Abstract
Introduction
Ifosfamide (IF) is a cytostatic that exhibits adverse nephrotoxic properties. Clinically, IF-induced nephrotoxicity takes various forms, depending on applied dose and length of treatment.
Objectives
The aim of the study was to evaluate the two proteins: osteopontin (OP) and fatty acid binding protein (FABP), as markers of kidney function in rats treated with ifosfamide.
Material and Methods
Rats receiving a single IF dose (250 mg/kg b.w.; group 1) or treated with five consecutive IF doses administrated on following days (50mg/kg b.w.; group 3), compared with control groups 2 and 4, respectively, were studied. Kidney function was assessed using classical (urea, creatinine) and novel (FABP, OP) laboratory parameters and by histopathology.
Results
Single IF dose administration resulted in significant total proteinuria with urinary concentrations and 24-hour excretions of both FABP and OP comparable to the appropriate control. In rats treated with five consecutive IF doses, the urinary concentrations and 24-hour excretion of both FABP and OP were significantly higher compared to the appropriate control. The development of cystitis was revealed in groups 1 and 3, which was not accompanied by significant histopathological kidney damage.
Conclusions
Both OP and FABP may be useful laboratory markers of tubulopathy in the early stage of chronic nephrotoxicity of ifosfamide.
1 Introduction
Ifosfamide [3-(2-chloroethyl)-2-[(2-chloroethyl)-amino] tetrahydro-2H-1,3,2-oxazaphosphorin-2-oxide] is a cytostatic that belongs to the family of the oxazaphosphorine alkylating agents [1]. Besides ifosfamide (IF), the group also contains cyclophosphamide (CP), trofosfamide and mafosfamide [2].
Ifosfamide was discovered in the 1960s and introduced into common clinical practice in the 1970s [3]. It exerts antineoplastic activity and it is also administrated in the treatment of osteosarcoma, neuroma, multiple myeloma and leukemias [2]. IF is a prodrug and to acquire antineoplastic activity, it must be metabolized to cytotoxic agents. The main IF metabolic pathway is oxidation, mainly involving hepatic cytochrome enzymes. IF oxidation takes place via two major routes. One pathway, with the involvement of CYP3A4, CYP2B6 and CYP2C9 (with less participation of CYP2C8 and CYP2C19), leads to the formation of isophosphoramide mustard and acrolein. The second pathway, catalyzed by CYP3A4, CYP3A5 and CYP2B6, leads to the formation of chloroacetaldehyde (CAA) [1,2]. Those metabolites are responsible for the cytotoxic effects of IF result from their ability of attaching to the nucleophilic N-7 position of one of the nitrogenous base of DNA, guanine (the other alkylation sites include N-1 and N-3 position of adenine, N-3 position of cytosine and O-6 of guanine). Depending on the position of the alkylated nitrogenous DNA base, intra- and interstrand DNA cross-links are formed and the final consequence of this cross-link formation is the activation of apoptotic mechanisms, leading to cell death [2,4].
The administration of oxazaphosphorines is associated with many adverse drug reactions due to the fact that the same active metabolites produce toxic effects. Similarly to the other cytostatics, common side effects observed during oxazaphosphorine’s pharmacotherapy include: alopecia, myelosuppression (manifested by anemia, neutropenia and thrombocytopenia), nausea, vomiting and hypersensitivity reactions [2,4]. The more specific toxicities related to both IF and CP involve hemorrhagic cystitis, neurotoxicity and nephrotoxicity [1]. The urinary bladder is damaged mostly by CP, whereas IF is regarded to impair kidney functions. The observed differences in toxicity between CP and IF results from the quantitative aspects of the metabolism of those drugs and the amounts of the synthetized acrolein, phosphoramide mustard and chloroacetaldehyde. The latter is considered to be a potent nephrotoxic compound [2]. Both IF and CP undergo similar metabolic pathways with production of the same metabolites. However, approximately 45% of the administrated IF dose is converted to CAA, while metabolism of CP mainly leads to acrolein overproduction. Therefore, the main CP toxicity profile is related to acrolein-induced cystitis, while IF is regarded as the more potent nephrotoxic agent [1,2].
The average incidence of IF-related kidney dysfunction is reported to be as high as 5-30% [1]. The most frequent presentation of IF nephrotoxicity is in the form of disturbances of both the proximal and distal tubules, with potentially fatal Fanconi syndrome development [5,6]. IF-induced tubulopathy is not fully elucidated but it is hypothesized that it is strongly related to CAA, which interferes with the biochemical processes of the nephron cells (especially the tubules), with impairment of cell energy homeostasis, intensification of oxidative stress and finally morphological and functional disturbances of the tubules [2].
The currently used laboratory diagnostics of kidney function (e.g. tubular handling of sodium, glucose, phosphate, calcium, bicarbonate, amino acids and urinary acidification and concentration as functional markers of proximal and distal tubules, respectively) offer only a general assessment of renal tubular secretion and resorption. Similarly, due to numerous limitations, creatinine and urea assessment is also not an ideal method reflecting glomerular filtration. Also, proteinuria is a general sign of various kidney diseases of different pathophysiological background. However, despite all reservations, all of those parameters are currently widely used as the present routine laboratory diagnostics of kidney function [7,8].
However, there is an ongoing search for novel bio-markers characterized by improved sensitivity and selectivity in the biochemical assessment of kidney activity, especially reflecting early renal tubular dysfunction. The current research focuses on “renal troponins” - proteins released by renal tubules cells into the blood and urine in response to their damage, analogous to the troponins released by damaged cardiomyocytes [9,10]. Two of the “kidney troponins”, currently being investigated are osteopontin (OP) and fatty acid binding protein (FABP). In kidneys, both compounds exert antioxidant activity and they are overproduced and released into urine by injured kidney tubules in response to oxidative stress. As mentioned above, CAA is an intermediate IF metabolite, generating an enhanced synthesis of oxygen and nitrogen free radicals, contributing to kidney damage. Therefore FABP and OP are considered to be indirect evidence of oxidative stress in the kidneys [11,12]. The urinary assessment of those proteins can be adopted as markers of nephrotoxicity induced by IF.
The aim of our study was to assess the usefulness of osteopontin and fatty acid binding protein as markers of kidney function in rats treated with ifosfamide. The results of this experimental study may be of clinical relevance. The findings may be a rationale for the wider introduction of the laboratory assessment of both OP and FABP as new markers of nephrotoxicity in oncological patients treated with ifosfamide.
2 Methods
Ethical approval: The experiment described in this paper was carried out following the approval of the 2nd Local Ethical Commission operating in the Institute of Pharmacology of the Polish Academy of Science in Krakow. Procedures of the experiment were consistent with EU law (Directive EU 2010/63 of the Parliament and of the Council of 22 September 2010) and Polish law (The Law on protection of animals used for scientific and educational purposes, of 27 May 2015).
2.1 Experimental animals
The experiment was carried out on 40 albino Wistar rats aged 10 weeks, with an average initial body weight of 251.89 ± 20.76 g; equal numbers of males and females. The randomization of both females and males rats in all studied groups resulted from the necessity to avoid potential gender-related differences (e.g. hormonal) affecting the metabolism of ifosfamide and the endogenous production of nephrotoxic CAA in the course of metabolic pathway of the drug. Therefore, each group included both male and female rats. We did not perform the analysis of the results obtained separately for females and males individuals, as it would require a significant increase of the number of study animals.
2.2 Housing and husbandry
During the entire time of the experiment animals stayed in the animal house, ensuring the following standard and monitored living conditions: with the ventilation system allowing air turnover of 8-10 times per hour, at 20-24°C, humidity of 60-70%, 130-325 lux lighting, with a light-darkness cycles of 12h-12h , with noise < 30 dB, fed with standard laboratory feed (Labofeed, Kcynia, Poland) and with access to water ad libitum. The experiment was performed following a preliminary 10 day quarantine.
