Abstract
Folate metabolism plays quite a critical role in Parkinson’s disease (PD). Previous published research works have studied the link existing between the folate metabolism genetic polymorphisms and PD susceptibility; nevertheless, the results continue having controversies and inconclusiveness. Accordingly, we carried out the present meta-analysis for the assessment of the potential link between the folate metabolism genetic polymorphisms and the susceptibility to PD. In addition we carried out a literature search in the PubMed, EMBASE, Cochrane Library, and WanFang databases till November 10, 2018. The odds ratios (ORs) with corresponding 95% credible interval (95%CI) were put to use for evaluating the strength of the association of three folate metabolism genetic polymorphism ( C677T, A1298C, and A2756G) with the susceptibility to PD. Each statistical analysis was carried out with the use of STATA 15.0. An aggregate of twenty-one case-control investigations were retrieved, which involved 3,944 PD patients and 4,412 controls. We discovered the existence of no substantial link between the C677T and A1298C polymorphism and PD risk in any genetic framework comparisons. With regard to A2756G polymorphism, we discovered that there was an association between the A2756G genetic polymorphism and an augmented threat of PD in the co-dominant genetic framework (GG vs. AA: OR=1.86, 95%CI=1.02-3.37, P=0.042) and the recessive genetic model (GG vs. GA+AA: OR=1.90, 95%CI=1.06-3.41, P=0.031). To summarize, our research work indicates that the A2756G polymorphism of the folate metabolism gene had an association with an augmented threat of PD. Also, A1298C polymorphisms is unlikely to significantly contribute towards the susceptibility to PD. Further large-scale case-control studies are still required.
1 Introduction
Parkinson’s disease (PD) is termed as the second most frequently prevalent neurodegenerative disorder following the Alzheimer’s disease, which impacts approximately 1% of the individuals aged more than 60 across the globe and 4-5% of people aged more than 85 years [1, 2] . Clinically, PD is manifested by the classical motor symptoms, which include not only the tremor, but also the rigidity, bradykinesia, and postural instability, significantly impacting the patients’ quality of life [2, 3]. These medicinal presentations constitute the results of dopaminergic neuron loss in the substantia nigra, leading the lowered degrees of dopamine in the striatum and disrupted motor control. Despite the fact that the reason leading to the neuronal loss is not clear, more and more evidence has suggested that mitochondrial impairment, endothelial damage, inflammatory process, and oxidative stress are considered as playing key roles when it comes to the selective dopaminergic cell death in the brain of those patients, who have PD [4, 5].
Homocysteine (Hcy) has been observed as enhancing mitochondrial dysfunction, apoptosis, and oxidative stress, together with being a contributing determinant in the pathophysiological process to a number of neurodegenerative diseases that include PD [6, 7]. Hcy is a sulfur-containing amino acid, which is derived from the demethylation of methionine by means of the methionine cycle and the folate cycle [8]. Methylenetetrahydrofolate reductase (MTHFR) is a folate-reliant enzyme that catalyzes 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. The output is termed as the dominant form of circulating folate, besides providing a methyl group for the re-methylation of Hcy back to methionine [8]. Three common variants (C677T, A1298C, and A2756G) in the MTHFR gene, which lower the function of MTHFR, in addition to further resulting into the metabolic disruption of Hcy, have been indicated as having association with the risk of patients with PD [8, 9].
A number of case-control investigations have explored the potential link that exists between the MTHFR genetic polymorphisms and susceptibility to PD, but the findings are still inconclusive [9, 10, 11, 12]. Hence, we carried out the present meta-analysis in order to clarify the link existing between the MTHFR polymorphisms and susceptibility to PD with the use of qualified data attained from the published case-control research works.
2 Materials and methods
The current meta-analysis was carried out in accordance with the consensus statement of the Meta-analysis of Observational Studies in Epidemiology recommendations [13]. All the analysis was carried out on the basis of previously published works; in this manner, ethical approval and patient consent were not required.
2.1 Identification of eligible studies
A literature search in the PubMed, as well as in the EMBASE, Cochrane Library, and WanFang databases was carried out to figure out the eligible studies, and the latest search was updated in November 2018. The terms presented as follows, together with their combinations, were searched: ‘methylenetetrahydrofolate reductase’ OR ‘MTHFR’ AND ‘Parkinson’s disease’ OR ‘PD’ AND ‘polymorphism*’ OR ‘variant*’. Each of the search was constrained to English or Chinese language papers.
2.2 Inclusion and exclusion
The qualified research works mandatorily require meeting the inclusion criteria as hereunder: (1) evaluate the link existing between the MTHFR polymorphisms and susceptibility to PD; (2) case-control research design; (3) enough amount of data for the calculation of the odds ratios (ORs), coupled with 95 percent confidence interval (95%CI). The research works were not counted on for any of the following reasons: (1) inadequate amount of information for extracting the data; (2) only case study; (3) case reports, reviews, letters; (4) studies carried out in non-humans, and duplicated publication. For the research works having copied data, the biggest or the latest publication was chosen.
2.3 Data extraction
All data were independently extracted by two investigators in accordance with the above-mentioned inclusion criteria. Moreover, the following information was extracted from all of the research works included: the first author, in addition to the year of publication, country, ethnicity, genotype distributions in cases as well as controls, besides the detection methodology of genotypes. The disagreement existing between scholars were removed through the consultation with a 3rd scholar.
2.4 Statistical analysis
The Hardy-Weinberg equilibrium (HWE) was calculated by the Chi-squared test in control groups in all of the research works; the P-value more than 0.001 demonstrated that the population was in genetic equilibrium. Besides that, the odds ratios (ORs) that had 95 percent confidence interval (CI) were adopted for the purpose of calculating the strength of the link between PD susceptibility and the MTHFR polymorphisms. The importance associated with the accumulated OR was investigated in accordance with the Z-test; in addition, P<0.05 was regarded as having statistical significance. The evaluation of the between-study heterogeneity was carried out with the help of the Q-test as well as I2 statistics [14]. Moreover, the application of the fixed-effects framework was made at Ph>0.1 or I2<50% [15]; or else, the random-effects framework was followed [16]. The sensitivity analysis was carried out through the omission of the single research work always for the evaluation of the robustness of the findings. Also, the underlying publication partiality was assessed with the help of the Begg’s funnel plots and Egger’s test [17, 18]. All of the statistical analysis was carried out with the use of the STATA version 15.0 software (Stata Corporation, College Station, TX, USA). A P-value below 0.05 was regarded as having statistical significance.
3 Results
3.1 Study characteristics
The flow chart presented in Figure 1 shed light on the research selection mechanism. An aggregate of 256 papers were figured out by means of the preliminary search of databases. In accordance with the inclusion and exclusion criteria presented earlier, an aggregate of 21 case-control research works, which involved 3,944 PD patients and 4,412 controls, were included [10, 11, 12, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]. Among the selected studies, 20 studies involving 3,712 PD patients and 4,167 controls were carried out for the purpose of evaluating MTHFR C677T polymorphism [10, 11, 12, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35]; 10 studies were carried out for the assessment of MTHFR A1298C polymorphism with an aggregate of 1,861 PD patients, together with 1,987 controls [12, 19, 23, 24, 25, 27, 31, 33, 34, 36]; 4 research works that contained 541 PD patients as well as 709 controls, were performed for the purpose of evaluating MTHFR A2756G polymorphism [19, 26, 29, 31]. Moreover, the key attributes of all of the investigations and HWE examination results were presented in Table 1. The allocations of the MTHFR genetic mutation in controls were observed as showing consistency with HWE in all studies (P>0.001).
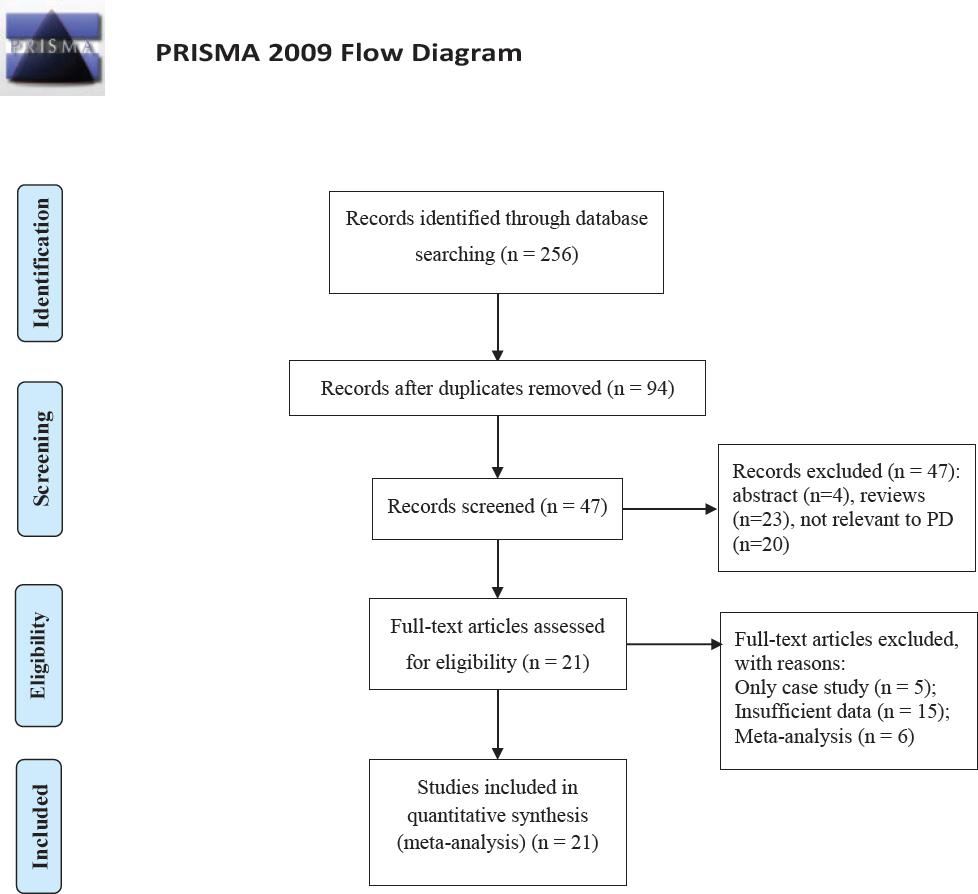
Flow diagram of included and excluded studies.
