PubMed PubMed Central
[8] Szkup-Jablonska M., Karakiewicz B., Grochans E., Jurczak A., Nowak-Staz G., Rotter I., Prokopowicz A., Effects of blood lead and cadmium levels on the functioning of children with behaviour disorders in the family environment, Ann. Agric. Environ. Med., 2012, 19, 241-246.Suche in Google Scholar
[9] Bar-Sela S., Reingold S., Richter E.D., Amyotrophic lateral sclerosis in a battery-factory worker exposed to cadmium, Int. J. Occup. Environ. Health., 2001, 7, 109-112.10.1179/oeh.2001.7.2.109Suche in Google Scholar
[10] Figueiredo-Pereira M.E., Yakushin S., Cohen G., Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells, J. Biol. Chem., 1998, 273, 12703-12709.10.1074/jbc.273.21.12703Suche in Google Scholar PubMed
[11] Lopez E., Arce C., Oset-Gasque M.J., Cañadas S., González M.P., Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture, Free Radic. Biol. Med., 2006, 40, 940-951.10.1016/j.freeradbiomed.2005.10.062Suche in Google Scholar PubMed
[12] Waisberg M., Joseph P., Hale B., Beyersmann D., Molecular and cellular mechanisms of cadmium carcinogenesis, Toxicology, 2003, 192, 95-117.10.1016/S0300-483X(03)00305-6Suche in Google Scholar
[13] Cuypers A., Plusquin M., Remans T., Jozefczak M., Keunen E., Gielen H., Opdenakker K., Nair A. R., Munters E., Artois T. J., Nawrot T., Vangronsveld J., Smeets K., Cadmium stress: an oxidative challenge, Biometals, 2010, 23, 927-940.10.1007/s10534-010-9329-xSuche in Google Scholar
[14] Yang M.S., Yu L.C., Pat S.W., Manipulation of energy and redox states in the C6 glioma cells by buthionine sulfoxamine and N-acetylcysteine and the effect on cell survival to cadmium toxicity, Cell Mol. Biol., 2007, 53, 56-61.Suche in Google Scholar
[15] Yang C.S., Tzou B.C., Liu Y.P., Tsai M.J., Shyue S.K., Tzeng S.F., Inhibition of cadmium-induced oxidative injury in rat primary astrocytes by the addition of antioxidants and the reduction of intracellular calcium, J. Cell Biochem., 2008, 103, 825-834.10.1002/jcb.21452Suche in Google Scholar
[16] Yan Y., Bian J.C., Zhong L.X., Zhang Y., Sun Y., Liu Z.P., Oxidative stress and apoptotoic changes of rat cerebral cortical neurons exposed to cadmium in vitro, Biomed. Environ. Sci., 2012, 25, 172-181.Suche in Google Scholar
[17] Gupta A., Gupta A., Murthy R.C., Chandra S.V. Neurochemical changes in developing rat brain after pre and postnatal cadmium exposure, Bull. Environ. Contam. Toxicol., 1993, 51, 12-17.10.1007/BF00200994Suche in Google Scholar
[18] Antonio M.T., Corredor L., Leret M.L., Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium, Toxicol. Lett., 2003, 143, 331-340.10.1016/S0378-4274(03)00194-2Suche in Google Scholar
[19] Shukla A., Shukla G.S., Srimal R.C., Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat, Hum. Exp. Toxicol., 1996, 15, 400-405.10.1177/096032719601500507Suche in Google Scholar
[20] Nemmiche S., Chabane-Sari D., Guiraud P., Role of alphatocopherol in cadmium-induced oxidative stress in Wistar rat’s blood, liver and brain, Chem. Biol. Interact., 2007, 170, 221-230.10.1016/j.cbi.2007.08.004Suche in Google Scholar