2.3 Sample size and allocating animals to experimental groups
After quarantine, animals were randomly assigned to individual groups (each of 10 animals, with equal ratios of males and females). The study was carried out on animals receiving a single dose of IF (group 1) with a matched control (group 2), and on animals receiving five doses of IF (group 3) with a matched control (group 4). During this stage of the experiment, the animals were in cages of two animals of the same sex
2.4 Experimental procedures in groups 1 and 2
Animals in group 1, after determination of body weight, received a single intraperitoneal dose of 250 mg/kg b.w. of ifosfamide (Holoxan, Baxter), and rats in the group 2 received a single intraperitoneal dose of saline. After the injection, each animal was placed for 24 hours in an individual metabolic cage, with unlimited but monitored access to water and feed. After 24 hours, body weight and temperature, the amount of consumed feed and water, and diuresis were measured. After this, animals were sacrificed by injection of pentobarbital (Morbital, Biowet, Puławy). First, the animals were placed in the anaesthetic chamber and treated with isoflurane. Then, the anaesthetic dose of 60 mg/kg b.w. of pentobarbital was administered, and blood was collected from the heart under deep anaesthesia. Finally, the additional dose of 100 mg/kg b.w. of the drug was administered. After confirmed cessation of vital functions, laparotomy was performed, with bilateral nephrectomy and cystectomy. For maintenance of their integrity, urinary bladders were collected along with 2-3 mm of the proximal urethra, carefully dried and emptied by delicate compression. Kidneys were hulled of the surrounding fat capsule. Collected organs were weighed on an analytical scale in order to determine their wet weight, and then placed in containers with 4% formalin solution, until preparation as histopathology specimens.
2.5 Experimental procedures in groups 3 and 4
Animals in group 3 received five intraperitoneal doses of 50 mg/kg b.w. of ifosfamide (Holoxan, Baxter). In group 4, placebo treatment with saline instead of IF was used. Each individual was weighed before each administration of the drug, in order to determine a precise dose. During the time of administration of subsequent doses, animals were housed in collective, unisex cages. After administration of the last dose of IF or saline, animals were placed for 24 hours in individual metabolic cages, for determination of the same parameters as those measured in groups 1 and 2. Further procedures were the same as those applied to animals in groups 1 and 2, described above.
2.6 Rationale for ifosfamide applied doses
The single IF administered dose to group 1 was identical with the total amount of the drug administered in the form of five divided doses to animals in the group 3, and was consistent with IF doses used in other experimental studies [13,14]. According to literature data, IF administered in a larger single dose (400 – 500 mg/kg b.w.) exerts an intensified nephrotoxic effect, manifested with Fanconi syndrome [15,16]. Moreover, the choice of the applied IF dose was also consistent with the clinical data. In accordance with the official Summary of the Holoxan Product Characteristics [17], a single, high dose of ifosfamide at 200 mg/kg b.w is associated with an increased risk of adverse effects, including uro- and nephrotoxicity.
The IF dosing regimen used in group 3 to induce kidney dysfunction was also based on literature data - the application of a total dose of 250-400 mg/kg b.w. of IF in rats for 3-5 days is a nephrotoxic dose [18,19]. Moreover, the dosing regimen used in our experiment is consistent with clinical practice - as recommended, ifosfamide is administered as monotherapy in adults in divided doses of 30-60 mg/kg, for 5 days [17].
2.7 Laboratory parameters determined in serum and urine
Blood was collected, clotted and centrifuged. Resulting serum was stored frozen at -80oC until tested. Similarly, urine samples were centrifuged and frozen. Biochemical measurements were done using the SIEMENS ADVIA 1200 laboratory analyser. Classic parameters of renal function were determined in serum: urea [mmol/L] and creatinine [μmol/L].
Urea [mmol/L], creatinine [μmol/L], and total protein [g/L] were determined in urine, and based on the 24-hour diuresis, a diurnal elimination of those compounds was also calculated, expressed as [mmol/24 h], [μmol/24h], and [mg/24 h], respectively. Urea clearance (CLurea) and creatinine clearance (CLcr) were also calculated, using the commonly used formulas:
Moreover, the immunoenzymatic method (ELISA) was used to determine urinary concentrations [ng/mL] of two proteins: osteopontin and fatty acid-binding protein. Commercial ELISA kits were used (Shanghai Sunred Biological Technology Co.) and the manufacturer’s instructions were followed. Similarly to creatinine and urea, considering the observed diuresis, a 24-hour elimination of those proteins with urine was estimated [ng/24 h].
2.8 The measurement of wet weight of kidneys and urinary bladders collected during the laparotomy, and their histopathological assessment
Directly after cystectomy and nephrectomy, collected organs were weighed on an analytical scale for determination of the bladder wet weight - BWW [mg] and kidney wet weight - KWW [mg]. The measurement of the wet weight is an indirect, approximate parameter of the organs’ functional status, reflecting advancement of edema due to the inflammation [20,21].
Microscopic specimens from urinary bladders and kidneys collected during the autopsy were prepared according to the methodology described in details in our previous work [22]. Final specimens were stained with hematoxylin - eosin (H&E).
The histopathological assessment was provided by a specialist in pathomorphology, using a classical light microscope (Delta Optical) at the 100x magnification.
2.9 Statistical methods
Results of determined living and laboratory parameters were expressed as mean ± SD and they were statistically analysed with comparison paired groups 1 and 2, and 3 and 4. First, normality of distribution was verified using the Shapiro-Wilk test. Then, the obtained results (group 1 vs. 2, and group 3 vs. 4) were analysed using the t-Student test (for values demonstrating a normal distribution) or the Mann-Whitney test (for values demonstrating no normal distribution). The level of statistical significance was p <0.05.
3 Results
3.1 Numbers of analysed and lost animals
All animals completed the experiment, although treatment with ifosfamide worsened their overall welfare, manifested by abnormalities in values of basic living parameters described below. Thus, in each of the studied groups we obtained results from 10 rats. Moreover, in the overall assessment, the IF-treated animals also showed reduced motor activity compared to individuals from the respective control groups.
3.2 The living parameters
Animals that received a single IF dose were characterised by significantly lower 24-hour water and feed intake, compared to controls. This was probably a reflection of generalised, systemic toxic effects of IF. Despite a significantly lower diurnal water intake, the diuresis was comparable in both groups 1 and 2. Moreover, in the case of rats treated with a single IF dose, a tendency for lower body weight and temperature was observed, although these changes were not statistically significant.
Contrary to groups 1 and 2, no difference related to 24-hour water intake was demonstrated in groups 3 and 4, with still comparable diuresis in both groups. Other parameters were not significantly different in those groups, except for diurnal feed intake.
Detailed data of living parameters in groups 1-4 are presented in Tables 1 and 2.
Results of parameters obtained in groups 1 and 2 during 24-hour monitoring in metabolic cages after administration of a single ifosfamide dose or saline, respectively (mean ± SD; NS – non-significant).
| body weight [g] | body temperature [°C] | feed intake [g/24h] | water intake [mL/24h] | diuresis [mL/24h] | |
|---|---|---|---|---|---|
| group 1 | 262.66 | 36.50 | 4.55 | 13.50 | 8.96 |
| ± 24.64 | ± 0.25 | ± 3.72 | ± 7.19 | ± 7.49 | |
| group 2 | 297.10 | 37.33 | 24.00 | 26.13 | 6.28 |
| ± 10.89 | ± 0.87 | ± 6.18 | ± 4.02 | ± 2.27 | |
| p value | NS | NS | < 0.01 | 0.01 | NS |
Results of parameters obtained in groups 3 and 4 during 24-hour monitoring in metabolic cages after administration of five doses of ifosfamide or saline, respectively (mean ± SD; NS – non-significant).
| body weight [g] | body temperature [°C] | feed intake [g/24h] | water intake [mL/24h] | diuresis [mL/24h] | |
|---|---|---|---|---|---|
| group 3 | 251.53 | 36.98 | 11.70 | 21.70 | 6.78 |
| ± 62.60 | ± 0.38 | ± 3.59 | ± 15.29 | ± 2.91 | |
| group 4 | 277.45 | 37.92 | 20.38 | 29.90 | 5.91 |
| ± 63.19 | ± 0.25 | ± 2.39 | ± 3.21 | ± 1.24 | |
| p value | NS | NS | < 0.01 | NS | NS |
3.3 The laboratory parameters assessed in serum
Significantly higher levels of serum urea [mmol/L] was found in rats receiving a single dose of IF compared to control animals (10.93 ± 2.12 and 6.34 ± 0.71 respectively; p=0.01). The creatinine level [μmol/L] was not significantly different in both groups (34.68 ± 7.48 vs. 28.87 ± 1.66 for groups 1 and 2, respectively).