Main characteristics of included studies in this meta-analysis.
| Author | Year | Country | Ethnicity | Control Source | Sample Size Case | Control | Genotyping Methods | HWE in controls |
|---|---|---|---|---|---|---|---|---|
| Dorszewska | 2007 | Poland | Caucasian | PB | 98 | 50 | PCR-RFLP | 0.495 |
| Lin | 2007 | China | Asian | PB | 94 | 146 | PCR-RFLP | 0.410 |
| Yasui | 2000 | Japan | Asian | PB | 90 | 53 | PCR | 0.194 |
| Harmon | 1997 | Ireland | Caucasian | PB | 188 | 184 | PCR | 0.132 |
| Białecka | 2012 | Poland | Caucasian | HB | 320 | 254 | PCR-RFLP | 0.855 |
| Caccamo | 2007 | Italy | Caucasian | PB | 49 | 86 | PCR | 0.376 |
| Camicioli | 2009 | Canada | Caucasian | HB | 51 | 49 | PCR | 0.804 |
| Fong | 2011 | China | Asian | HB | 211 | 218 | PCR | 0.494 |
| Wullner | 2005 | Germany | Caucasian | NA | 342 | 342 | PCR | 0.021 |
| Garcia | 2015 | Mexico | Caucasian | PB | 140 | 216 | TaqMan | 0.118 |
| Gorgone | 2012 | Italy | Caucasian | PB | 60 | 82 | PCR | 0.430 |
| Kumudini | 2014 | India | Asian | PB | 151 | 416 | PCR-RFLP | 0.352 |
| Liao | 2014 | China | Asian | PB | 765 | 717 | PCR | 0.013 |
| Religa | 2006 | Poland | Caucasian | HB | 114 | 100 | PCR | 0.901 |
| Rodriguez-Oroz | 2009 | Spain | Caucasian | PB | 77 | 28 | PCR-RFLP | 0.748 |
| Todorovic | 2006 | Serbia | Caucasian | PB | 113 | 53 | PCR | 0.583 |
| Yuan | 2016 | China | Asian | PB | 512 | 512 | PCR | 0.347 |
| Yuan | 2009 | China | Asian | HB | 76 | 110 | PCR-RFLP | 0.652 |
| Zahra | 2016 | Malta | Caucasian | PB | 100 | 311 | PCR | 0.382 |
| Chao | 2014 | China | Asian | HB | 161 | 240 | PCR | 0.198 |
| Momose | 2002 | Japan | Asian | PB | 232 | 245 | PCR | 0.849 |
Abbreviations: HWE, Hardy-Weinberg equilibrium; PCR-RFLP, Polymerase chain reaction-restriction fragment length polymorphism; PB, Population-based; HB, Hospital-based.
3.2 MTHFR C677T and PD Susceptibility
With regard to the MTHFR C677T polymorphism, as revealed by the meta-analysis, there was no statistically significant association in any of the genetic frameworks in the population, in general (Figure 2A). For the stratification in accordance with ethnicity, no substantial link in any of the genetic frameworks in Caucasians or Asians (Figure 2B) was observed. In the meantime, we conducted the stratified analysis in accordance with the source of controls, substantially augmenting the risk of PD discovered in the allelic genetic framework (T vs. C: OR=1.18, 95%CI=1.01-1.39, P=0.043), co-dominant genetic framework (TC vs. CC: OR=1.24, 95%CI=1.02-1.51, P=0.034), and dominant genetic framework (TT+TC vs. CC: OR=1.27, 95%CI=1.03-1.55, P=0.023) in the hospital-based study (Figure 2C and 2D). Table 2 summarizes the meta-analysis findings between MTHFR C677T polymorphism and PD susceptibility.

Forest plot of the association between MTHFR C677T polymorphism and PD susceptibility: (A) allelic genetic model in the overall populations; (B) allelic genetic model, stratified by ethnicity; (C) allelic and (D) co-dominant genetic model, stratified by source of control.
Summary ORs and 95%CI of the association between C677T polymorphism and Parkinson disease susceptibility.
| Genetic models | Study subjects | No. of studies | OR (95%CI) | p-meta | Test of heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | Model | Test of Egger’s | |||||
| T vs. C | Overall | 20 | 1.07(0.94-1.22) | 0.297 | 66.5 | <0.001 | R | 0.206 |
| Asian | 8 | 1.08(0.89-1.32) | 0.429 | 71.8 | 0.001 | R | ||
| Caucasian | 12 | 1.06(0.89-1.27) | 0.497 | 61.0 | 0.003 | R | ||
| PB | 13 | 1.03(0.86-1.22) | 0.781 | 69.6 | <0.001 | R | ||
| HB | 6 | 1.18(1.01-1.39) | 0.043 | 22.5 | 0.265 | R | ||
| TC vs. CC | Overall | 20 | 1.06(0.90-1.25) | 0.494 | 56.3 | 0.001 | R | 0.581 |
| Asian | 8 | 1.06(0.81-1.40) | 0.676 | 70.9 | 0.001 | R | ||
| Caucasian | 12 | 1.09(0.90-1.30) | 0.377 | 23.4 | 0.213 | R | ||
| PB | 13 | 0.93(0.76-1.14) | 0.491 | 48.6 | 0.025 | F | ||
| HB | 6 | 1.24(1.02-1.51) | 0.034 | 0 | 0.426 | F | ||
| TT vs. CC | Overall | 20 | 1.08(0.83-1.42) | 0.567 | 58.6 | 0.001 | R | 0.064 |
| Asian | 8 | 1.07(0.74-1.54) | 0.719 | 53.9 | 0.034 | R | ||
| Caucasian | 12 | 1.05(0.70-1.57) | 0.812 | 62.3 | 0.002 | R | ||
| PB | 13 | 1.03(0.70-1.52) | 0.878 | 66.3 | <0.001 | R | ||
| HB | 6 | 1.30(0.96-1.77) | 0.094 | 0 | 0.469 | R | ||
| TT+TC vs. CC | Overall | 20 | 1.08(0.91-1.29) | 0.386 | 64.6 | <0.001 | R | 0.075 |
| Asian | 8 | 1.09(0.82-1.45) | 0.547 | 75.3 | <0.001 | R | ||
| Caucasian | 12 | 1.09(0.88-1.34) | 0.442 | 45.0 | 0.045 | R | ||
| PB | 13 | 0.98(0.78-1.22) | 0.842 | 63.5 | 0.001 | R | ||
| HB | 6 | 1.27(1.03-1.55) | 0.023 | 11.5 | 0.342 | R | ||
| TT vs. CC+CT | Overall | 20 | 1.06(0.86-1.30) | 0.942 | 43.3 | 0.021 | F | 0.186 |
| Asian | 8 | 0.99(0.79-1.24) | 0.926 | 15.5 | 0.308 | R | ||
| Caucasian | 12 | 1.04(0.74-1.47) | 0.814 | 55.9 | 0.009 | R | ||
| PB | 13 | 1.07(0.78-1.45) | 0.690 | 56.6 | 0.006 | R | ||
| HB | 6 | 1.17(0.88-1.54) | 0.283 | 0 | 0.557 | R | ||
Abbreviations: Bold values indicate statistically significant results; OR, Odds ratio; CI, Confidence interval; p-meta, p-value of pooled effect; R, Random-effect model; F, Fixed-effect model;
3.3 MTHFR A1298C and PD Susceptibility
With regard to MTHFR A1298C polymorphism, the findings, on the basis of all of the involved research works, did not provide any proof of a link between MTHFR A1298C polymorphism and PD risk in any of the genetic framework in the population, in general (Figure 3A). For the stratified analysis in accordance with ethnicity, no substantial link in any genetic models in Caucasians or Asians (Figure 3B) was discovered. In the subgroup analysis in accordance with the source of controls, the significantly augmented susceptibility of PD was discovered in the allelic genetic framework (C vs. A: OR=1.17, 95%CI=1.02-1.35, P=0.029), co-dominant genetic model (CA vs. AA: OR=1.25, 95%CI=1.04-1.51, P=0.016), and dominant genetic model (TT+TC vs. CC: OR=1.25, 95%CI=1.05-1.49, P=0.014) in the population-based study (Figure 3C and 3D). Table 3 provides the summary of the findings of comparisons.
