The effects of propranolol on heart rate variability and quantitative, mechanistic, pain profiling: a randomized placebo-controlled crossover study
-
Kristian Kjær Petersen
, Hjalte Holm Andersen
Abstract
Background and aims
The autonomic nervous system (ANS) is capable of modulating pain. Aberrations in heart rate variability (HRV), reflective of ANS activity, are associated with experimental pain sensitivity, chronic pain, and more recently, pain modulatory mechanisms but the underlying mechanisms are still unclear. HRV is lowered during experimental pain as well as in chronic pain conditions and HRV can be increased by propranolol, which is a non-selective β-blocker. Sensitization of central pain pathways have been observed in several chronic pain conditions and human mechanistic pain biomarkers for these central pain pathways include temporal summation of pain (TSP) and conditioned pain modulation (CPM). The current study aimed to investigate the effect of the β-blocker propranolol, and subsequently assessing the response to standardized, quantitative, mechanistic pain biomarkers.
Methods
In this placebo-controlled, double-blinded, randomized crossover study, 25 healthy male volunteers (mean age 25.6 years) were randomized to receive 40 mg propranolol and 40 mg placebo. Heart rate, blood pressure, and HRV were assessed before and during experimental pain tests. Cuff pressure pain stimulation was used for assessment of pain detection (cPDTs) and pain tolerance (cPTTs) thresholds, TSP, and CPM. Offset analgesia (OA) was assessed using heat stimulation.
Results
Propranolol significantly reduced heart rate (p<0.001), blood pressure (p<0.02) and increased HRV (p<0.01) compared with placebo. No significant differences were found comparing cPDT (p>0.70), cPTT (p>0.93), TSP (p>0.70), OA-effect (p>0.87) or CPM (p>0.65) between propranolol and placebo.
Conclusions
The current study demonstrated that propranolol increased HRV, but did not affect pressure pain sensitivity or any pain facilitatory or modulatory outcomes.
Implications
Analgesic effects of propranolol have been reported in clinical pain populations and the results from the current study could indicate that increased HRV from propranolol is not associated with peripheral and central pain pathways in healthy male subjects.
1 Introduction
Propranolol is a non-selective β-blocker originally used to treat portal hypertension [1], but has also been applied as an anxiolytic [2] and migraine prophylactic [3]. Propranolol exerts its antihypertensive effects by blocking both the β-1 (resulting in a reduction of cardiac output and splanchnic blood flow) and β-2 (resulting in splanchnic vasoconstriction due to unopposed activation of adrenergic α-1 receptors) receptors [4]. In addition, propranolol has indirect-acting parasympathomimetic effects, whereby it increases heart rate variability (HRV) [5] – a common measure of the relative contributions of parasympathetic activity in the autonomic control of the heart.
Low HRV has been proposed as a marker for cardiovascular diseases [6], [7], [8] and accumulating evidence suggests a close relationship between the autonomic nervous system (ANS) and pain processing [9], [10], [11], where HRV is found lowered during experimental pain as well as in chronic pain conditions [9], [12]. Furthermore, reduced HRV has also been associate with increased pain in fibromyalgia [13], and increased post-surgical pain [14]. Administration of propranolol has been shown to alleviate pain in fibromyalgia [15], and temporomandibular joint disorder (TMD) [16]. In addition, propranolol can minimize opioid-induced mechanical and thermal hyperalgesia [17], indicating that propranolol can modulate peripheral and central pain pathways. Administration of Catechol-O-methyltransferase inhibitors in rodents produces increased pain sensitivity at multiple body sites [18], [19], but this pain sensitivity can be blocked by administration of the nonselective β-adrenergic receptor antagonists such propranolol [19], [20]. Likewise, intramuscular injection of serotonin in humans generates pain [21], which again can be reduced by co-administration of propranolol [22]. There are several mechanisms of which propranolol could mediate the analgesic effects such as peripheral blocking of the β-2-receptors [20], [23], or by blocking of the serotonin receptors in the central nervous system [24]. In spite of the evidence implicating ANS activity and in pain processing and the clinical usefulness of propranolol, it is unknown whether pain facilitatory and modulatory mechanisms are affected by ANS output under normal conditions.