Animals treated with five doses of IF were characterised by a significant increase of both urea (7.13 ± 1.03 [mmol/L]), and creatinine (34.09 ± 3.73 [μmol/L]) levels compared to the control group (6.34 ± 0.76 [mmol/L]; p=0.05 and 28.85 ± 1.77 [μmol/L]; p<0.01; respectively).
3.4 The laboratory parameters assessed in urine
Animals treated with a single IF dose were characterised by statistically significant proteinuria – the total protein concentration in urine of animals from group 1 was nearly 5 times higher, which was accompanied by a nearly 5-fold higher 24-hour elimination of protein with urine. The other assessed low weight molecular parameters (urea, creatinine) had comparable concentrations and diurnal elimination values, although the calculated urea clearance in rats treated with IF was nearly twice as low compared to the control group.
Contrary to animals treated with a single dose of IF, neither significant differences related to urinary protein concentration nor their diurnal elimination were observed in rats treated with five doses of IF. Also, values of urinary concentration, 24-hour elimination and clearance of creatinine were not different in control animals and those treated with IF. Rats in the group 3 demonstrated a significantly reduced urea clearance, with a decrease in urea urinary concentration.
Detailed data of living parameters in groups 1-4 are presented in Tables 3 and 4.
Biochemical parameters assessed in the urine of rats from groups 1 and 2 (mean ± SD; NS – non-significant).
| urea [mmol/L] | urea excretion [mmol/24h] | urea clearance [mL/min] | creatinine [μmol/L] | creatinine excretion [μmol/24h] | creatinine clearance [mL/min] | total protein [g/L] | protein excretion [mg/24h] | |
|---|---|---|---|---|---|---|---|---|
| group 1 | 949.25 | 5.72 | 0.38 | 6750.10 | 42.21 | 0.85 | 3.91 | 23.57 |
| ± 532.44 | ± 0.61 | ± 0.10 | ± 4340.89 | ± 13.76 | ± 0.24 | ± 1.51 | ± 14.83 | |
| group 2 | 1003.97 | 5.97 | 0.67 | 6100.00 | 36.23 | 0.87 | 0.81 | 5.34 |
| ± 176.71 | ± 2.28 | ± 0.31 | ± 1028.35 | ± 13.32 | ± 0.33 | ± 0.41 | ± 4.19 | |
| p value | NS | NS | 0.03 | NS | NS | NS | 0.01 | 0.04 |
Biochemical parameters assessed in the urine of rats from groups 3 and 4 (mean ± SD; NS – non-significant).
| urea [mmol/L] | urea excretion [mmol/24h] | urea clearance [mL/min] | creatinine [μmol/L] | creatinine excretion [μmol/24h] | creatinine clearance [mL/min] | total protein [g/L] | protein excretion [mg/24h] | |
|---|---|---|---|---|---|---|---|---|
| group 3 | 701.58 | 4.71 | 0.47 | 6237.50 | 42.21 | 0.87 | 0.63 | 4.59 |
| ± 207.21 | ± 2.28 | ± 0.24 | ± 1582.89 | ± 19.74 | ± 0.45 | ± 0.55 | ± 3.38 | |
| group 4 | 1058.63 | 5.93 | 0.66 | 8162.50 | 45.56 | 1.10 | 1.05 | 5.72 |
| ± 193.43 | ± 1.28 | ± 0.15 | ± 2285.32 | ± 14.14 | ± 0.37 | ± 0.65 | ± 4.15 | |
| p value | < 0.01 | NS | 0.05 | NS | NS | NS | NS | NS |
3.5 The assessment of FABP and osteopontin
Rats that received a single IF dose demonstrated similar urinary concentrations of FABP and osteopontin in urine, and similar values of 24-hour elimination of those proteins with urine, comparing to corresponding control animals. The urinary concentration of FABP was 10.46 ± 2.12 in rats treated with a single IF dose vs. 10.69 ± 3.68 in appropriate controls [ng/mL], while the concentrations of osteopontin in those groups were: 21.53 ± 5.70 vs. 24.43 ± 7.91 [ng/ mL], respectively. The 24-hour urinary excretion of FABP was 97.34 ± 50.91 in the group with IF treatment vs. 64.18 ± 28.48 [ng/24h] in the controls, while the 24-hour urinary osteopontin elimination with urine was 199.71 ± 92.63 and 146.38 ± 61.42 [ng/24h], respectively.
On the other hand, a statistically significant increase of urinary concentration of both proteins was observed in rats treated with five doses of IF. In rats treated with 5 consecutive IF doses, urinary FABP concentration was 17.63 ± 4.88 and osteopontin 33.44 ± 10.75 vs. 12.46 ± 4.19 and 21.37 ± 5.42 [ng/mL] found in the appropriate control groups, respectively. These findings were accompanied by statistically significant increased 24-hour urinary elimination of both proteins. In rats from group 3, 24-hour urinary FABP excretion was 109.72 ± 26.34 compared to 74.68 ± 31.44 [ng/24h] in controls. The difference was more emphasized for osteopontin – 208.91 ± 67.69 vs. 128.95 ± 50.66 [ng/24h], respectively. The aforementioned results are illustrated in Figures 1-4.
![Figure 1 Urinary FABP concentrations [ng/mL] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).](/document/doi/10.1515/med-2019-0063/asset/graphic/j_med-2019-0063_fig_001.jpg)
Urinary FABP concentrations [ng/mL] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).
![Figure 2 Urinary osteopontin concentrations [ng/mL] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).](/document/doi/10.1515/med-2019-0063/asset/graphic/j_med-2019-0063_fig_002.jpg)
Urinary osteopontin concentrations [ng/mL] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).
![Figure 3 24-hour urinary FABP excretion [ng/24h] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).](/document/doi/10.1515/med-2019-0063/asset/graphic/j_med-2019-0063_fig_003.jpg)
24-hour urinary FABP excretion [ng/24h] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).
![Figure 4 24-hour urinary osteopontin excretion [ng/24h] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).](/document/doi/10.1515/med-2019-0063/asset/graphic/j_med-2019-0063_fig_004.jpg)
24-hour urinary osteopontin excretion [ng/24h] in study groups (comparison for paired groups: 1-2 and 3-4; both appropriate control groups are marked as black bars; * - p<0.01).
3.6 The assessment of wet weight and the histopathological assessment of urinary bladders and kidneys
The measurement of the wet weight of collected urinary bladders and kidneys demonstrated no significant differences between groups. BWW [mg] was 120 ± 54; 134 ± 27; 150 ± 40 and 160 ± 58 in groups 1-4, respectively. The measurement of KWW [mg] indicated the weight of the left kidney of 910 ± 17 and 870 ± 21 in groups 1 and 2, and 951 ± 36 and 933 ± 25 in groups 3 and 4. The weight of the right kidney was 944 ± 14; 880 ± 22; 891 ± 37 and 950 ± 26 in groups 1-4, respectively.
Advanced histopathological disturbances were revealed in urinary bladders collected from rats in the group 1. Clusters of dilated tubules, cysts and hemorrhagic foci were visible in most of assessed specimens. Kidneys from animals in group 1 were slightly more congested; besides this, their presentation was within the normal range as observed in the group 2 (control).
An inflammatory infiltration with signs of exfoliation, reactive hypertrophy and moderate hyperemia was observed in bladder specimens collected from rats in group 3. The structure of the kidneys in this group was similar to that observed in the group 1; however the dilatation of tubules was revealed. Besides these findings, the kidneys of rats undergoing chronic IF therapy did not demonstrate significant abnormalities compared to the kidney histology of the control animals.
The examples of microscopic images of bladders and kidneys, observed in the studied rats treated with single and five consecutive doses of ifosfamide, together with appropriate control groups, are shown in Figures 5 and 6.