[21] Zhang Y.M., Liu X.Z., Lu H., Mei L., Liu Z.P., Lipid peroxidation and ultrastructural modifications in brain after perinatal exposure to lead and/ or cadmium in rat pups, Biomed. Environ. Sci., 2009, 22, 423-429.10.1016/S0895-3988(10)60021-9Suche in Google Scholar
[22] Solecki D.J., Liu X.L., Tomoda T., Fang V., Hatten M.E., Activated Notch2 signaling inhibits differentiation of cerebellar granule neurons precursors by maintaining proliferation, Neuron, 2001, 31, 557-568.10.1016/S0896-6273(01)00395-6Suche in Google Scholar
[23] Lopez E., Fiqueroa S., Oset-Gasque M. J., González M. P., Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture, Br. J. Pharmacol., 2003, 138, 901-911.10.1038/sj.bjp.0705111Suche in Google Scholar PubMed PubMed Central
[24] Fern R., Black J.A., Ransom B.R., Waxman S.G., Cd(2+)- induced injury in CNS white matter, J. Neurophysiol., 1996, 76, 3264-3273.10.1152/jn.1996.76.5.3264Suche in Google Scholar
[25] Poliandri A.H., Velardez M.O., Cabilla J.P., Bodo C.C., Machiavelli L.I., Quinteros A.F. Duvilanski B.H., Nitric oxide protects anterior pituitary cells from cadmium-induced apoptosis, Free Radic. Biol. Med., 2004, 37, 1463-1471.10.1016/j.freeradbiomed.2004.07.017Suche in Google Scholar
[26] Yoshida K., Ikeda S., Nakanishi J., Assessment of human health risk of dioxins in Japan, Chemosphere, 2000, 40, 177-185.10.1016/S0045-6535(99)00253-2Suche in Google Scholar
[27] Yumiko N., Jun-ya Y., Aimi K., Kanna H., Kaori K., Masaya S., Yasuo O., Increase in intracellular Cd2+ concentration of rat cerebellar granule neurons incubated with cadmium chloride: Cadmium cytotoxicity under external Ca2+-free condition, Toxicol. In Vitro, 2006, 20, 211-216.10.1016/j.tiv.2005.06.006Suche in Google Scholar
[28] Liu J., Qu W., Kadiiska M.B., Role of oxidative stress in cadmium toxicity and carcinogenesis, Toxicol. Appl. Pharmacol., 2009, 238, 209-214.10.1016/j.taap.2009.01.029Suche in Google Scholar
[29] Casalino E., Calzaretti G., Sblano C., Landriscina C., Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium, Toxicology, 2002, 179, 37-50.10.1016/S0300-483X(02)00245-7Suche in Google Scholar
[30] Okabe M., Hosokawa T., Saito S., Saito T., Kurasaki M., Shimizu H., Richard M.J., Co-localization of Cu/Zn-superoxide dismutase (SOD-1), nitric oxide synthase (NOS), and Zn/ Cu-metallothionein (MT) in rat brain, In: Trace elements in man and animals, 10 (Eds. A. M. Roussel, R. A. Anderson, and A. E. Favrier), 105-109, Plenum Publishers, New York, 2000.10.1007/0-306-47466-2_20Suche in Google Scholar
[31] Hussain T., Shukla G.S., Chandra S.V., Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies, Pharmacol. Toxicol., 1987, 60, 355-359.10.1111/j.1600-0773.1987.tb01526.xSuche in Google Scholar PubMed
[32] Kofod P., Bauer R., Danielsen E., Larsen E., Bjerrum M.J., 113Cd-NMR investigation of a cadmium-substitution copper, zing-containing superoxide dismutase from yeast, Eur. J. Biochem., 1991, 198, 607-611.10.1111/j.1432-1033.1991.tb16057.xSuche in Google Scholar PubMed
© 2017 Dhouha Karoui-Kharrat et al.
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 License.