Pain inhibition is commonly assessed using conditioned pain modulation (CPM), which is a proxy measure of the balance between descending pain inhibition and facilitation in humans [25]. CPM is functional in healthy subjects, but impaired in several chronic pain conditions such as osteoarthritis [26], fibromyalgia [27] and chronic pancreatic pain [28]. Recently, offset analgesia (OA) has been suggested as another measure of descending pain inhibition [29] and is observed as a disproportionally reduction in perceived pain following a slight decrease in painful stimulus intensity [30]. OA has been suggested to act via different pain pathways than CPM [31], [32], but the specific pathways of OA are largely unknown [29]. Patients with neuropathic pain display impairments in both OA [33] and CPM [34]. Temporal summation of pain (TSP) is considered a measure of the mechanisms responsible for pain facilitation in the central nervous system and has been found associated with pain progression [35], [36], [37]. The potential role of the ANS in the modulation of central pain processing is unclear, and studies have yet to investigate the effect of pharmacologically augmented parasympathomimetic activity on pain sensitivity, TSP, OA, or CPM.
The aim of this study was to investigate the effect of propranolol on CPM, with secondary outcome measures being experimental pain assessments such as pressure pain thresholds, TSP, and OA. We hypothesized that propranolol would increase HRV and in turn decrease pain sensitivity.
2 Methods
2.1 Study design
The study used a randomized, placebo-controlled, double-blinded, crossover design, with the two experimental sessions being separated by at least 1 week. A single 40 mg dose of propranolol was used as a drug model of parasympathomimetic activation. An identical looking capsule (containing 40 mg calcium) was administrated as placebo. On each study day, the subjects were administered either propranolol or placebo in a randomized order. The experimental assessments were conducted 2 h after administration, corresponding to the expected peak plasma concentration of propranolol [38]. The experimental sequence for pain assessments was: pressure pain thresholds, TSP, OA, and CPM. HRV was recorded prior to and during the CPM testing in each session.
2.2 Participants
Izumi et al. [39] found a CPM effect of 12 kPa (SD: 10 kPa) and this study was designed to find a change in CPM of at least 75%, with a power of 80% with a significant level of 0.05, why 25 healthy men, mean age 25.6 (range: 20–37) years, were recruited from July 2016 to January 2017. Participants were excluded if they suffered from any concomitant pain conditions, used any analgesics, lacked understanding of the procedures, had any history of alcohol or drug misuse, were diagnosed with cardio-vascular diseases, asthma, diabetes, or had known decreased function of the liver or kidneys. All participants were given oral and written information and signed written informed consent prior to the initiation of the study. The study complied with the Helsinki Declaration, was approved by the local Ethical Committee (reference number: N-20120043), and registered at ClinicalTrials.gov (registration number: NCT02808611).
2.3 Cardiovascular and heart rate variability measures
Blood pressure was recorded with subjects relaxing in a supine position for 5 min before the commencement of the experimental tests using the Omron Automatic Blood Pressure Monitor, Model: M3 (Imron Healthcare, Kyoto, Japan). Heart rate and HRV were assessed using a Polar RS800CX heart rate monitor (Polar Electro, Kempele, Finland) and all measurements were recorded for 5 min. An elastic chest band with built-in recording electrodes (wetted before use) was placed horizontally immediately below the papilla mammaria. The Polar RS800CX has been used in a number of empirical investigations, and is reliable for assessments in a supine position at rest [40]. The device records inter-beat intervals (IBI) at a sampling frequency of 1,000 Hz, providing a temporal resolution of 1 ms for each R–R interval. Timestamps were inserted for the baseline recordings (prior to experimental tests) and during CPM paradigms. The following time-domain measures were derived from analysis in Kubios HRV: mean IBI (ms), the square root of the mean squared difference of successive R–R intervals (rMSSD, ms), and the percentage of adjacent cycles that are greater than 50 ms apart (pNN50, %), which is in line with previous studies in this field [41], [42], [43]. Measures from the frequency-domain were not analysis, since they have recently been heavily criticized. Both rMSSD and pNN50 reflect vagal-parasympathetic activity [9].