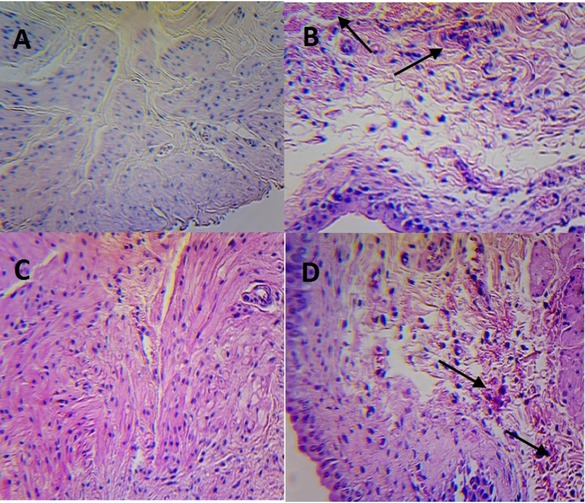
Urinary bladders in rats treated with a single IF dose (part B) and with five consecutive IF doses (part D) with appropriate control groups (part A and C, respectively). H&E staining, magnification 100x.
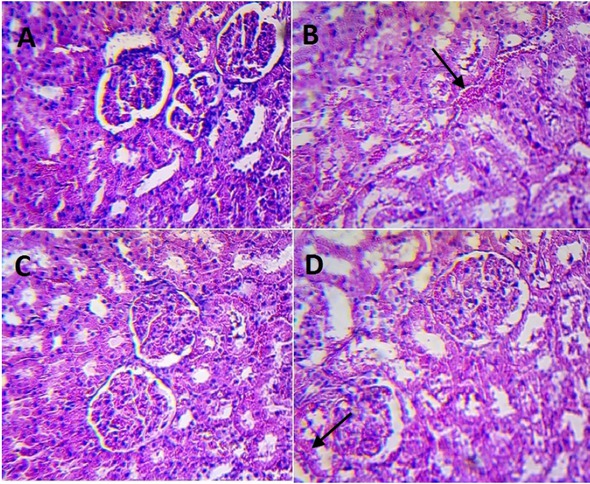
Kidneys in rats treated with a single IF dose (part B) and with five consecutive IF doses (part D) with appropriate control groups (part A and C, respectively). H&E staining, magnification 100x.
The aforementioned histopathological disturbances found in microscopic images of bladders and kidneys in the study groups have been indicated in Figures 5 and 6, respectively, using arrows.
4 Discussion
4.1 Scientific interpretation of obtained data
As was mentioned in the Introduction, one of the main metabolites of ifosfamide, chloroacetaldehyde, has nephrotoxic properties. Considering the presence of isoforms CYP3A4, CYP3A5 and CYP2B6 synthesizing CAA in kidneys, this organ is the most prone to injury as a result of impairment of mitochondrial oxidative phosphorylation in proximal tubules [2,23]. CAA causes reduction of glutathione in tubules, inhibits some transporters responsible for re-absorption of proteins from pre-urine and blocks the NADH oxidoreductase-ubiquinone complex, an element of the mitochondrial respiratory chain. Hence, CAA accounts for injury of renal tubules in the mechanism of energy deficit and intensification of oxidative stress [24,25].
Ifosfamide, depending on the dose and duration of treatment, causes a broad spectrum of glomerular and/or tubular dysfunctions, including Fanconi syndrome, with the loss of phosphates, carbonates, potassium, calcium and magnesium [5,26]. Dysfunction mostly affecting the distal part of the nephron was also described, manifested with impairment of urine acidification and concentration [26]. The characteristic feature of nephrotoxicity caused by IF is also increased urinary elimination of proteins that undergo glomerular filtration, but are not taken back up in injured proximal tubules, or are excreted by cells of renal tubules in response to their injury [5,26]. It can be adopted in estimation of the kidney functioning.
The current progress in diagnostics and pathophysiology of kidney diseases enables the determination of blood and/or urinary proteins that allow early detection of nephron injury. Those biomarkers appear in urine in broadly understood nephron dysfunctions (cystatin C, beta-2-microglobulin, neutrophil gelatinase-associated lipocalin – NGAL); some of them are more specific for the site of injury (e.g. proximal tubules – interleukin-18, kidney injury molecule 1 (KIM-1), N-acetyl-beta-D-glucosaminidase (NAG), liver-type fatty acid binding protein (L-FABP); distal tubules – clusterin, renal papillary antigen 1 (RPA-1), heart-type fatty acid binding protein (H-FABP), osteopontin) [27, 28, 29]. New markers also allow drawing general conclusions regarding the etiological basis of kidney injury (they may be classified as parameters of renal oxidative stress, structural injury of the glomeruli or tubules, or immunological processes in kidneys or renal fibrosis) [30].
Among proteins currently investigated for their usefulness in detailed laboratory diagnostics of kidney dysfunction, there are also the two proteins discussed above: fatty-acid binding protein and osteopontin.
FABP is engaged in intracellular regulation of lipid metabolism, allowing translocation of free fatty acids (FAs) to various cellular organelles, and intensifying the activity of enzymes that catalyse oxidation and esterification of fatty acids [31]. Moreover, FABP is also one of the intracellular antioxidants – free fatty acids may become over-oxidized; therefore by binding FAs, FABP demonstrates cytoprotective properties [31,32]. The presence of FABP of heart-type (H-FABP) in blood plasma is biochemical evidence of extended ischaemia of the myocardium. In this pathophysiological condition, compensatory antioxidative mechanisms (including H-FABP) become overactive, and the protein is released into the blood from injured cardiomyocytes [33]. Both heart- and liver type isoforms of the fatty acid binding protein are also present in renal tubules (H-FABP – in proximal, and L-FABP in distal ones) [34]. These proteins are synthesized and released into the urine by renal tubules, and partially resorbed from the glomerular filtrate [32]. The total amount of FABP eliminated with urine is considered to be the indirect marker of oxidative injury of renal tubules, similarly to the cardiomyocyte injury discussed above.
Osteopontin is one of proteins of the extracellular matrix of bones participating in the dynamic regulation of bone mineral density. The protein binds to osseous hydroxyapatite and stimulates activity of osteoclasts, acting as an inhibitor of crystallisation [35]. OP is also excreted in the distal part of nephron, acting as an inhibitor of precipitation of mineral compounds of urine, thus preventing formation of urinary stones [36]. Moreover, similarly to FABP, the protein also plays an important antioxidative role in renal tubules. OP inhibits the activity of nitrous oxide synthase and reduces the production of reactive nitrogen species [37]. Therefore, both OP and FABP have a nephroprotective effect and may be treated as indirect biomarkers of oxidative injury of renal tubules. Therefore, higher amounts of FABP and OP found in urine may be indirect evidence for injury of renal tubules by free radicals, overproduced in response to CAA. We demonstrated normal urinary concentrations and elimination of FABP and OP in rats receiving a single dose of IF. Increased amounts of those proteins were noted in urine of animals receiving a chronic treatment with IF. Therefore, it may be hypothesised that progressive administration of IF leads to intensification of oxidative stress with concurrent, compensative antioxidative reaction. In our opinion, such a reaction cannot rapidly and fully occur after single administration of a high IF dose, thus no significant quantitative abnormalities related to OP and FABP were determined in the urine of rats in group 1. It should be also underlined that FABP and OP abnormalities observed in rats receiving 5 subsequent doses of IF were not accompanied by any significant changes of the low molecular weight laboratory parameters used for the assessment of the kidney function, which may suggest that dysregulation of FABP and OP may precede possible disturbances of classical kidney parameters. Additionally, our experiment demonstrated no significant histopathological disorders of the kidneys. Thus, the intensified elimination of FABP and OP, probably also preceding structural abnormalities of kidneys, may be regarded as a manifestation of developing tubulopathy in rats receiving five doses of IF.
The problem of applicability of new protein biomarkers to the assessment of drug-induced nephrotoxicity was also discussed in one of our earlier studies focusing on the urinary concentration and elimination of kidney injury molecule 1 (KIM-1) in rats treated with single and repeated doses of cyclophosphamide or ifosfamide [22]. The experiment also revealed the increased 24-hour urinary elimination of KIM-1 in rats treated with repeated IF doses, but again, the same finding was not observed in animals receiving a single dose of ifosfamide [22]. The results, therefore, are consistent with those ones obtained in the presently described experiment. KIM-1 is another “renal troponin”, also indicating dysfunction of proximal tubules. Under physiological conditions KIM-1 is not found in urine [38,39]. Toxic and ischaemic factors that injure proximal tubules and infringe integrity of their cytoskeleton cause release of KIM-1 from apical membranes of tubules. Thus, this protein can be also measured in urine as a marker of developing of tubular necrosis and early stage of acute kidney injury (AKI) [5,6,23,40, 41, 42].