Artikel in diesem Heft
- Research Articles
- Field Performance and Genetic Fidelity of Micropropagated Plants of Coffea canephora (Pierre ex A. Froehner)
- Research Articles
- Foliage maturity of Quercus ilex affects the larval development of a Croatian coastal population of Lymantria dispar (Lepidoptera: Erebidae)
- Research Articles
- Structural and functional impact of SNPs in P-selectin gene: A comprehensive in silico analysis
- Research Articles
- High embryogenic ability and regeneration from floral axis of Amorphophallus konjac (Araceae)
- Research Articles
- Terpene content of wine from the aromatic grape variety ‘Irsai Oliver’ (Vitis vinifera L.) depends on maceration time
- Research Articles
- Light and smell stimulus protocol reduced negative frontal EEG asymmetry and improved mood
- Research Articles
- Swailing affects seed germination of plants of European bio-and agricenosis in a different way
- Research Articles
- Survey analysis of soil physicochemical factors that influence the distribution of Cordyceps in the Xiahe Region of Gansu Province
- Research Articles
- Production of biogas: relationship between methanogenic and sulfate-reducing microorganisms
- Research Articles
- Pentraxin 3 and atherosclerosis among type 2 diabetic patients
- Research Articles
- Evaluation of ribosomal P0 peptide as a vaccine candidate against Argulus siamensis in Labeo rohita
- Research Articles
- Variation of autosomes and X chromosome STR in breast cancer and gynecological cancer tissues
- Research Articles
- Response of antioxidant enzymes to cadmium-induced cytotoxicity in rat cerebellar granule neurons
- Research Articles
- Cardiac hypertrophy and IGF-1 response to testosterone propionate treatment in trained male rats
- Research Articles
- BRAF-activated non-protein coding RNA (BANCR) advances the development of esophageal squamous cell carcinoma via cell cycle
- Research Articles
- The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties
- Research Articles
- Simple Protocol for immunoglobulin G Purification from Camel “Camelus dromedarius” Serum
- Research Articles
- Expression of psbA1 gene in Synechocystis sp. PCC 6803 is influenced by CO2
- Research Articles
- Frequency of Thrombophilic Gene Mutations in Patients with Deep Vein Thrombosis and in Women with Recurrent Pregnancy Loss
- Research Articles
- Evaluation of anticancer properties of a new α-methylene-δ-lactone DL-249 on two cancer cell lines
- Research Articles
- Impact of heated waters on water quality and macroinvertebrate community in the Narew River (Poland)
- Research Articles
- Effects of Some Additives on In Vitro True Digestibility of Wheat and Soybean Straw Pellets
- Research Articles
- RNAi-mediated gene silencing in Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae)
- Research Articles
- New pathway of icariin-induced MSC osteogenesis: transcriptional activation of TAZ/Runx2 by PI3K/Akt
- Research Articles
- Tudor-SN protein expression in colorectal cancer and its association with clinical characteristics
- Research Articles
- Proteomic and bioinformatics analysis of human saliva for the dental-risk assessment
- Research Articles
- Reverse transcriptase sequences from mulberry LTR retrotransposons: characterization analysis
- Research Articles
- Strain Stimulations with Different Intensities on Fibroblast Viability and Protein Expression
- Research Articles
- miR-539 mediates osteoblast mineralization by regulating Distal-less genes 2 in MC3T3-E1 cell line
- Research Articles
- Diversity of Intestinal Microbiota in Coilia ectenes from Lake Taihu, China
- Research Articles
- The production of arabitol by a novel plant yeast isolate Candida parapsilosis 27RL-4
- Research Articles
- Effectiveness of Azospirillum brasilense Sp245 on young plants of Vitis vinifera L.
- Research Articles
- Changes of photochemical efficiency and epidermal polyphenols content of Prosopis glandulosa and Prosopis juliflora leaves exposed to cadmium and copper
- Research Articles
- Ultraweak photon emission in strawberry fruit during ripening and aging is related to energy level
- Research Articles
- Molecular cloning, characterization and evolutionary analysis of leptin gene in Chinese giant salamander, Andrias davidianus
- Research Articles
- Longevity and stress resistance are affected by activation of TOR/Myc in progenitor cells of Drosophila gut
- Research Articles
- Curcumin attenuates oxidative stress in liver in Type 1 diabetic rats
- Research Articles
- Risk factors of long-term postoperative renal function after partial nephrectomy in a solitary kidney
- Research Articles
- Developmental anomalies of the right hepatic lobe: systematic comparative analysis of radiological features
- Review articles
- Genetic Defects Underlie the Non-syndromic Autosomal Recessive Intellectual Disability (NS-ARID)
- Review articles
- Research Progress on Tissue Culture and Genetic Transformation of Kenaf (Hibiscus cannabinus)
- Topical Issue On Precision Medicine
- MiR-107 inhibits proliferation of lung cancer cells through regulating TP53 regulated inhibitor of apoptosis 1 (TRIAP1)
- Topical Issue On Precision Medicine
- The functional role of exosome microRNAs in lung cancer
- Topical Issue On Precision Medicine
- The diagnostic value of serum microRNA-183 and TK1 as biomarkers for colorectal cancer diagnosis
- Topical Issue On Precision Medicine
- Screening feature modules and pathways in glioma using EgoNet
- Topical Issue On Precision Medicine
- Isoliquiritigenin inhibits colorectal cancer cells HCT-116 growth by suppressing the PI3K/AKT pathway