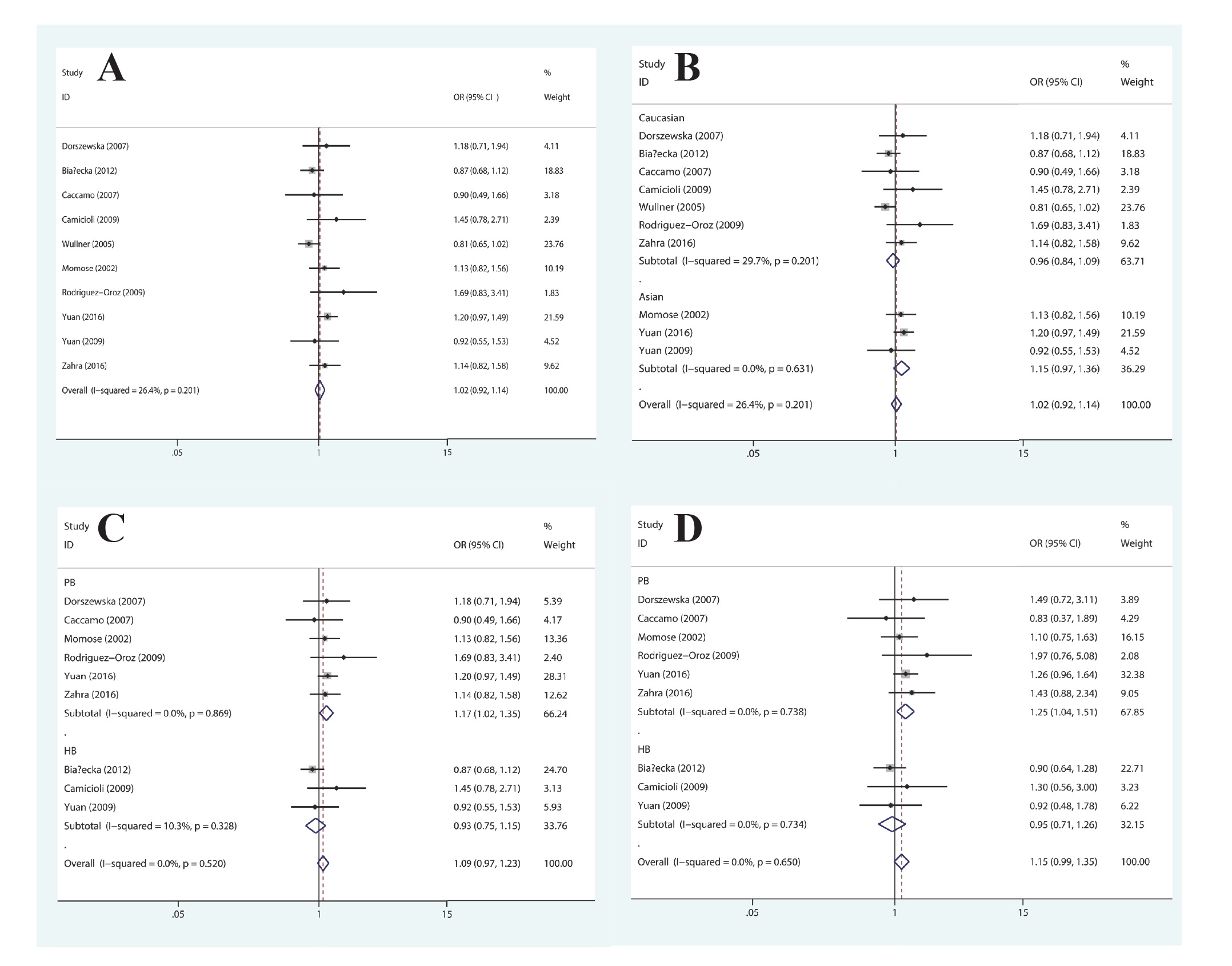
Forest plot of the association between MTHFR A1298C polymorphism and PD susceptibility: (A) allelic genetic model in the overall populations; (B) allelic genetic model, stratified by ethnicity; (C) allelic and (D) co-dominant genetic model, stratified by source of control.
Summary ORs and 95%CI of the association between A1298C polymorphism and Parkinson disease susceptibility.
| Genetic models | Study subjects | No. of studies | OR (95%CI) | p-meta | Test of heterogeneity | Test of Egger’s | |||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | Model | |||||||
| C vs. A | Overall | 10 | 1.02(0.92-1.14) | 0.652 | 26.4 | 0.201 | F | 0.275 | |
| Asian | 3 | 1.15(0.97-1.36) | 0.115 | 0 | 0.631 | F | |||
| Caucasian | 7 | 0.96(0.84-1.09) | 0.503 | 29.7 | 0.201 | F | |||
| PB | 6 | 1.17(1.02-1.35) | 0.029 | 0 | 0.869 | F | |||
| HB | 3 | 0.93(0.75-1.15) | 0.515 | 10.3 | 0.328 | F | |||
| CA vs. AA | Overall | 10 | 1.11(0.97-1.28) | 0.144 | 0 | 0.608 | F | 0.499 | |
| Asian | 3 | 1.17(0.95-1.45) | 0.131 | 0 | 0.638 | F | |||
| Caucasian | 7 | 1.06(0.88-1.28) | 0.542 | 0 | 0.439 | F | |||
| PB | 6 | 1.25(1.04-1.51) | 0.016 | 0 | 0.738 | F | |||
| HB | 3 | 0.95(0.71-1.26) | 0.703 | 0 | 0.734 | F | |||
| CC vs. AA | Overall | 10 | 0.93(0.72-1.19) | 0.557 | 0 | 0.491 | F | 0.121 | |
| Asian | 3 | 1.21(0.75-1.94) | 0.437 | 0 | 0.830 | F | |||
| Caucasian | 7 | 0.83(0.62-1.13) | 0.237 | 5.4 | 0.386 | F | |||
| PB | 6 | 1.24(0.86-1.79) | 0.251 | 0 | 0.975 | F | |||
| HB | 3 | 0.85(0.52-1.40) | 0.527 | 6.1 | 0.345 | F | |||
| CC+CA vs. AA | Overall | 10 | 1.08(0.95-1.23) | 0.261 | 11.5 | 0.337 | F | 0.378 | |
| Asian | 3 | 1.18(0.97-1.44) | 0.107 | 0 | 0.611 | F | |||
| Caucasian | 7 | 1.01(0.84-1.20) | 0.942 | 23.4 | 0.250 | F | |||
| PB | 6 | 1.25(1.05-1.49) | 0.014 | 0 | 0.757 | F | |||
| HB | 3 | 0.93(0.71-1.22) | 0.603 | 0 | 0.505 | F | |||
| CC vs. AA+AC | Overall | 10 | 0.88(0.69-1.12) | 0.310 | 0 | 0.726 | F | 0.095 | |
| Asian | 3 | 1.15(0.72-1.84) | 0.561 | 0 | 0.873 | F | |||
| Caucasian | 7 | 0.80(0.60-1.06) | 0.124 | 0 | 0.656 | F | |||
| PB | 6 | 1.09(0.76-1.54) | 0.642 | 0 | 0.973 | F | |||
| HB | 3 | 0.87(0.54-1.41) | 0.574 | 0 | 0.427 | F | |||
Abbreviations: Bold values indicate statistically significant results; OR, Odds ratio; CI, Confidence interval; p-meta, p-value of pooled effect; R, Random-effect model; F, Fixed-effect model; NA, Not available.
3.4 MTHFR A2756G and PD Susceptibility
In terms of MTHFR A2756G polymorphism, there was statistically significant association discovered in the co-dominant genetic model (GG vs. AA: OR=1.86, 95%CI=1.02-3.37, P=0.042) and recessive genetic model (GG vs. AA+AG: OR=1.90, 95%CI=1.06-3.41, P=0.031) in the overall population (Figure 4A and 4B). With regard to the subgroup analysis in accordance with ethnicity, we found no substantial link in any genetic models in Caucasians or Asians. Additionally, in the subgroup analysis in accordance with the source of controls, MTHFR A2756G polymorphism was found to be linked to the increased susceptibility of PD in the co-dominant genetic model (GG vs. AA: OR=2.22, 95%CI=1.10-4.49, P=0.027) and recessive genetic model (GG vs. AA+AG: OR=2.29, 95%CI=1.15-4.56, P=0.018) in the population-based study (Figure 4C and 4D). Table 4 presents the summary findings of comparisons.