4.2 Study limitations
The unambiguous confirmation of our hypothesis would require a parallel assessment of plasma FABP and OP concentrations to exclude extra-renal sources of these proteins. However, even assuming the increased synthesis of these proteins in the peripheral tissues, their increased excretion into urine suggests kidney dysfunction. Under physiological conditions, most of FABP and OP undergo to some degree the tubular resorption following filtration in the glomeruli. Thus, the significant differences related to urinary excretion of those proteins found in IF-treated rats and control ones suggest kidney dysfunction. Moreover, the obtained results do not clearly indicate a possible place of injury (proximal or distal tubules). We assayed total FABP, without making a distinction between both isoforms of FABP (L-FABP and H-FABP). As mentioned above, H-FABP is the isoform more specific for proximal tubules, whereas L-FABP for distal ones [34]. However, considering also the presence of osteopontin in urine (the protein more specific for the distal part of nephron) [43], it could be assumed that IF administered repeatedly to rats in group 3 may cause development of tubulopathy also involving distal tubules. The analysis of the results of classical, low molecular weight parameters brought conflicting results. In animals treated with both single and repeated IF doses, decreased urea clearance accompanied by normal urine volume were demonstrated and it may suggest the impaired urine concentration. On the other hand, however, to confirm the aforementioned assumption, urine osmolality analysis should be conducted. Hence, results obtained in our experiment are ambiguous and allow only for confirmation of the general tubulopathy development, with no indication of its precise localization.
4.3 Generalisation of findings to clinical practice
The findings of our study are bringing some new aspects to clinical practice. Considering both results obtained in the currently described experiment and previously performed studies, it may be stated, that IF administered repeatedly contributes to progressive injury of renal tubules without any structural damage at the level of light microscopy. It is possible that kidney damage occurs later than the adopted time perspective in our experiment. According to the above assumption, it should be noted that the structural changes in the kidney are preceded by an increased urinary excretion of our study proteins. Therefore, the novel biomarkers, such as OP, FABP or KIM-1, already appearing in urine in the absence of any signs of structural damage of kidneys, should be considered to reflect an early stage of kidney injury. The application of laboratory surveillance based on FABP and OP monitoring will ensure better safety of pharmacotherapy with ifosfamide.
5 Conclusions
The single dose of 250 mg/kg b.w. of ifosfamide administered to rats caused cystitis and a significant proteinuria. However, urinary elimination of the fatty acid binding protein and osteopontin was normal, as was the histological structure of kidneys.
The treatment with five repeated doses of 50 mg/kg b.w. of ifosfamide resulted in development of cystitis in study rats with increased excretion of the fatty acid binding protein and osteopontin with urine, but with still normal histological structure of kidneys.
The obtained results suggest that assessment of urinary concentration and excretion of the fatty acid binding protein and osteopontin may offer an advantage to the usually performed laboratory analysis (urea, creatinine) with respect to early detection of chronic, but not acute, nephrotoxic effect of ifosfamide, probably manifesting by functional, not yet structural tubulopathy.
The performed experiment suggests that patients treated with ifosfamide should be monitored to improve the safety of the implemented pharmacotherapy.
Acknowledgments
The research described in this paper was not supported by specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Conflict of interest: Authors state no conflict of interest.
-
Author contribution to study:
Łukasz Dobrek – study design, data collection, analysis and interpretation, literature search, manuscript preparation, research funding
Zbigniew Arent – data collection and analysis, research funding
Klaudia Nalik-Iwaniak – data collection, husbandry and care of animals used in the experiment
Kinga Fic – data collection, husbandry and care of animals used in the experiment
Marta Kopańska – literature search, research funding
References
[1] Wang D, Wang H. Oxazaphosphorine bioactivation and detoxification. The role of xenobiotic receptors. Acta Pharm Sin B 2012; 2, 21 pages10.1016/j.apsb.2012.02.004Search in Google Scholar PubMed PubMed Central
[2] Sloderbach A, Górska A, Sikorska M, Misiura K, Hładoń B. Klasyczne oksazafosforiany – metabolizm i właściwości terapeutyczne (Classical oxazaphosphorines – metabolism and therapeutic properties – new implications). Postepy Hig Med Dosw (online) 2013; 67: 1235-1253. (in Polish)10.5604/17322693.1079389Search in Google Scholar PubMed
[3] Liang J, Huang M, Duan W, Yu XQ, Zhou S. Design of new oxazaphosphorine anticancer drugs. Curr Pharm Des 2007; 13: 963-97810.2174/138161207780414296Search in Google Scholar PubMed
[4] Zhang J, Tian Q, Zhu YZ, Xu AL, Zhou SF. Reversal of resistance to oxazaphosphorines. Curr Cancer Drug Targets 2006; 6: 385-40710.2174/156800906777723967Search in Google Scholar PubMed
[5] Furlanut M, Franceschi L. Pharmacology of ifosfamide. Oncology 2003; 65(suppl. 2): 2-610.1159/000073350Search in Google Scholar PubMed
[6] Sharbaf FG, Farhangi H, Assadi F. Prevention of chemotherapy-induced nephrotoxicity in children with cancer. Int J Prev Med 2017; 8: 7610.4103/ijpvm.IJPVM_40_17Search in Google Scholar PubMed PubMed Central
[7] Thomas D, Zachariah S, Elamin AEE, Hashim ALO. Limitations of serum creatinine as a marker of renal function. Sch Acad J Pharm 2017; 6: 168-170Search in Google Scholar
[8] Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AAK, Vernekar SN. Markers of renal function tests. N Am J Med Sci 2010; 2: 170-173Search in Google Scholar
[9] Lombi F, Muryan A, Canzonieri R, Trimarchi H. Biomarkers in acute kidney injury: evidence or paradigm? Nefrologia 2016; 36: 339-34610.1016/j.nefroe.2016.10.006Search in Google Scholar
[10] Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10: 147-15510.2215/CJN.12191213Search in Google Scholar PubMed PubMed Central
[11] Shen P, Li W, Jiang J, He L. Liver fatty acid binding protein protects renal function through down-regulation of oxidative stress in IgA nephropathy. Int J Clin Pathol 2017; 10: 1131-1139Search in Google Scholar
[12] Wolak T, Kim H, Ren Y, Kim J, Vaziri ND, Nicholas SB. Osteopontin modulates angiotensin II–induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int 2009; 76: 32-4310.1038/ki.2009.90Search in Google Scholar PubMed
[13] Borner K, Kisro J, Bruggemann SK, Hagenah W, Peters SO, Wagner T. Metabolism of ifosfamide to chloroacetaldehyde contributes to antitumor activity in vivo. Drugs Metab Dispos 2000; 28: 573-576Search in Google Scholar
[14] Mancini L, Payne GS, Dzik-Jurasz ASK, Leach LO. Ifosfamide pharmacokinetics and hepatobiliary uptake in vivo investigated using single- and double-resonance 31P MRS. Magn Reson Med 2003; 50: 249-25510.1002/mrm.10540Search in Google Scholar PubMed
[15] Sayed-Ahmed MM, Hafez MM, Aldelemy ML, Aleisa AM, Al-Rejaie SS, Al-Hosaini KA, Al-Harbi NO, Al-Harbi MM, Al-Shabanah OA. Downregulation of oxidative and nitrosative apoptotic signaling by L-carnitine in ifosfamide-induced Fanconi syndrome rat model. Oxid Med Cell Longev, Volume 2012, Article ID 696704, 9 pages10.1155/2012/696704Search in Google Scholar PubMed PubMed Central
[16] Ozguven AA, Yılmaz O, Taneli F, Ulman C, Vatansever S, Onag A. Protective role of ketamine against hemorrhagic cystitis in rats receiving ifosfamide. Indian J Pharmacol 2014; 46: 147-15110.4103/0253-7613.129301Search in Google Scholar PubMed PubMed Central
[17] Charakterystyka produktu leczniczego Holoxan http://pub.rejestrymedyczne.csioz.gov.pl (accessed on 14. 02. 2019; in Polish)Search in Google Scholar
[18] Garimella-Krovi S, Springate JE. Effect of glutathione depletion on ifosfamide nephrotoxicity in rats. Int J Biomed Sci 2008; 4: 171-174Search in Google Scholar
[19] Chen N, Aleksa K, Woodland C, Rieder M, Koren G. N-Acetylcysteine prevents ifosfamide-induced nephrotoxicity in rats. Br J Pharmacol 2008; 153: 1364-137210.1038/bjp.2008.15Search in Google Scholar PubMed PubMed Central
[20] Lee G, Romih R, Zupancic D. Cystitis: from urothelial cell biology to clinical applications. BioMed Res Int 2014; Article ID 473536, 10 pages10.1155/2014/473536Search in Google Scholar PubMed PubMed Central