2.4 Sudomotor activity
Skin conductance measurements were performed by galvanic skin resistance measurements with a DermaLab USB Hydration eight-pin probe (Cortex Technology ApS, Hadsund, Denmark), as a measure of sudomotor activity and a proxy for sympathetic activity in the ANS. The probe was gently wiped off in a cotton cloth before each assessment. Measurements were performed in duplicate on the index and middle fingers and an average was used for statistical analysis. Skin conductance was assessed before the experimental tests and 30 s after cuff or CPT conditioned pain in according with a previous study [41].
2.5 Experimental pain assessments
2.5.1 Pressure pain thresholds
Pressure stimulation was applied by a computer-controlled cuff algometer (Cortex Technology ApS, Hadsund, Denmark and Aalborg University, Aalborg, Denmark). A 13 cm wide air-cuff (VBM, Sulz am Neckar, Germany) was wrapped around the belly of the gastrocnemius muscle, centering approximately at the level of the lower leg with the maximum circumference, and was inflated at a rate of 1 kPa/s. The participants were instructed to rate the pain intensity of the cuff pressure stimulus on a handheld 10 cm computerized VAS where zero denotes “no pain”, and 10 denotes “worst imaginable pain”. The pressure at VAS=1 was defined as cuff pressure pain detection threshold (cPDT) [44], [45], and the pressure at which participants felt the pain became intolerable was defined as the pressure pain tolerance threshold (cPTT).
2.5.2 Temporal summation of pain
Ten identical pressure stimuli, equivalent to a pressure at individual cPTT-level, with 1 s duration and 1 s inter-stimulus interval, were applied to induce TSP. Subjects were asked to rate their pain intensity continuously during sequential stimulation on the VAS. In addition, subjects were instructed not to return the VAS to zero in-between the 10 stimulations. The VAS score after each stimulus were extracted, as commonly done when assessing TSP using cuff algometry [46], [47], [48]. For analysis of TSP, the mean VAS score was calculated in the interval from the first to the end of the fourth stimulus (VAS-I) and in the interval from the eighth to the end of the 10th stimulus (VAS-II). Temporal summation of pain was defined as the difference between VAS-I and VAS-II (i.e. VAS-II minus VAS-I), which is commonly used when assessing TSP using cuff algometry [46], [47].
2.5.3 Offset analgesia
Heat stimulations were applied using the Pathway Neurosensory Analyzer (Medoc ltd., Ramat Yishai, Israel) and the ATS 30×30 mm squared probe. First, a constant control stimulus of 48°C was applied to the dorsal forearm for 30 s. After a short break, the offset analgesia paradigm was applied in three intervals T1 (5 s), T2 (5 s), and T3 (20 s) with temperatures during the trials selected as follows: T1=48°C, T2=49°C, and T3=48°C. The subjects were asked to assess the pain of the thermal stimuli using the electronic VAS (VAS0−10 with 0=“no pain” and 10=“worst imaginable pain”). The analgesic effects have been documented to take place after the decrease of the temperature from T2 to T3 (49°C–48°C) [30], [49]. The average pain ratings following the decrease from T2 to T3, i.e. in the time interval between 16 s and 20 s (due to the delay of the thermodes to reach the target temperature and the responses latency) were calculated. The window-time used for statistical analysis of OA-effect was adapted based on previous studies [30], [49].
2.5.4 Conditioned pain modulation
CPM was measured using two protocols for test stimuli (TS) and two conditioning stimuli (CS). cPDT was applied as the TS on the dominant leg and one protocol applied 70% of cPTT as CS on the non-dominant leg while the other protocol applied the cold pressor test as CS where the subjects were instructed to immerse the non-dominant hand up to the wrist into the stirred ice-cold water (0–4°C). The subjects were allowed to withdraw their hand from the ice-water if it became too painful, but were instructed re-immerse their hand and aim the pain rating for approximately VAS=7, which has previously been applied in comparable studies [50], [51]. Both CS were applied for 5 min to allow for the HRV measures to be conducted.