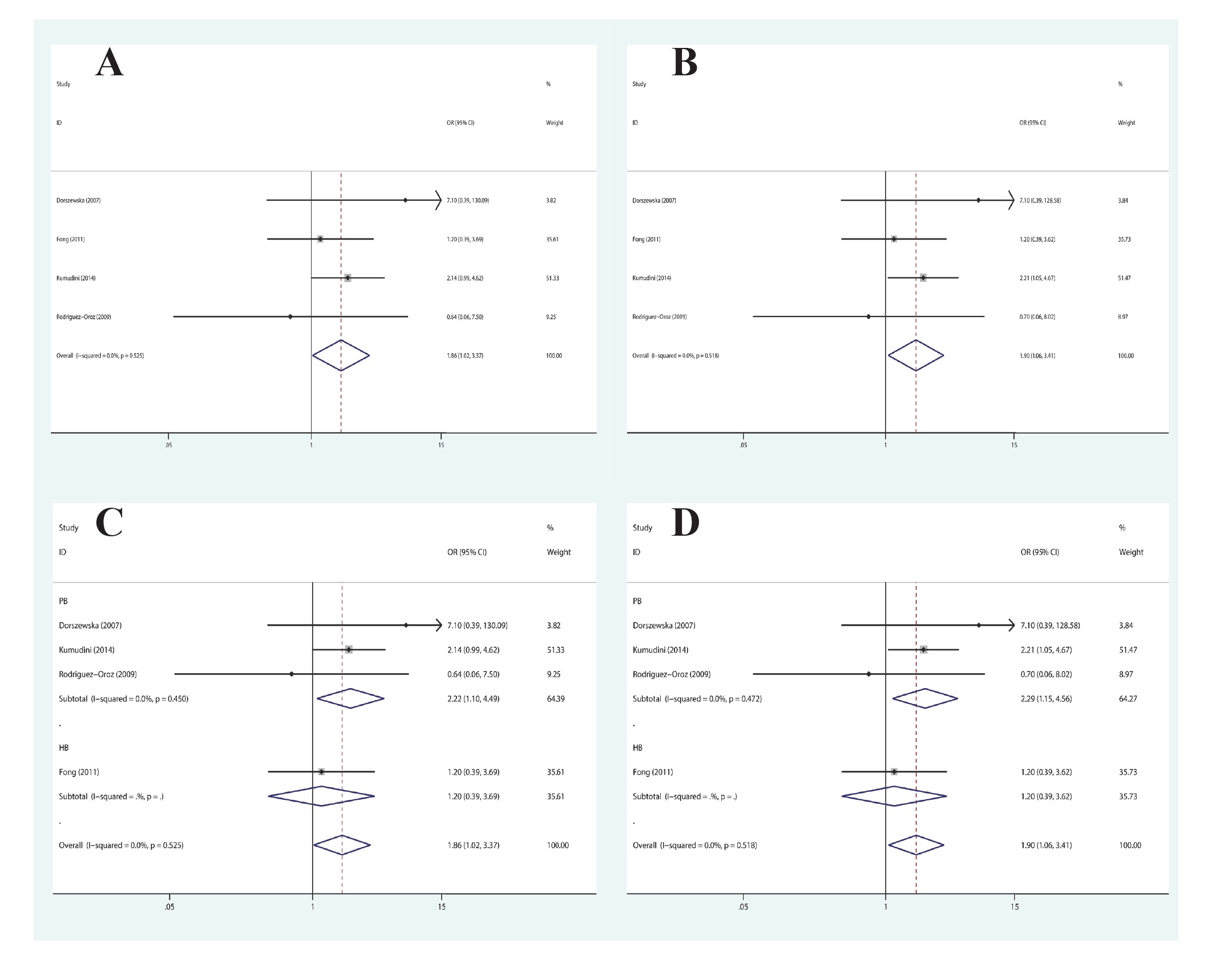
Forest plot of the association between MTHFR A2756G polymorphism and PD susceptibility: (A) co-dominant and (B) recessive genetic model in the overall populations; (C) co-dominant and (D) recessive genetic model, stratified by source of control.
Summary ORs and 95%CI of the association between A2756G polymorphism and Parkinson disease susceptibility.
| Genetic models | Study subjects | No. of studies | OR (95%CI) | p-meta | Test of heterogeneity | Test of Egger’s | ||
|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | Model | ||||||
| G vs. A | Overall | 4 | 1.09(0.90-1.33) | 0.373 | 0 | 0.699 | F | 0.748 |
| Asian | 2 | 1.09(0.88-1.35) | 0.428 | 0 | 0.687 | F | ||
| Caucasian | 2 | 1.11(0.68-1.79) | 0.683 | 21.0 | 0.260 | F | ||
| PB | 3 | 1.13(0.88-1.44) | 0.343 | 0 | 0.527 | F | ||
| HB | 1 | 1.04(0.75-1.44) | 0.818 | NA | NA | F | ||
| GA vs. AA | Overall | 4 | 0.96(0.75-1.23) | 0.742 | 0 | 0.943 | F | 0.406 |
| Asian | 2 | 0.97(0.74-1.28) | 0.852 | 0 | 0.747 | F | ||
| Caucasian | 2 | 0.90(0.51-1.59) | 0.711 | 0 | 0.642 | F | ||
| PB | 3 | 0.92(0.67-1.27) | 0.614 | 0 | 0.892 | F | ||
| HB | 1 | 1.02(0.69-1.51) | 0.921 | NA | NA | F | ||
| GG vs. AA | Overall | 4 | 1.86(1.02-3.37) | 0.042 | 0 | 0.525 | F | 0.943 |
| Asian | 2 | 1.76(0.93-3.32) | 0.084 | 0 | 0.408 | F | ||
| Caucasian | 2 | 2.53(0.45-14.21) | 0.292 | 40.3 | 0.195 | F | ||
| PB | 3 | 2.22(1.10-4.49) | 0.027 | 0 | 0.450 | F | ||
| HB | 1 | 1.20(0.39-3.69) | 0.744 | NA | NA | F | ||
| GG+GA vs. AA | Overall | 4 | 1.02(0.80-1.30) | 0.856 | 11.5 | 0.887 | F | 0.582 |
| Asian | 2 | 1.03(0.79-1.35) | 0.827 | 0 | 0.987 | F | ||
| Caucasian | 2 | 0.99(0.57-1.73) | 0.972 | 23.4 | 0.430 | F | ||
| PB | 3 | 1.02(0.75-1.38) | 0.919 | 0 | 0.727 | F | ||
| HB | 1 | 1.03(0.70-1.52) | 0.870 | NA | NA | F | ||
| GG vs. AA+AG | Overall | 4 | 1.90(1.06-3.41) | 0.031 | 0 | 0.518 | F | 0.933 |
| Asian | 2 | 1.79(0.96-3.35) | 0.066 | 0 | 0.366 | F | ||
| Caucasian | 2 | 2.62(0.47-14.66) | 0.274 | 36.7 | 0.209 | F | ||
| PB | 3 | 2.29(1.15-4.56) | 0.018 | 0 | 0.472 | F | ||
| HB | 1 | 1.20(0.39-3.62) | 0.752 | NA | NA | F | ||
3.5 Sensitivity analysis
For the purpose of assessing the stability of the findings of the meta-analysis, we carried out a sensitivity analysis with the help of the sequentially excluded separate research works. The results, having statistical similarity, were attained subsequent to the sequential exclusion of all studies (Figure 5).