[21] Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol 2015; 4: 57-7310.5527/wjn.v4.i1.57Search in Google Scholar PubMed PubMed Central
[22] Dobrek Ł, Skowron B, Baranowska A, Malska-Woźniak A, Thor P. Urinary kidney injury molecule-1 excretion in rats with experimental cystitis induced by oxazaphosphorines. Przegl Lek 2016; 73: 805-812Search in Google Scholar
[23] Nissim I, Horyn O, Daikhin Y, Nissim I, Luhovyy B, Phillips PC, Yudkoff M. Ifosfamide-induced nephrotoxicity: mechanism and prevention. Cancer Res 2006; 66: 7824-783110.1158/0008-5472.CAN-06-1043Search in Google Scholar PubMed
[24] Elfawy HA, Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci 2019; 218: 165-18410.1016/j.lfs.2018.12.029Search in Google Scholar PubMed
[25] Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, Vessières E, Ayer A, Henrion D, Lenaers G, Reynier P, Procaccio V. Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice. PLOS One 2015; 10: e014429010.1371/journal.pone.0144290Search in Google Scholar PubMed PubMed Central
[26] Ruggiero A , Ferrara P, Attina G, Rizzo D, Riccardi R. Renal toxicity and chemotherapy in children with cancer. Br J Clin Pharmacol 2017; 83: 2605-261410.1111/bcp.13388Search in Google Scholar PubMed PubMed Central
[27] Fuchs TC, Hewitt P. Biomarkers for drug-induced renal damage and nephrotoxicity – an overview for applied toxicology. The AAPS Journal 2011; 13: 615-63110.1208/s12248-011-9301-xSearch in Google Scholar PubMed PubMed Central
[28] Rysz J, Gluba-Brzózka A, Franczyk B, Jabłonowski Z, Ciałkowska-Rysz A. Novel biomarkers in the diagnosis of chronic kidney disease and the prediction of its outcome. Int J Mol Sci 2017; 18: 1702, 17 pages10.3390/ijms18081702Search in Google Scholar PubMed PubMed Central
[29] Xie HG, Wang SK, Cao CC, Harpur E. Qualified kidney biomarkers and their potential significance in drug safety evaluation and prediction. Pharmacol Ther 2013; 137: 100-10710.1016/j.pharmthera.2012.09.004Search in Google Scholar PubMed
[30] Tesch GH. Review: Serum and urine biomarkers of kidney disease: a pathophysiological perspective. Nephrology 2010; 15: 609-61610.1111/j.1440-1797.2010.01361.xSearch in Google Scholar PubMed
[31] Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids 2015; 93: 9-1610.1016/j.plefa.2014.10.001Search in Google Scholar PubMed PubMed Central
[32] Charlton JR, Portilla D, Okusa MD. A basic science view of acute kidney injury biomarkers. Nephrol Dial Transplant 2014; 29: 1301-131110.1093/ndt/gft510Search in Google Scholar PubMed PubMed Central
[33] Chacko S, Haseeb S, Glover BM, Wallbridge D, Harper A. The role of biomarkers in the diagnosis and risk stratification of acute coronary syndrome. Future Sci OA 2017; 4: FSO25110.4155/fsoa-2017-0036Search in Google Scholar PubMed PubMed Central
[34] Erenler AK, Yardan T, Duran L, Baydin A. Usefulness of heart-type fatty acid binding protein in the emergency department. J Pak Med Assoc 2013; 63: 1176-1181Search in Google Scholar
[35] Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 2013; 93: 348-35410.1007/s00223-013-9698-6Search in Google Scholar PubMed
[36] Yasui T, Okada A, Hamamoto S, Ando R, Taguchi K, Tozawa K, Kohri K. Pathophysiology-based treatment of urolithiasis. Int J Urol 2017; 24: 32-3810.1111/iju.13187Search in Google Scholar PubMed
[37] Rittling SR. Osteopontin in macrophage function. Expert Rev Mol Med 2011; 13: e1510.1017/S1462399411001839Search in Google Scholar PubMed
[38] Lim AI, Tang SCW, Lai KN, Leung JCK. Kidney injury molecule-1: more than just an injury marker of tubular epithelial cells? J Cell Physiol 2013; 228: 917-92410.1002/jcp.24267Search in Google Scholar PubMed
[39] Yin C, Wang N. Kidney injury molecule-1 in kidney disease. Ren Fail 2016; 38: 1567-157310.1080/0886022X.2016.1193816Search in Google Scholar PubMed
[40] Barrera-Chimal J, Bobadilla NA. Are recently reported biomarkers helpful for early and accurate diagnosis of acute kidney injury? Biomarkers 2012; 17: 385-39310.3109/1354750X.2012.680070Search in Google Scholar PubMed
[41] Kashani K, Kellum JA. Novel biomarkers indicating repair or progression after acute kidney injury. Curr Opin Nephrol Hypertens 2015; 24: 21-2710.1097/MNH.0000000000000090Search in Google Scholar PubMed
[42] Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017; 55: 1074-108910.1515/cclm-2016-0973Search in Google Scholar PubMed
[43] Kaleta B. The role of osteopontin in kidney diseases. Inflamm Res 2019; 68(2): 93-10210.1007/s00011-018-1200-5Search in Google Scholar PubMed
© 2019 Łukasz Dobrek et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Research Article
- Prostate Cancer-Specific of DD3-driven oncolytic virus-harboring mK5 gene
- Case Report
- Pediatric acute paradoxical cerebral embolism with pulmonary embolism caused by extremely small patent foramen ovale
- Research Article
- Associations between ambient temperature and acute myocardial infarction
- Case Report
- Discontinuation of imatinib mesylate could improve renal impairment in chronic myeloid leukemia
- Research Article
- METTL3 promotes the proliferation and mobility of gastric cancer cells
- The C677T polymorphism of the methylenetetrahydrofolate reductase gene and susceptibility to late-onset Alzheimer’s disease
- microRNA-1236-3p regulates DDP resistance in lung cancer cells
- Review Article
- The link between thyroid autoimmunity, depression and bipolar disorder
- Research Article
- Effects of miR-107 on the Chemo-drug sensitivity of breast cancer cells
- Analysis of pH dose-dependent growth of sulfate-reducing bacteria
- Review Article
- Musculoskeletal clinical and imaging manifestations in inflammatory bowel diseases
- Research Article
- Regional hyperthermia combined with chemotherapy in advanced gastric cancer
- Analysis of hormone receptor status in primary and recurrent breast cancer via data mining pathology reports
- Morphological and isokinetic strength differences: bilateral and ipsilateral variation by different sport activity
- The reliability of adjusting stepped care based on FeNO monitoring for patients with chronic persistent asthma
- Comparison of the clinical outcomes of two physiological ischemic training methods in patients with coronary heart disease
- Analysis of ticagrelor’s cardio-protective effects on patients with ST-segment elevation acute coronary syndrome accompanied with diabetes
- Computed tomography findings in patients with Samter’s Triad: an observational study
- Case Report
- A spinal subdural hematoma induced by guidewire-based lumbar drainage in a patient with ruptured intracranial aneurysms
- Research Article
- High expression B3GAT3 is related with poor prognosis of liver cancer
- Effects of light touch on balance in patients with stroke
- Oncoprotein LAMTOR5 activates GLUT1 via upregulating NF-κB in liver cancer
- Effects of budesonide combined with noninvasive ventilation on PCT, sTREM-1, chest lung compliance, humoral immune function and quality of life in patients with AECOPD complicated with type II respiratory failure
- Prognostic significance of lymph node ratio in ovarian cancer
- Case Report
- Brainstem anaesthesia after retrobulbar block
- Review Article
- Treating infertility: current affairs of cross-border reproductive care
- Research Article
- Serum inflammatory cytokines comparison in gastric cancer therapy
- Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes
- MRI and bone scintigraphy for breast cancer bone metastase: a meta-analysis
- Comparative study of back propagation artificial neural networks and logistic regression model in predicting poor prognosis after acute ischemic stroke
- Analysis of the factors affecting the prognosis of glioma patients
- Compare fuhrman nuclear and chromophobe tumor grade on chromophobe RCC
- Case Report
- Signet ring B cell lymphoma: A potential diagnostic pitfall
- Research Article
- Subparaneural injection in popliteal sciatic nerve blocks evaluated by MRI
- Loneliness in the context of quality of life of nursing home residents
- Biological characteristics of cervical precancerous cell proliferation
- Effects of Rehabilitation in Bankart Lesion in Non-athletes: A report of three cases
- Management of complications of first instance of hepatic trauma in a liver surgery unit: Portal vein ligation as a conservative therapeutic strategy
- Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect
- Comparison of laparoscopy and open radical nephrectomy of renal cell cancer
- Case Report
- A severe complication of myocardial dysfunction post radiofrequency ablation treatment of huge hepatic hemangioma: a case report and literature review