The CPM-effect was calculated as the differences in pressure needed to evoke cPDT while conditioned subtracted from cPDT at baseline (unconditioned). A 15 min break was included between the two CPM tests to avoid carry-over effects [52]. Subjects completed both CPM protocols that were randomized in order. Pain ratings from both cuff and CPT conditioned stimuli were recorded.
2.5.5 Statistical analysis
All values are presented as mean and standard error of mean (±SEM) if not otherwise indicated. Visual inspection confirmed normal distribution of data. Data were tested for normality using QQ-plots and the Kolmogorov-Smirnov normality test. The rMSSD data were log-transforms to achieve normality.
The effects of propranolol compared to placebo on ANS activity and sensory tests were analysis using repeated measures analysis of variance (rm-ANOVA) with drug (propranolol, placebo) as the main factor. For OA, the paradigm (constant, OA-paradigm) factor was added to investigate the difference in pain rating from a constant 48°C stimulus to a OA-paradigm. For CPM, a paradigm (cPDTbaseline, cPDTconditioned) factor was added to investigate the inhibitory response from a baseline cPDT to cPDT during a conditioned stimuli. To investigate changes in HRV measures during the conditioned stimuli, a time (baseline, during conditioned) factor was added for both CPT and tonic cuff conditioning stimuli.
The statistical analyses were performed by SPSS (version 23, IBM Corporation, NY, USA). p-Values <0.05 were considered as significant.
3 Results
3.1 The effect of propranolol on heart rate and blood pressure
Propranolol significantly reduced heart rate (F(1,24)=25.89, p<0.001) as well as diastolic and systolic blood pressure (F(1,24)=6.89, p=0.015) compared with placebo (Table 1). No adverse event were observed.
Mean and standard deviation (SD) of heart rate and blood pressure measures 2 h after administration of propranolol or placebo in 25 healthy male subjects.
| Propranolol | Placebo | Effect size (Cohen’s d) | |
|---|---|---|---|
| Heart rate (beats/min) | 55.99 (SD: 6.21)a | 61.64 (SD: 7.30) | 1.96 |
| Blood pressure (systolic/diastolic, mmHg) | 112.04/68.44a (SD: 13.42/9.42) | 116.08/70.28 (SD: 10.81/7.71) | 0.53/0.21 |
-
The effect size was calculated using Cohen’s d. mmHg=millimeter of mercury. aIndicate p<0.05 comparing propranolol to placebo.
3.1.1 Pressure pain sensitivity
No statistical drug effect was found for cPDT (F(1,24)=0.15, p=0.70) or cPTT (F(1,24)=0.01, p=0.93) when comparing propranolol to placebo, indicating that propranolol did not influence pressure pain sensitivity (Fig. 1).