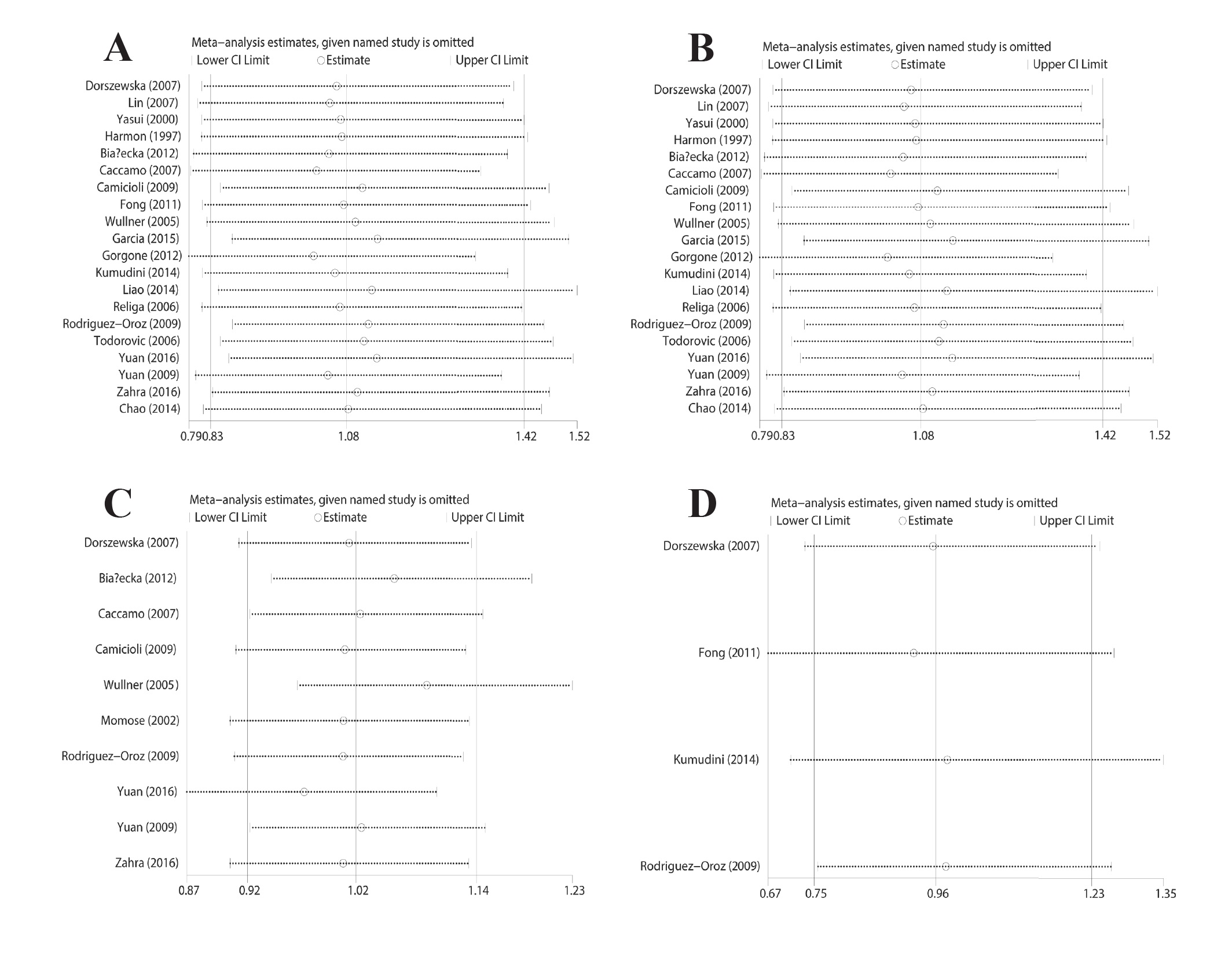
Sensitivity analysis of studies on MTHFR genetic polymorphism and PD: (A) allelic and (B) co-dominant genetic model for C677T polymorphism; (C) allelic genetic model for A1298C polymorphism; (D) allelic genetic model for A2756G polymorphism.
3.6 Publication bias
The evaluation of the publication bias was carried out in accordance with the Begg’s funnel plots and Egger’s test. None of the shapes of the funnel plots indicated any proof of significant asymmetry (Figure 6). Nevertheless, the Egger’s test yielded no proof suggesting publication bias. Moreover, the key results as presented in Table 2, Table 3, 4.
Begg’s funnel plots of publication bias for the association between MTHFR genetic polymorphism and PD: (A) allelic and (B) co-dominant genetic model for C677T polymorphism; (C) allelic and (D) co-dominant genetic model for A1298C polymorphism.
4 Discussion
Parkinson’s disease (PD) is termed as a frequently found neurodegenerative illness that symptoms worsened gradually, ultimately impacting the quality of life of patients. As fully acknowledged, PD is termed as a multifactorial illness, which is a result of intricate gene-gene and gene-environment contacts [37]. Even though the therapy steps have advanced continuously for controlling symptoms, so far no methodology exists for the prevention of the incidence of PD. The latest observations have shed light on the involving homocysteine (Hcy) in the pathogenesis of PD [6, 8, 38]. There are some research works that have discovered an augmented incidence of the illness in those subjects, who carry the MTHFR genotype and identified a major role for Hcy in the promotion of the susceptibility and growth of PD; on the other hand, other research works reached the contrary conclusion [11, 12, 24, 26, 34].
The MTHFR gene is located on chromosome 1p36.3 spanning more than 20 kb and containing a noncoding exon as well as 11 coding exons [39]. In addition, the encoded enzyme catalyzes the conversion of 5,10-methylenetetrahydrofolate, a carbon donor in nucleotide biosynthesis, to 5-methyltetrahydrofolate, the key form of circulatory folate, together with providing a methyl group for the re-methylation of Hcy back to methionine [8]. Genetic mutations in the MTHFR gene have the potential to causing the autosomal recessive homocystinuria because of the MTHFR shortage. In addition, gene variants have been extensively studied in lots of illness, which also include PD.
Even though the augmented number of case-control research works studied the link between MTHFR genetic polymorphism and PD risk; however, the findings remain controversial. In the year 2014, a large case-control study by Liao was conducted for the purpose of investigating the function of the MTHFR genetic polymorphism in PD susceptibility and the results demonstrated that the MTHFR C677T genetic polymorphism had an association with the decreased PD susceptibility [26]. Fong et al. carried out study for the discovery of the link of MTHFR genetic polymorphism with PD susceptibility; the results shed light on the fact that the augmented PD susceptibility is expected to have more significance in the carriers that have the polymorphisms of MTHFR gene [26].
The present meta-analysis is carried out on the basis of the systematic review, which follows the PRISMA guidelines. The robustness of a systematic review involves including the papers that are not based on the selection of authors but the number of papers retrieved from electronic databases. That is why it depends not only on the quality but also the specimen size of investigations involved in the systematic review. Furthermore, a meta-analysis is termed as a methodology pooling the effect sizes of the current scientific literature. A meta-analysis can be carried out on two or more papers. Therefore, it is of great significance to perform a meta-analysis of all of the qualified research works for the clarification of the impacts of the MTHFR genetic polymorphism with PD susceptibility. As illustrated by the current meta-analysis, the A2756G polymorphism in MTHFR gene had an association with an increased risk of PD. However, the C677T and A1298C polymorphisms are not likely to perform a significant function in the risk to PD. Moreover, the meta-analysis carried out by Liu et al. in the year 2018 demonstrated that MTHFR (C677T and A1298C) polymorphism is likely to have an association with the augmented PD susceptibility, which was different from our result [40].
The possible constraints of the meta-analysis require consideration. At first, the specimen in this meta-analysis was small in size, which was likely to give rise to the partiality of the findings in the course of the evaluation of the link between MTHFR genetic polymorphisms and risk to PD. Second, the genuine data in some of the research works was deficient that was likely to constrain enough statistical power for the evaluation of the underlying impacts of gene-gene and gene-environment contacts on the growth of PD. Third, the meta-analysis just counts on the research works that were published in English or Chinese that are likely to give rise to the selection partiality in the accumulated findings. Fourthly, a substantial difference exists in numbers between PD cases and controls that is likely to exert an influence on the dependability of our findings. Based on the above reasons, the pooled estimates of our meta-analysis require careful interpretation.
To conclude, the current meta-analysis illustrated the fact that the MTHFR A2756G genetic polymorphism confers susceptibility to PD, but no link was observed to exist between the C677T and A1298C polymorphisms and PD susceptibility. More efficiently formulated investigations, which have larger specimens, are required for the purpose of clarifying the link of the MTHFR genetic polymorphism with PD risk.
-
Conflict of interest: The authors declare no existence of any conflicting interest.