- Solar urticaria, a disease with many dark sides: is omalizumab the right therapeutic response? Reflections from a clinical case report
- Research Article
- Binge eating disorder and related features in bariatric surgery candidates
- Propofol versus 4-hydroxybutyric acid in pediatric cardiac catheterizations
- Nasointestinal tube in mechanical ventilation patients is more advantageous
- The change of endotracheal tube cuff pressure during laparoscopic surgery
- Correlation between iPTH levels on the first postoperative day after total thyroidectomy and permanent hypoparathyroidism: our experience
- Case Report
- Primary angiosarcoma of the kidney: case report and comprehensive literature review
- Research Article
- miR-107 enhances the sensitivity of breast cancer cells to paclitaxel
- Incidental findings in dental radiology are concerning for family doctors
- Suffering from cerebral small vessel disease with and without metabolic syndrome
- A meta-analysis of robot assisted laparoscopic radical prostatectomy versus laparoscopic radical prostatectomy
- Indications and outcomes of splenectomy for hematological disorders
- Expression of CENPE and its prognostic role in non-small cell lung cancer
- Barbed suture and gastrointestinal surgery. A retrospective analysis
- Using post transplant 1 week Tc-99m DTPA renal scan as another method for predicting renal graft failure
- The pseudogene PTTG3P promotes cell migration and invasion in esophageal squamous cell carcinoma
- Lymph node ratio versus TNM system as prognostic factor in colorectal cancer staging. A single Center experience
- Review Article
- Minimally invasive pilonidal sinus treatment: A narrative review
- Research Article
- Anatomical workspace study of Endonasal Endoscopic Transsphenoidal Approach
- Hounsfield Units on Lumbar Computed Tomography for Predicting Regional Bone Mineral Density
- Communication
- Aspirin, a potential GLUT1 inhibitor in a vascular endothelial cell line
- Research Article
- Osteopontin and fatty acid binding protein in ifosfamide-treated rats
- Familial polyposis coli: the management of desmoid tumor bleeding
- microRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1
- PYCR1 is associated with papillary renal cell carcinoma progression
- Prediction of recurrence-associated death from localized prostate cancer with a charlson comorbidity index–reinforced machine learning model
- Colorectal cancer in the elderly patient: the role of neo-adjuvant therapy
- Association between MTHFR genetic polymorphism and Parkinson’s disease susceptibility: a meta-analysis
- Metformin can alleviate the symptom of patient with diabetic nephropathy through reducing the serum level of Hcy and IL-33
- Case Report
- Severe craniofacial trauma after multiple pistol shots
- Research Article
- Echocardiography evaluation of left ventricular diastolic function in elderly women with metabolic syndrome
- Tailored surgery in inguinal hernia repair. The role of subarachnoid anesthesia: a retrospective study
- The factors affecting early death in newly diagnosed APL patients
- Review Article
- Oncological outcomes and quality of life after rectal cancer surgery
- Research Article
- MiR-638 repressed vascular smooth muscle cell glycolysis by targeting LDHA
- microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma
- Analyzing the semantic space of the Hippocratic Oath
- Fournier’s gangrene and intravenous drug abuse: an unusual case report and review of the literature
- Evaluation of surgical site infection in mini-invasive urological surgery
- Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway
- Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients
- Case Report
- Appendix bleeding with painless bloody diarrhea: A case report and literature review
- Research Article
- Protective effects of specneuzhenide on renal injury in rats with diabetic nephropathy
- PBF, a proto-oncogene in esophageal carcinoma
- Use of rituximab in NHL malt type pregnant in I° trimester for two times
- Cancer- and non-cancer related chronic pain: from the physiopathological basics to management
- Case report
- Non-surgical removal of dens invaginatus in maxillary lateral incisor using CBCT: Two-year follow-up case report
- Research Article
- Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU
- Accuracy of tumor perfusion assessment in Rat C6 gliomas model with USPIO
- Lemann Index for Assessment of Crohn’s Disease: Correlation with the Quality of Life, Endoscopic Disease activity, Magnetic Resonance Index of Activity and C- Reactive Protein
- Case report
- Münchausen syndrome as an unusual cause of pseudo-resistant hypertension: a case report
- Research Article
- Renal artery embolization before radical nephrectomy for complex renal tumour: which are the true advantages?
- Prognostic significance of CD276 in non-small cell lung cancer
- Potential drug-drug interactions in acute ischemic stroke patients at the Neurological Intensive Care Unit
- Effect of vitamin D3 on lung damage induced by cigarette smoke in mice
- CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge miRNA-767-5p
- Case report
- Partial hydatidiform mole and coexistent live fetus: a case report and review of the literature
- Research Article
- Effect of NGR1 on the atopic dermatitis model and its mechanisms
- Clinical features of infertile men carrying a chromosome 9 translocation
- Review Article
- Expression and role of microRNA-663b in childhood acute lymphocytic leukemia and its mechanism
- Case Report
- Mature cystic teratoma of the pancreas: A rare cystic neoplasm
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Case Report
- Predictive factors of intestinal necrosis in acute mesenteric ischemia
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Effects of dexmedetomidine on the RhoA /ROCK/ Nox4 signaling pathway in renal fibrosis of diabetic rats
- MicroRNA-181a-5p regulates inflammatory response of macrophages in sepsis
- Intraventricular pressure in non-communicating hydrocephalus patients before endoscopic third ventriculostomy
- CyclinD1 is a new target gene of tumor suppressor miR-520e in breast cancer
- CHL1 and NrCAM are primarily expressed in low grade pediatric neuroblastoma
- Epidemiological characteristics of postoperative sepsis
- Association between unstable angina and CXCL17: a new potential biomarker
- Cardiac strains as a tool for optimization of cardiac resynchronization therapy in non-responders: a pilot study
- Case Report
- Resuscitation following a bupivacaine injection for a cervical paravertebral block
- Research Article
- CGF treatment of leg ulcers: A randomized controlled trial
- Surgical versus sequential hybrid treatment of carotid body tumors
Articles in the same Issue
- Research Article
- Prostate Cancer-Specific of DD3-driven oncolytic virus-harboring mK5 gene
- Case Report
- Pediatric acute paradoxical cerebral embolism with pulmonary embolism caused by extremely small patent foramen ovale
- Research Article
- Associations between ambient temperature and acute myocardial infarction
- Case Report
- Discontinuation of imatinib mesylate could improve renal impairment in chronic myeloid leukemia
- Research Article
- METTL3 promotes the proliferation and mobility of gastric cancer cells
- The C677T polymorphism of the methylenetetrahydrofolate reductase gene and susceptibility to late-onset Alzheimer’s disease
- microRNA-1236-3p regulates DDP resistance in lung cancer cells
- Review Article
- The link between thyroid autoimmunity, depression and bipolar disorder
- Research Article
- Effects of miR-107 on the Chemo-drug sensitivity of breast cancer cells
- Analysis of pH dose-dependent growth of sulfate-reducing bacteria
- Review Article
- Musculoskeletal clinical and imaging manifestations in inflammatory bowel diseases
- Research Article
- Regional hyperthermia combined with chemotherapy in advanced gastric cancer
- Analysis of hormone receptor status in primary and recurrent breast cancer via data mining pathology reports
- Morphological and isokinetic strength differences: bilateral and ipsilateral variation by different sport activity
- The reliability of adjusting stepped care based on FeNO monitoring for patients with chronic persistent asthma
- Comparison of the clinical outcomes of two physiological ischemic training methods in patients with coronary heart disease
- Analysis of ticagrelor’s cardio-protective effects on patients with ST-segment elevation acute coronary syndrome accompanied with diabetes
- Computed tomography findings in patients with Samter’s Triad: an observational study
- Case Report
- A spinal subdural hematoma induced by guidewire-based lumbar drainage in a patient with ruptured intracranial aneurysms
- Research Article