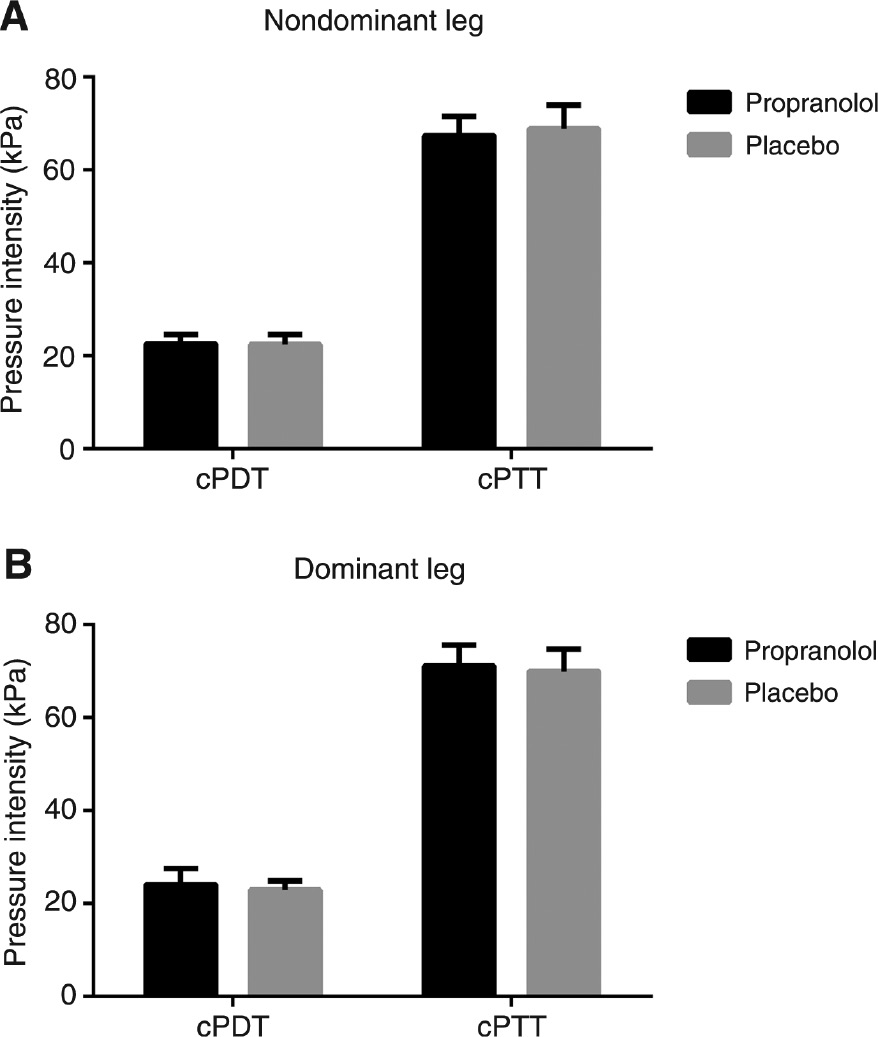
Cuff pressure detection (cPDT) and tolerance threshold (cPTT) assess on the (A) non-dominant and the (B) dominant lower leg assessed by cuff algometry for healthy males subjects following administration of propranolol and placebo.
3.1.2 Temporal summation of pain
The rm-ANOVA showed no difference in TSP comparing propranolol and placebo (F(1,24)=0.16, p=0.70), indicating that propranolol did not influence pain facilitation in the central nervous system (Fig. 2).
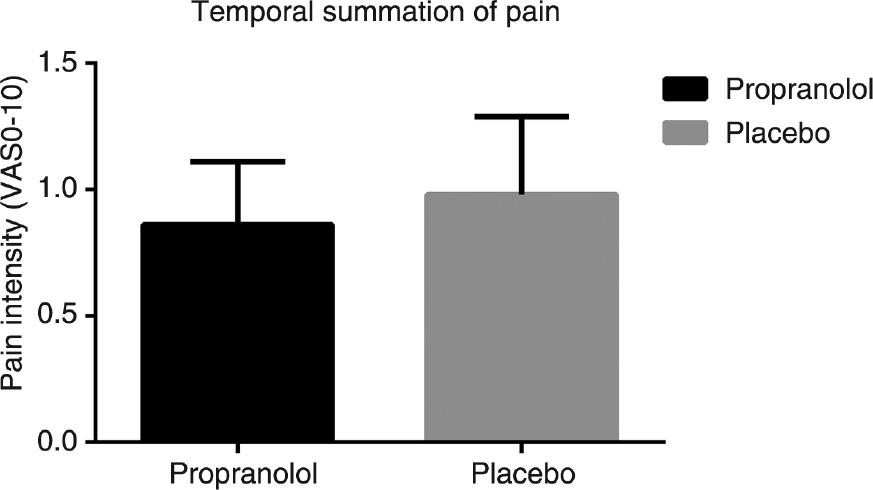
Temporal summation of pain for healthy male subjects administered propranolol and placebo.
Temporal summation of pain was assessed by 10 identical pressure stimuli and the mean VAS score was calculated in the interval from the first to the end of the fourth stimulus (VAS-I) and in the interval from the eighth to the end of the tenth stimulus (VAS-II). Temporal summation of pain was defined as the difference between VAS-I and VAS-II.