References
[1] de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525-53510.1016/S1474-4422(06)70471-9Search in Google Scholar
[2] Elbaz A, Carcaillon L, Kab S, Moisan F. Epidemiology of Parkinson’s disease. Rev Neurol (Paris). 2016;172:14-2610.1016/j.neurol.2015.09.012Search in Google Scholar PubMed
[3] Berganzo K, Tijero B, Gonzalez-Eizaguirre A, et al. Motor and non-motor symptoms of Parkinson’s disease and their impact on quality of life and on different clinical subgroups. Neurologia. 2016;31:585-59110.1016/j.nrleng.2014.10.016Search in Google Scholar
[4] reenamyre JT, Hastings TG. Biomedicine. Parkinson’s--divergent causes, convergent mechanisms. Science. 2004;304:1120-112210.1126/science.1098966Search in Google Scholar PubMed
[5] Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues Clin Neurosci. 2017;19:71-8010.31887/DCNS.2017.19.1/rpalSearch in Google Scholar
[6] Bonetti F, Brombo G, Zuliani G. The relationship between hyperhomocysteinemia and neurodegeneration. Neurodegener Dis Manag. 2016;6:133-14510.2217/nmt-2015-0008Search in Google Scholar PubMed
[7] Kruman, II, Culmsee C, Chan SL, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920-692610.1523/JNEUROSCI.20-18-06920.2000Search in Google Scholar
[8] Moretti R, Caruso P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int J Mol Sci. 2019;20. pii: E23110.3390/ijms20010231Search in Google Scholar PubMed PubMed Central
[9] de Lau LM, Koudstaal PJ, van Meurs JB, Uitterlinden AG, Hofman A, Breteler MM. Methylenetetrahydrofolate reductase C677T genotype and PD. Ann Neurol. 2005;57:927-93010.1002/ana.20509Search in Google Scholar PubMed
[10] Gorgone G, Curro M, Ferlazzo N, et al. Coenzyme Q10, hyperhomocysteinemia and MTHFR C677T polymorphism in levodopa-treated Parkinson’s disease patients. Neuromolecular Med. 2012;14:84-9010.1007/s12017-012-8174-1Search in Google Scholar PubMed
[11] Liao Q, Li NN, Mao XY, et al. MTHFR C677T variant reduces risk of sporadic Parkinson’s disease in ethnic Chinese. Acta Neurol Scand. 2014;130:e30-3410.1111/ane.12245Search in Google Scholar PubMed
[12] Yuan L, Song Z, Deng X, Xiong W, Yang Z, Deng H. Association of the MTHFR rs1801131 and rs1801133 variants in sporadic Parkinson’s disease patients. Neurosci Lett. 2016;616:26-3110.1016/j.neulet.2016.01.031Search in Google Scholar PubMed
[13] Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-201210.1001/jama.283.15.2008Search in Google Scholar PubMed
[14] Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672-367310.1093/bioinformatics/bti536Search in Google Scholar PubMed
[15] Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748Search in Google Scholar
[16] DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139-14510.1016/j.cct.2015.09.002Search in Google Scholar PubMed PubMed Central
[17] Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-110110.2307/2533446Search in Google Scholar
[18] Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-63410.1136/bmj.315.7109.629Search in Google Scholar PubMed PubMed Central
[19] Dorszewska J1, Florczak J, Rozycka A, et al. Oxidative DNA damage and level of thiols as related to polymorphisms of MTHFR, MTR, MTHFD1 in Alzheimer’s and Parkinson’s diseases. Acta Neurobiol Exp (Wars). 2007;67:113-12910.55782/ane-2007-1639Search in Google Scholar
[20] Lin JJ, Yueh KC, Liu CS, Liu JT, Lin SZ. 5,10-methylenetetrahydrofolate reductase C677T gene polymorphism can influence age at onset of Parkinson’s disease. Acta Neurol Taiwan. 2007;16:150-157Search in Google Scholar
[21] Yasui K, Kowa H, Nakaso K, et al. Plasma homocysteine and MTHFR C677T genotype in levodopa-treated patients with PD. Neurology. 2000;55:437-44010.1212/WNL.55.3.437Search in Google Scholar PubMed
[22] Harmon DL, Ramsbottom D, Whitehead AS, et al. The thermolabile variant of 5,10-methylenetetrahydrofolate reductase is not associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1997;62:67110.1136/jnnp.62.6.671Search in Google Scholar PubMed PubMed Central
[23] Bialecka M, Kurzawski M, Roszmann A, et al. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet Genomics. 2012;22:716-72410.1097/FPC.0b013e32835693f7Search in Google Scholar PubMed
[24] Caccamo D, Gorgone G, Currò M, et al. Effect of MTHFR Polymorphisms on Hyperhomocysteinemia in Levodopa-treated Parkinsonian Patients. Neuromolecular Med. 2007;9:249-25410.1007/s12017-007-8006-xSearch in Google Scholar PubMed
[25] Camicioli RM, Bouchard TP, Somerville MJ. Homocysteine is not associated with global motor or cognitive measures in nondemented older Parkinson’s disease patients. Mov Disord. 2009;24:176-18210.1002/mds.22227Search in Google Scholar PubMed
[26] Fong CS, Shyu HY, Shieh JC, et al. Association of MTHFR, MTR, and MTRR polymorphisms with Parkinson’s disease among ethnic Chinese in Taiwan. Clin Chim Acta. 2011;412:332-33810.1016/j.cca.2010.11.004Search in Google Scholar PubMed
[27] Fregni F, DaSilva D, Potvin K, et al. Treatment of chronic visceral pain with brain stimulation. Ann Neurol. 2005;58:971-97210.1002/ana.20651Search in Google Scholar PubMed
[28] García S, Coral-Vázquez RM, Gallegos-Arreola MP, et al. Association of the rs1801133 variant in the MTHFR gene and sporadic Parkinson’s disease. Folia Neuropathol. 2015;53:24-2810.5114/fn.2015.49971Search in Google Scholar PubMed
[29] Kumudini N, Uma A, Naushad SM, Mridula R, Borgohain R, Kutala VK. Association of seven functional polymorphisms of one-carbon metabolic pathway with total plasma homocysteine levels and susceptibility to Parkinson’s disease among South Indians. Neurosci Lett. 2014;568:1-510.1016/j.neulet.2014.03.044Search in Google Scholar PubMed
[30] Religa D, Czyzewski K, Styczynska M, et al. Hyperhomocysteinemia and methylenetetrahydrofolate reductase polymorphism in patients with Parkinson’s disease. Neurosci Lett. 2006;404:56-6010.1016/j.neulet.2006.05.040Search in Google Scholar PubMed
[31] Rodriguez-Oroz MC, Lage PM, Sanchez-Mut J, et al. Homocysteine and cognitive impairment in Parkinson’s disease: a biochemical, neuroimaging, and genetic study. Mov Disord. 2009;24:1437-144410.1002/mds.22522Search in Google Scholar PubMed
[32] Todorović Z, Džoljić E, Novaković I, et al. Homocysteine serum levels and MTHFR C677T genotype in patients with Parkinson’s disease, with and without levodopa therapy. J Neurol Sci. 2006;248:56-6110.1016/j.jns.2006.05.040Search in Google Scholar PubMed
[33] Yuan R-Y, Sheu J-J, Yu J-M, et al. Methylenetetrahydrofolate reductase polymorphisms and plasma homocysteine in levodopa-treated and non-treated Parkinson’s disease patients. J Neurol Sci. 2009;287:64-6810.1016/j.jns.2009.09.007Search in Google Scholar PubMed
[34] Zahra C, Tabone C, Camilleri G, Felice AE, Farrugia R, Bezzina Wettinger S. Genetic causes of Parkinson’s disease in the Maltese: a study of selected mutations in LRRK2, MTHFR, QDPR and SPR. BMC Med Genet. 2016; 17: 6510.1186/s12881-016-0327-xSearch in Google Scholar PubMed PubMed Central
[35] Ning C, Liang S, Hui-Yan Y, et al. Association between methylenetetrahydrofolate reductase gene C677T polymorphism and Parkinson’s disease combined with hyperhomocysteinemia. Chin J Geriatr. 2014; 33: 121-125Search in Google Scholar
[36] Momose Y, Murata M, Kobayashi K, et al. Association studies of multiple candidate genes for Parkinson’s disease using single nucleotide polymorphisms. Ann Neurol. 2002;51:133-13610.1002/ana.10079Search in Google Scholar PubMed
[37] Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol. 2003;53:S16-23; discussion S23-2510.1002/ana.10487Search in Google Scholar PubMed
[38] Sharma M, Tiwari M, Tiwari RK. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin Pharmacol Toxicol. 2015;117:287-29610.1111/bcpt.12424Search in Google Scholar PubMed
[39] Prasad AN, Rupar CA, Prasad C. Methylenetetrahydrofolate reductase (MTHFR) deficiency and infantile epilepsy. Brain Dev. 2011;33:758-76910.1016/j.braindev.2011.05.014Search in Google Scholar PubMed
[40] Liu L, Zhang L, Guo L, et al. MTHFR C677T and A1298C polymorphisms may contribute to the risk of Parkinson’s disease: A meta-analysis of 19 studies. Neurosci Lett. 2018;662:339-34510.1016/j.neulet.2017.10.060Search in Google Scholar PubMed
© 2019 Hong-Mei Diao et al. published by De Gruyter
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Research Article
- Prostate Cancer-Specific of DD3-driven oncolytic virus-harboring mK5 gene
- Case Report
- Pediatric acute paradoxical cerebral embolism with pulmonary embolism caused by extremely small patent foramen ovale
- Research Article
- Associations between ambient temperature and acute myocardial infarction
- Case Report
- Discontinuation of imatinib mesylate could improve renal impairment in chronic myeloid leukemia
- Research Article
- METTL3 promotes the proliferation and mobility of gastric cancer cells
- The C677T polymorphism of the methylenetetrahydrofolate reductase gene and susceptibility to late-onset Alzheimer’s disease
- microRNA-1236-3p regulates DDP resistance in lung cancer cells
- Review Article
- The link between thyroid autoimmunity, depression and bipolar disorder
- Research Article
- Effects of miR-107 on the Chemo-drug sensitivity of breast cancer cells
- Analysis of pH dose-dependent growth of sulfate-reducing bacteria
- Review Article
- Musculoskeletal clinical and imaging manifestations in inflammatory bowel diseases
- Research Article
- Regional hyperthermia combined with chemotherapy in advanced gastric cancer
- Analysis of hormone receptor status in primary and recurrent breast cancer via data mining pathology reports
- Morphological and isokinetic strength differences: bilateral and ipsilateral variation by different sport activity
- The reliability of adjusting stepped care based on FeNO monitoring for patients with chronic persistent asthma
- Comparison of the clinical outcomes of two physiological ischemic training methods in patients with coronary heart disease
- Analysis of ticagrelor’s cardio-protective effects on patients with ST-segment elevation acute coronary syndrome accompanied with diabetes