- High expression B3GAT3 is related with poor prognosis of liver cancer
- Effects of light touch on balance in patients with stroke
- Oncoprotein LAMTOR5 activates GLUT1 via upregulating NF-κB in liver cancer
- Effects of budesonide combined with noninvasive ventilation on PCT, sTREM-1, chest lung compliance, humoral immune function and quality of life in patients with AECOPD complicated with type II respiratory failure
- Prognostic significance of lymph node ratio in ovarian cancer
- Case Report
- Brainstem anaesthesia after retrobulbar block
- Review Article
- Treating infertility: current affairs of cross-border reproductive care
- Research Article
- Serum inflammatory cytokines comparison in gastric cancer therapy
- Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes
- MRI and bone scintigraphy for breast cancer bone metastase: a meta-analysis
- Comparative study of back propagation artificial neural networks and logistic regression model in predicting poor prognosis after acute ischemic stroke
- Analysis of the factors affecting the prognosis of glioma patients
- Compare fuhrman nuclear and chromophobe tumor grade on chromophobe RCC
- Case Report
- Signet ring B cell lymphoma: A potential diagnostic pitfall
- Research Article
- Subparaneural injection in popliteal sciatic nerve blocks evaluated by MRI
- Loneliness in the context of quality of life of nursing home residents
- Biological characteristics of cervical precancerous cell proliferation
- Effects of Rehabilitation in Bankart Lesion in Non-athletes: A report of three cases
- Management of complications of first instance of hepatic trauma in a liver surgery unit: Portal vein ligation as a conservative therapeutic strategy
- Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect
- Comparison of laparoscopy and open radical nephrectomy of renal cell cancer
- Case Report
- A severe complication of myocardial dysfunction post radiofrequency ablation treatment of huge hepatic hemangioma: a case report and literature review
- Solar urticaria, a disease with many dark sides: is omalizumab the right therapeutic response? Reflections from a clinical case report
- Research Article
- Binge eating disorder and related features in bariatric surgery candidates
- Propofol versus 4-hydroxybutyric acid in pediatric cardiac catheterizations
- Nasointestinal tube in mechanical ventilation patients is more advantageous
- The change of endotracheal tube cuff pressure during laparoscopic surgery
- Correlation between iPTH levels on the first postoperative day after total thyroidectomy and permanent hypoparathyroidism: our experience
- Case Report
- Primary angiosarcoma of the kidney: case report and comprehensive literature review
- Research Article
- miR-107 enhances the sensitivity of breast cancer cells to paclitaxel
- Incidental findings in dental radiology are concerning for family doctors
- Suffering from cerebral small vessel disease with and without metabolic syndrome
- A meta-analysis of robot assisted laparoscopic radical prostatectomy versus laparoscopic radical prostatectomy
- Indications and outcomes of splenectomy for hematological disorders
- Expression of CENPE and its prognostic role in non-small cell lung cancer
- Barbed suture and gastrointestinal surgery. A retrospective analysis
- Using post transplant 1 week Tc-99m DTPA renal scan as another method for predicting renal graft failure
- The pseudogene PTTG3P promotes cell migration and invasion in esophageal squamous cell carcinoma
- Lymph node ratio versus TNM system as prognostic factor in colorectal cancer staging. A single Center experience
- Review Article
- Minimally invasive pilonidal sinus treatment: A narrative review
- Research Article
- Anatomical workspace study of Endonasal Endoscopic Transsphenoidal Approach
- Hounsfield Units on Lumbar Computed Tomography for Predicting Regional Bone Mineral Density
- Communication
- Aspirin, a potential GLUT1 inhibitor in a vascular endothelial cell line
- Research Article
- Osteopontin and fatty acid binding protein in ifosfamide-treated rats
- Familial polyposis coli: the management of desmoid tumor bleeding
- microRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1
- PYCR1 is associated with papillary renal cell carcinoma progression
- Prediction of recurrence-associated death from localized prostate cancer with a charlson comorbidity index–reinforced machine learning model
- Colorectal cancer in the elderly patient: the role of neo-adjuvant therapy
- Association between MTHFR genetic polymorphism and Parkinson’s disease susceptibility: a meta-analysis
- Metformin can alleviate the symptom of patient with diabetic nephropathy through reducing the serum level of Hcy and IL-33
- Case Report
- Severe craniofacial trauma after multiple pistol shots
- Research Article
- Echocardiography evaluation of left ventricular diastolic function in elderly women with metabolic syndrome
- Tailored surgery in inguinal hernia repair. The role of subarachnoid anesthesia: a retrospective study
- The factors affecting early death in newly diagnosed APL patients
- Review Article
- Oncological outcomes and quality of life after rectal cancer surgery
- Research Article
- MiR-638 repressed vascular smooth muscle cell glycolysis by targeting LDHA
- microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma
- Analyzing the semantic space of the Hippocratic Oath
- Fournier’s gangrene and intravenous drug abuse: an unusual case report and review of the literature
- Evaluation of surgical site infection in mini-invasive urological surgery
- Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway
- Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients
- Case Report
- Appendix bleeding with painless bloody diarrhea: A case report and literature review
- Research Article
- Protective effects of specneuzhenide on renal injury in rats with diabetic nephropathy
- PBF, a proto-oncogene in esophageal carcinoma
- Use of rituximab in NHL malt type pregnant in I° trimester for two times
- Cancer- and non-cancer related chronic pain: from the physiopathological basics to management
- Case report
- Non-surgical removal of dens invaginatus in maxillary lateral incisor using CBCT: Two-year follow-up case report
- Research Article
- Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU
- Accuracy of tumor perfusion assessment in Rat C6 gliomas model with USPIO
- Lemann Index for Assessment of Crohn’s Disease: Correlation with the Quality of Life, Endoscopic Disease activity, Magnetic Resonance Index of Activity and C- Reactive Protein
- Case report
- Münchausen syndrome as an unusual cause of pseudo-resistant hypertension: a case report
- Research Article
- Renal artery embolization before radical nephrectomy for complex renal tumour: which are the true advantages?
- Prognostic significance of CD276 in non-small cell lung cancer
- Potential drug-drug interactions in acute ischemic stroke patients at the Neurological Intensive Care Unit
- Effect of vitamin D3 on lung damage induced by cigarette smoke in mice
- CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge miRNA-767-5p
- Case report
- Partial hydatidiform mole and coexistent live fetus: a case report and review of the literature
- Research Article
- Effect of NGR1 on the atopic dermatitis model and its mechanisms
- Clinical features of infertile men carrying a chromosome 9 translocation
- Review Article
- Expression and role of microRNA-663b in childhood acute lymphocytic leukemia and its mechanism
- Case Report
- Mature cystic teratoma of the pancreas: A rare cystic neoplasm
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Case Report
- Predictive factors of intestinal necrosis in acute mesenteric ischemia
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Effects of dexmedetomidine on the RhoA /ROCK/ Nox4 signaling pathway in renal fibrosis of diabetic rats
- MicroRNA-181a-5p regulates inflammatory response of macrophages in sepsis
- Intraventricular pressure in non-communicating hydrocephalus patients before endoscopic third ventriculostomy
- CyclinD1 is a new target gene of tumor suppressor miR-520e in breast cancer
- CHL1 and NrCAM are primarily expressed in low grade pediatric neuroblastoma
- Epidemiological characteristics of postoperative sepsis
- Association between unstable angina and CXCL17: a new potential biomarker
- Cardiac strains as a tool for optimization of cardiac resynchronization therapy in non-responders: a pilot study
- Case Report
- Resuscitation following a bupivacaine injection for a cervical paravertebral block
- Research Article
- CGF treatment of leg ulcers: A randomized controlled trial
- Surgical versus sequential hybrid treatment of carotid body tumors