- Topical Issue On Precision Medicine
- Association between Caveolin-1 expression and pathophysiological progression of femoral nerves in diabetic foot amputation patients
- Topical Issue On Precision Medicine
- Biomarkers in patients with myocardial fibrosis
- Topical Issue On Precision Medicine
- Dysregulated pathways for off-pump coronary artery bypass grafting
- Topical Issue On Precision Medicine
- Individualized identification of disturbed pathways in sickle cell disease
- Topical Issue On Precision Medicine
- The prognostic value of serum PCT, hs-CRP, and IL-6 in patients with sepsis
- Topical Issue On Precision Medicine
- Sevoflurane-medicated the pathway of chemokine receptors bind chemokines in patients undergoing CABG
- Topical Issue On Precision Medicine
- The functional role of microRNAs in laryngeal carcinoma
- Topical Issue On Precision Medicine
- Revealing pathway cross-talk related to diabetes mellitus by Monte Carlo Cross-Validation analysis
- Topical Issue On Precision Medicine
- Correlation between CDKAL1 rs10946398C>A single nucleotide polymorphism and type 2 diabetes mellitus susceptibility: A meta-analysis
- Special Issue on Agricultural and Biological Sciences
- Effects of environmental variables on seedling distribution of rare and endangered Dacrydium pierrei
- Special Issue on Agricultural and Biological Sciences
- Study on synthesis and properties of nanoparticles loaded with amaryllidaceous alkaloids
- Special Issue on Agricultural and Biological Sciences
- Bacterial Infection Potato Tuber Soft Rot Disease Detection Based on Electronic Nose
- Special Issue on Agricultural and Biological Sciences
- Effects of subsoiling on maize yield and water-use efficiency in a semiarid area
Artikel in diesem Heft
- Research Articles
- Field Performance and Genetic Fidelity of Micropropagated Plants of Coffea canephora (Pierre ex A. Froehner)
- Research Articles
- Foliage maturity of Quercus ilex affects the larval development of a Croatian coastal population of Lymantria dispar (Lepidoptera: Erebidae)
- Research Articles
- Structural and functional impact of SNPs in P-selectin gene: A comprehensive in silico analysis
- Research Articles
- High embryogenic ability and regeneration from floral axis of Amorphophallus konjac (Araceae)
- Research Articles
- Terpene content of wine from the aromatic grape variety ‘Irsai Oliver’ (Vitis vinifera L.) depends on maceration time
- Research Articles
- Light and smell stimulus protocol reduced negative frontal EEG asymmetry and improved mood
- Research Articles
- Swailing affects seed germination of plants of European bio-and agricenosis in a different way
- Research Articles
- Survey analysis of soil physicochemical factors that influence the distribution of Cordyceps in the Xiahe Region of Gansu Province
- Research Articles
- Production of biogas: relationship between methanogenic and sulfate-reducing microorganisms
- Research Articles
- Pentraxin 3 and atherosclerosis among type 2 diabetic patients
- Research Articles
- Evaluation of ribosomal P0 peptide as a vaccine candidate against Argulus siamensis in Labeo rohita
- Research Articles
- Variation of autosomes and X chromosome STR in breast cancer and gynecological cancer tissues
- Research Articles
- Response of antioxidant enzymes to cadmium-induced cytotoxicity in rat cerebellar granule neurons
- Research Articles
- Cardiac hypertrophy and IGF-1 response to testosterone propionate treatment in trained male rats
- Research Articles
- BRAF-activated non-protein coding RNA (BANCR) advances the development of esophageal squamous cell carcinoma via cell cycle
- Research Articles
- The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties
- Research Articles
- Simple Protocol for immunoglobulin G Purification from Camel “Camelus dromedarius” Serum
- Research Articles
- Expression of psbA1 gene in Synechocystis sp. PCC 6803 is influenced by CO2
- Research Articles
- Frequency of Thrombophilic Gene Mutations in Patients with Deep Vein Thrombosis and in Women with Recurrent Pregnancy Loss
- Research Articles
- Evaluation of anticancer properties of a new α-methylene-δ-lactone DL-249 on two cancer cell lines
- Research Articles
- Impact of heated waters on water quality and macroinvertebrate community in the Narew River (Poland)
- Research Articles
- Effects of Some Additives on In Vitro True Digestibility of Wheat and Soybean Straw Pellets
- Research Articles
- RNAi-mediated gene silencing in Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae)
- Research Articles
- New pathway of icariin-induced MSC osteogenesis: transcriptional activation of TAZ/Runx2 by PI3K/Akt
- Research Articles
- Tudor-SN protein expression in colorectal cancer and its association with clinical characteristics
- Research Articles
- Proteomic and bioinformatics analysis of human saliva for the dental-risk assessment
- Research Articles
- Reverse transcriptase sequences from mulberry LTR retrotransposons: characterization analysis
- Research Articles
- Strain Stimulations with Different Intensities on Fibroblast Viability and Protein Expression
- Research Articles
- miR-539 mediates osteoblast mineralization by regulating Distal-less genes 2 in MC3T3-E1 cell line
- Research Articles
- Diversity of Intestinal Microbiota in Coilia ectenes from Lake Taihu, China
- Research Articles
- The production of arabitol by a novel plant yeast isolate Candida parapsilosis 27RL-4
- Research Articles
- Effectiveness of Azospirillum brasilense Sp245 on young plants of Vitis vinifera L.