- Computed tomography findings in patients with Samter’s Triad: an observational study
- Case Report
- A spinal subdural hematoma induced by guidewire-based lumbar drainage in a patient with ruptured intracranial aneurysms
- Research Article
- High expression B3GAT3 is related with poor prognosis of liver cancer
- Effects of light touch on balance in patients with stroke
- Oncoprotein LAMTOR5 activates GLUT1 via upregulating NF-κB in liver cancer
- Effects of budesonide combined with noninvasive ventilation on PCT, sTREM-1, chest lung compliance, humoral immune function and quality of life in patients with AECOPD complicated with type II respiratory failure
- Prognostic significance of lymph node ratio in ovarian cancer
- Case Report
- Brainstem anaesthesia after retrobulbar block
- Review Article
- Treating infertility: current affairs of cross-border reproductive care
- Research Article
- Serum inflammatory cytokines comparison in gastric cancer therapy
- Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes
- MRI and bone scintigraphy for breast cancer bone metastase: a meta-analysis
- Comparative study of back propagation artificial neural networks and logistic regression model in predicting poor prognosis after acute ischemic stroke
- Analysis of the factors affecting the prognosis of glioma patients
- Compare fuhrman nuclear and chromophobe tumor grade on chromophobe RCC
- Case Report
- Signet ring B cell lymphoma: A potential diagnostic pitfall
- Research Article
- Subparaneural injection in popliteal sciatic nerve blocks evaluated by MRI
- Loneliness in the context of quality of life of nursing home residents
- Biological characteristics of cervical precancerous cell proliferation
- Effects of Rehabilitation in Bankart Lesion in Non-athletes: A report of three cases
- Management of complications of first instance of hepatic trauma in a liver surgery unit: Portal vein ligation as a conservative therapeutic strategy
- Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect
- Comparison of laparoscopy and open radical nephrectomy of renal cell cancer
- Case Report
- A severe complication of myocardial dysfunction post radiofrequency ablation treatment of huge hepatic hemangioma: a case report and literature review
- Solar urticaria, a disease with many dark sides: is omalizumab the right therapeutic response? Reflections from a clinical case report
- Research Article
- Binge eating disorder and related features in bariatric surgery candidates
- Propofol versus 4-hydroxybutyric acid in pediatric cardiac catheterizations
- Nasointestinal tube in mechanical ventilation patients is more advantageous
- The change of endotracheal tube cuff pressure during laparoscopic surgery
- Correlation between iPTH levels on the first postoperative day after total thyroidectomy and permanent hypoparathyroidism: our experience
- Case Report
- Primary angiosarcoma of the kidney: case report and comprehensive literature review
- Research Article
- miR-107 enhances the sensitivity of breast cancer cells to paclitaxel
- Incidental findings in dental radiology are concerning for family doctors
- Suffering from cerebral small vessel disease with and without metabolic syndrome
- A meta-analysis of robot assisted laparoscopic radical prostatectomy versus laparoscopic radical prostatectomy
- Indications and outcomes of splenectomy for hematological disorders
- Expression of CENPE and its prognostic role in non-small cell lung cancer
- Barbed suture and gastrointestinal surgery. A retrospective analysis
- Using post transplant 1 week Tc-99m DTPA renal scan as another method for predicting renal graft failure
- The pseudogene PTTG3P promotes cell migration and invasion in esophageal squamous cell carcinoma
- Lymph node ratio versus TNM system as prognostic factor in colorectal cancer staging. A single Center experience
- Review Article
- Minimally invasive pilonidal sinus treatment: A narrative review
- Research Article
- Anatomical workspace study of Endonasal Endoscopic Transsphenoidal Approach
- Hounsfield Units on Lumbar Computed Tomography for Predicting Regional Bone Mineral Density
- Communication
- Aspirin, a potential GLUT1 inhibitor in a vascular endothelial cell line
- Research Article
- Osteopontin and fatty acid binding protein in ifosfamide-treated rats
- Familial polyposis coli: the management of desmoid tumor bleeding
- microRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1
- PYCR1 is associated with papillary renal cell carcinoma progression
- Prediction of recurrence-associated death from localized prostate cancer with a charlson comorbidity index–reinforced machine learning model
- Colorectal cancer in the elderly patient: the role of neo-adjuvant therapy
- Association between MTHFR genetic polymorphism and Parkinson’s disease susceptibility: a meta-analysis
- Metformin can alleviate the symptom of patient with diabetic nephropathy through reducing the serum level of Hcy and IL-33
- Case Report
- Severe craniofacial trauma after multiple pistol shots
- Research Article
- Echocardiography evaluation of left ventricular diastolic function in elderly women with metabolic syndrome
- Tailored surgery in inguinal hernia repair. The role of subarachnoid anesthesia: a retrospective study
- The factors affecting early death in newly diagnosed APL patients
- Review Article
- Oncological outcomes and quality of life after rectal cancer surgery
- Research Article
- MiR-638 repressed vascular smooth muscle cell glycolysis by targeting LDHA
- microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma
- Analyzing the semantic space of the Hippocratic Oath
- Fournier’s gangrene and intravenous drug abuse: an unusual case report and review of the literature
- Evaluation of surgical site infection in mini-invasive urological surgery
- Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway
- Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients
- Case Report
- Appendix bleeding with painless bloody diarrhea: A case report and literature review
- Research Article
- Protective effects of specneuzhenide on renal injury in rats with diabetic nephropathy
- PBF, a proto-oncogene in esophageal carcinoma
- Use of rituximab in NHL malt type pregnant in I° trimester for two times
- Cancer- and non-cancer related chronic pain: from the physiopathological basics to management
- Case report
- Non-surgical removal of dens invaginatus in maxillary lateral incisor using CBCT: Two-year follow-up case report
- Research Article
- Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU
- Accuracy of tumor perfusion assessment in Rat C6 gliomas model with USPIO
- Lemann Index for Assessment of Crohn’s Disease: Correlation with the Quality of Life, Endoscopic Disease activity, Magnetic Resonance Index of Activity and C- Reactive Protein
- Case report
- Münchausen syndrome as an unusual cause of pseudo-resistant hypertension: a case report
- Research Article
- Renal artery embolization before radical nephrectomy for complex renal tumour: which are the true advantages?
- Prognostic significance of CD276 in non-small cell lung cancer
- Potential drug-drug interactions in acute ischemic stroke patients at the Neurological Intensive Care Unit
- Effect of vitamin D3 on lung damage induced by cigarette smoke in mice
- CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge miRNA-767-5p
- Case report
- Partial hydatidiform mole and coexistent live fetus: a case report and review of the literature
- Research Article
- Effect of NGR1 on the atopic dermatitis model and its mechanisms
- Clinical features of infertile men carrying a chromosome 9 translocation
- Review Article
- Expression and role of microRNA-663b in childhood acute lymphocytic leukemia and its mechanism
- Case Report
- Mature cystic teratoma of the pancreas: A rare cystic neoplasm
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Case Report
- Predictive factors of intestinal necrosis in acute mesenteric ischemia
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Effects of dexmedetomidine on the RhoA /ROCK/ Nox4 signaling pathway in renal fibrosis of diabetic rats
- MicroRNA-181a-5p regulates inflammatory response of macrophages in sepsis
- Intraventricular pressure in non-communicating hydrocephalus patients before endoscopic third ventriculostomy
- CyclinD1 is a new target gene of tumor suppressor miR-520e in breast cancer
- CHL1 and NrCAM are primarily expressed in low grade pediatric neuroblastoma
- Epidemiological characteristics of postoperative sepsis
- Association between unstable angina and CXCL17: a new potential biomarker
- Cardiac strains as a tool for optimization of cardiac resynchronization therapy in non-responders: a pilot study
- Case Report
- Resuscitation following a bupivacaine injection for a cervical paravertebral block
- Research Article
- CGF treatment of leg ulcers: A randomized controlled trial
- Surgical versus sequential hybrid treatment of carotid body tumors
Articles in the same Issue
- Research Article
- Prostate Cancer-Specific of DD3-driven oncolytic virus-harboring mK5 gene
- Case Report
- Pediatric acute paradoxical cerebral embolism with pulmonary embolism caused by extremely small patent foramen ovale
- Research Article
- Associations between ambient temperature and acute myocardial infarction
- Case Report
- Discontinuation of imatinib mesylate could improve renal impairment in chronic myeloid leukemia
- Research Article
- METTL3 promotes the proliferation and mobility of gastric cancer cells
- The C677T polymorphism of the methylenetetrahydrofolate reductase gene and susceptibility to late-onset Alzheimer’s disease
- microRNA-1236-3p regulates DDP resistance in lung cancer cells
- Review Article
- The link between thyroid autoimmunity, depression and bipolar disorder
- Research Article
- Effects of miR-107 on the Chemo-drug sensitivity of breast cancer cells
- Analysis of pH dose-dependent growth of sulfate-reducing bacteria
- Review Article
- Musculoskeletal clinical and imaging manifestations in inflammatory bowel diseases
- Research Article