3.1.3 Offset analgesia
A significantly decreased pain rating was found for the OA-paradigm compared with the baseline-paradigm (F(1,24)=15.70, p=0.001), indicative of functional OA in the study sample. No significant drug effect was found (F(1,24)=0.03, p=0.87), indicating that propranolol did not affect offset pain modulation (Fig. 3).
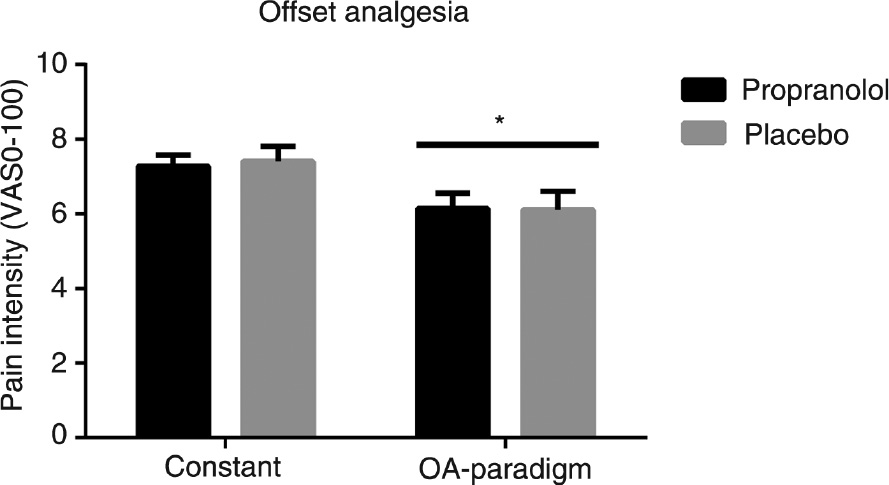
Pain rating to a constant 48°C heat stimulus and to an offset analgesia (OA) paradigm applied to healthy males subjects after administration of either propranolol or placebo. *Indicates p<0.05 comparing the constant heat stimulus to the OA-paradigm.
3.1.4 Conditioned pain modulation
Pain ratings to CPT (mean VAS: 6.65, SEM: 0.29) was significantly increased compared with cuff (mean VAS: 6.06, SEM: 0.27) conditioning stimuli (p=0.048). cPDT significantly increased during conditioning pain stimulation using both the CPT and a tonic cuff stimulus (rm-ANOVA: F(1,24)>17.49, p<0.001). There was no effect of drug (rm-ANOVA: F(1,24)=0.22, p=0.65), signifying functional CPM was unaffected by propranolol (Fig. 4).
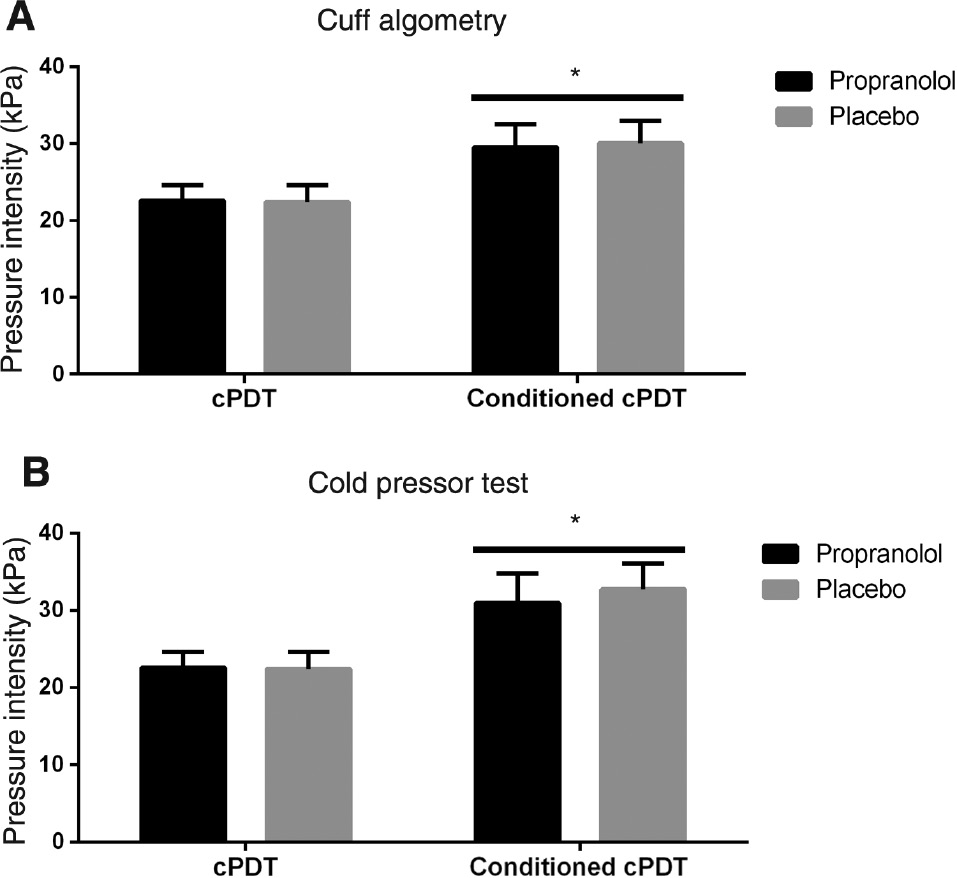
Unconditioned cuff pressure detection threshold (cPDT) and conditioned cPDT with (A) cuff algometry or (B) the cold pressor test (CPT). *Indicates p<0.05 comparing conditioned cPDT to unconditioned cPDT.
3.1.5 The effect of propranolol and tonic cuff and cold pressor test stimuli on the autonomic nervous system