- Research Articles
- Changes of photochemical efficiency and epidermal polyphenols content of Prosopis glandulosa and Prosopis juliflora leaves exposed to cadmium and copper
- Research Articles
- Ultraweak photon emission in strawberry fruit during ripening and aging is related to energy level
- Research Articles
- Molecular cloning, characterization and evolutionary analysis of leptin gene in Chinese giant salamander, Andrias davidianus
- Research Articles
- Longevity and stress resistance are affected by activation of TOR/Myc in progenitor cells of Drosophila gut
- Research Articles
- Curcumin attenuates oxidative stress in liver in Type 1 diabetic rats
- Research Articles
- Risk factors of long-term postoperative renal function after partial nephrectomy in a solitary kidney
- Research Articles
- Developmental anomalies of the right hepatic lobe: systematic comparative analysis of radiological features
- Review articles
- Genetic Defects Underlie the Non-syndromic Autosomal Recessive Intellectual Disability (NS-ARID)
- Review articles
- Research Progress on Tissue Culture and Genetic Transformation of Kenaf (Hibiscus cannabinus)
- Topical Issue On Precision Medicine
- MiR-107 inhibits proliferation of lung cancer cells through regulating TP53 regulated inhibitor of apoptosis 1 (TRIAP1)
- Topical Issue On Precision Medicine
- The functional role of exosome microRNAs in lung cancer
- Topical Issue On Precision Medicine
- The diagnostic value of serum microRNA-183 and TK1 as biomarkers for colorectal cancer diagnosis
- Topical Issue On Precision Medicine
- Screening feature modules and pathways in glioma using EgoNet
- Topical Issue On Precision Medicine
- Isoliquiritigenin inhibits colorectal cancer cells HCT-116 growth by suppressing the PI3K/AKT pathway
- Topical Issue On Precision Medicine
- Association between Caveolin-1 expression and pathophysiological progression of femoral nerves in diabetic foot amputation patients
- Topical Issue On Precision Medicine
- Biomarkers in patients with myocardial fibrosis
- Topical Issue On Precision Medicine
- Dysregulated pathways for off-pump coronary artery bypass grafting
- Topical Issue On Precision Medicine
- Individualized identification of disturbed pathways in sickle cell disease
- Topical Issue On Precision Medicine
- The prognostic value of serum PCT, hs-CRP, and IL-6 in patients with sepsis
- Topical Issue On Precision Medicine
- Sevoflurane-medicated the pathway of chemokine receptors bind chemokines in patients undergoing CABG
- Topical Issue On Precision Medicine
- The functional role of microRNAs in laryngeal carcinoma
- Topical Issue On Precision Medicine
- Revealing pathway cross-talk related to diabetes mellitus by Monte Carlo Cross-Validation analysis
- Topical Issue On Precision Medicine
- Correlation between CDKAL1 rs10946398C>A single nucleotide polymorphism and type 2 diabetes mellitus susceptibility: A meta-analysis
- Special Issue on Agricultural and Biological Sciences
- Effects of environmental variables on seedling distribution of rare and endangered Dacrydium pierrei
- Special Issue on Agricultural and Biological Sciences
- Study on synthesis and properties of nanoparticles loaded with amaryllidaceous alkaloids
- Special Issue on Agricultural and Biological Sciences
- Bacterial Infection Potato Tuber Soft Rot Disease Detection Based on Electronic Nose
- Special Issue on Agricultural and Biological Sciences
- Effects of subsoiling on maize yield and water-use efficiency in a semiarid area