- Regional hyperthermia combined with chemotherapy in advanced gastric cancer
- Analysis of hormone receptor status in primary and recurrent breast cancer via data mining pathology reports
- Morphological and isokinetic strength differences: bilateral and ipsilateral variation by different sport activity
- The reliability of adjusting stepped care based on FeNO monitoring for patients with chronic persistent asthma
- Comparison of the clinical outcomes of two physiological ischemic training methods in patients with coronary heart disease
- Analysis of ticagrelor’s cardio-protective effects on patients with ST-segment elevation acute coronary syndrome accompanied with diabetes
- Computed tomography findings in patients with Samter’s Triad: an observational study
- Case Report
- A spinal subdural hematoma induced by guidewire-based lumbar drainage in a patient with ruptured intracranial aneurysms
- Research Article
- High expression B3GAT3 is related with poor prognosis of liver cancer
- Effects of light touch on balance in patients with stroke
- Oncoprotein LAMTOR5 activates GLUT1 via upregulating NF-κB in liver cancer
- Effects of budesonide combined with noninvasive ventilation on PCT, sTREM-1, chest lung compliance, humoral immune function and quality of life in patients with AECOPD complicated with type II respiratory failure
- Prognostic significance of lymph node ratio in ovarian cancer
- Case Report
- Brainstem anaesthesia after retrobulbar block
- Review Article
- Treating infertility: current affairs of cross-border reproductive care
- Research Article
- Serum inflammatory cytokines comparison in gastric cancer therapy
- Behavioural and psychological symptoms in neurocognitive disorders: Specific patterns in dementia subtypes
- MRI and bone scintigraphy for breast cancer bone metastase: a meta-analysis
- Comparative study of back propagation artificial neural networks and logistic regression model in predicting poor prognosis after acute ischemic stroke
- Analysis of the factors affecting the prognosis of glioma patients
- Compare fuhrman nuclear and chromophobe tumor grade on chromophobe RCC
- Case Report
- Signet ring B cell lymphoma: A potential diagnostic pitfall
- Research Article
- Subparaneural injection in popliteal sciatic nerve blocks evaluated by MRI
- Loneliness in the context of quality of life of nursing home residents
- Biological characteristics of cervical precancerous cell proliferation
- Effects of Rehabilitation in Bankart Lesion in Non-athletes: A report of three cases
- Management of complications of first instance of hepatic trauma in a liver surgery unit: Portal vein ligation as a conservative therapeutic strategy
- Matrix metalloproteinase 2 knockdown suppresses the proliferation of HepG2 and Huh7 cells and enhances the cisplatin effect
- Comparison of laparoscopy and open radical nephrectomy of renal cell cancer
- Case Report
- A severe complication of myocardial dysfunction post radiofrequency ablation treatment of huge hepatic hemangioma: a case report and literature review
- Solar urticaria, a disease with many dark sides: is omalizumab the right therapeutic response? Reflections from a clinical case report
- Research Article
- Binge eating disorder and related features in bariatric surgery candidates
- Propofol versus 4-hydroxybutyric acid in pediatric cardiac catheterizations
- Nasointestinal tube in mechanical ventilation patients is more advantageous
- The change of endotracheal tube cuff pressure during laparoscopic surgery
- Correlation between iPTH levels on the first postoperative day after total thyroidectomy and permanent hypoparathyroidism: our experience
- Case Report
- Primary angiosarcoma of the kidney: case report and comprehensive literature review
- Research Article
- miR-107 enhances the sensitivity of breast cancer cells to paclitaxel
- Incidental findings in dental radiology are concerning for family doctors
- Suffering from cerebral small vessel disease with and without metabolic syndrome
- A meta-analysis of robot assisted laparoscopic radical prostatectomy versus laparoscopic radical prostatectomy
- Indications and outcomes of splenectomy for hematological disorders
- Expression of CENPE and its prognostic role in non-small cell lung cancer
- Barbed suture and gastrointestinal surgery. A retrospective analysis
- Using post transplant 1 week Tc-99m DTPA renal scan as another method for predicting renal graft failure
- The pseudogene PTTG3P promotes cell migration and invasion in esophageal squamous cell carcinoma
- Lymph node ratio versus TNM system as prognostic factor in colorectal cancer staging. A single Center experience
- Review Article
- Minimally invasive pilonidal sinus treatment: A narrative review
- Research Article
- Anatomical workspace study of Endonasal Endoscopic Transsphenoidal Approach
- Hounsfield Units on Lumbar Computed Tomography for Predicting Regional Bone Mineral Density
- Communication
- Aspirin, a potential GLUT1 inhibitor in a vascular endothelial cell line
- Research Article
- Osteopontin and fatty acid binding protein in ifosfamide-treated rats
- Familial polyposis coli: the management of desmoid tumor bleeding
- microRNA-27a-3p down-regulation inhibits malignant biological behaviors of ovarian cancer by targeting BTG1
- PYCR1 is associated with papillary renal cell carcinoma progression
- Prediction of recurrence-associated death from localized prostate cancer with a charlson comorbidity index–reinforced machine learning model
- Colorectal cancer in the elderly patient: the role of neo-adjuvant therapy
- Association between MTHFR genetic polymorphism and Parkinson’s disease susceptibility: a meta-analysis
- Metformin can alleviate the symptom of patient with diabetic nephropathy through reducing the serum level of Hcy and IL-33
- Case Report
- Severe craniofacial trauma after multiple pistol shots
- Research Article
- Echocardiography evaluation of left ventricular diastolic function in elderly women with metabolic syndrome
- Tailored surgery in inguinal hernia repair. The role of subarachnoid anesthesia: a retrospective study
- The factors affecting early death in newly diagnosed APL patients
- Review Article
- Oncological outcomes and quality of life after rectal cancer surgery
- Research Article
- MiR-638 repressed vascular smooth muscle cell glycolysis by targeting LDHA
- microRNA-16 via Twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma
- Analyzing the semantic space of the Hippocratic Oath
- Fournier’s gangrene and intravenous drug abuse: an unusual case report and review of the literature
- Evaluation of surgical site infection in mini-invasive urological surgery
- Dihydromyricetin attenuates inflammation through TLR4/NF-kappaB pathway
- Clinico-pathological features of colon cancer patients undergoing emergency surgery: a comparison between elderly and non-elderly patients
- Case Report
- Appendix bleeding with painless bloody diarrhea: A case report and literature review
- Research Article
- Protective effects of specneuzhenide on renal injury in rats with diabetic nephropathy
- PBF, a proto-oncogene in esophageal carcinoma
- Use of rituximab in NHL malt type pregnant in I° trimester for two times
- Cancer- and non-cancer related chronic pain: from the physiopathological basics to management
- Case report
- Non-surgical removal of dens invaginatus in maxillary lateral incisor using CBCT: Two-year follow-up case report
- Research Article
- Risk factors and drug resistance of the MDR Acinetobacter baumannii in pneumonia patients in ICU
- Accuracy of tumor perfusion assessment in Rat C6 gliomas model with USPIO
- Lemann Index for Assessment of Crohn’s Disease: Correlation with the Quality of Life, Endoscopic Disease activity, Magnetic Resonance Index of Activity and C- Reactive Protein
- Case report
- Münchausen syndrome as an unusual cause of pseudo-resistant hypertension: a case report
- Research Article
- Renal artery embolization before radical nephrectomy for complex renal tumour: which are the true advantages?
- Prognostic significance of CD276 in non-small cell lung cancer
- Potential drug-drug interactions in acute ischemic stroke patients at the Neurological Intensive Care Unit
- Effect of vitamin D3 on lung damage induced by cigarette smoke in mice
- CircRNA-UCK2 increased TET1 inhibits proliferation and invasion of prostate cancer cells via sponge miRNA-767-5p
- Case report
- Partial hydatidiform mole and coexistent live fetus: a case report and review of the literature
- Research Article
- Effect of NGR1 on the atopic dermatitis model and its mechanisms
- Clinical features of infertile men carrying a chromosome 9 translocation
- Review Article
- Expression and role of microRNA-663b in childhood acute lymphocytic leukemia and its mechanism
- Case Report
- Mature cystic teratoma of the pancreas: A rare cystic neoplasm
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Case Report
- Predictive factors of intestinal necrosis in acute mesenteric ischemia
- Research Article
- Application of exercised-based pre-rehabilitation in perioperative period of patients with gastric cancer
- Effects of dexmedetomidine on the RhoA /ROCK/ Nox4 signaling pathway in renal fibrosis of diabetic rats
- MicroRNA-181a-5p regulates inflammatory response of macrophages in sepsis
- Intraventricular pressure in non-communicating hydrocephalus patients before endoscopic third ventriculostomy
- CyclinD1 is a new target gene of tumor suppressor miR-520e in breast cancer
- CHL1 and NrCAM are primarily expressed in low grade pediatric neuroblastoma
- Epidemiological characteristics of postoperative sepsis
- Association between unstable angina and CXCL17: a new potential biomarker
- Cardiac strains as a tool for optimization of cardiac resynchronization therapy in non-responders: a pilot study
- Case Report
- Resuscitation following a bupivacaine injection for a cervical paravertebral block
- Research Article
- CGF treatment of leg ulcers: A randomized controlled trial
- Surgical versus sequential hybrid treatment of carotid body tumors