A significant drug effect was found at baseline (prior to experimental tests), showing that compared to placebo, administration of propranolol resulted in a significantly increased mean IBI (F(1,24)=28.85, p<0.001, Fig. 5A), rMSSD (F(1,24)=7.44, p=0.01, Fig. 5B), and pNN50 (F(1,24)=12.28, p=0.002, Fig. 5C). There was no effect of propranolol on skin conductance (F(1,24)=0.93, p=0.34; Fig. 5D) compared with placebo prior to experimental tests.
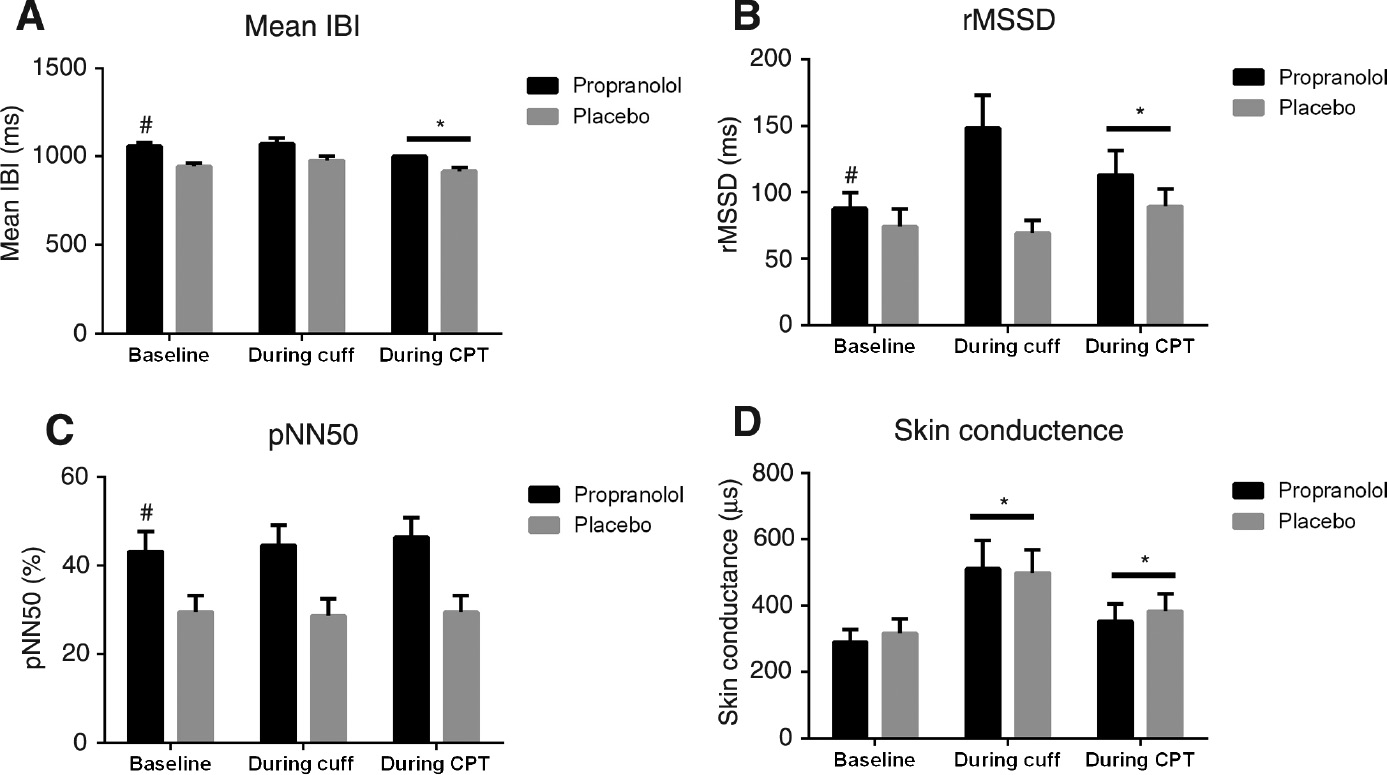
Measures of heart rate variability (A–C) and skin conductance (D) at baseline and during conditioning pain from cuff algometry and the cold pressor test (CPT).
IBI, The mean inter beat interval; rMSSD, the root mean squared difference of successive R–R intervals; pNN50, the percentage of adjacent cycles that are greater than 50 ms apart. #Indicate p<0.05 comparing propranolol to placebo and *indicate p<0.05 comparing conditioning stimuli to baseline.
A significant time effect comparing ANS activity during CPT to baseline (prior to CPT), showed increased heart rate (F(1,24)=8.71, p=0.01), rMSSD (F(1,24)=6.11, p=0.021, Fig. 5B), and decreased mean IBI (F(1,24)=8.70, p=0.01, Fig. 5A). In addition, a significant time effect was seen for both cuff and CPT compared with baseline (prior to cuff and CPT, respectively), which showed increased skin conductance (F(1,24)>15.89, p<0.005, Fig. 5D).
4 Discussion
The present randomized, placebo-controlled, crossover study showed that propranolol exerts a parasympathomimetic effect, decreasing heart rate and blood pressure, while increasing measures of vagally-mediated HRV, compared to placebo. However, propranolol did not affect the quantitative, mechanistic pain biomarkers (pressure pain thresholds, temporal summation of pain, offset analgesia, or conditioned pain modulation) in healthy male volunteers.
4.1 Pain and the automatous system
The parasympathetic vagus nerve influences pain. For instance, vagotomy increases pain, and stimulation of the vagus nerve reduces thermal pain sensitivity in both animal [53], [54], [55] and human [56], [57] studies. Afferent baroreceptor signaling has been suggested to modulate pain perception via medullary and mesencephalic neural circuitry that modulates descending pain inhibition [58], [59]. Lowered parasympathetic activity has been associated with increased ratings of pain in response to thermal stimuli in healthy subjects [60], [61], patients with fibromyalgia [62], and in patients with chemotherapy-induced polyneuropathy [63]. A recent study found propranolol to reduce measures of central sensitization in a migraine rat model [64] and two human experimental pain studies suggest that propranolol has potential antihyperalgesic effects although the mechanism(s) involved remain elusive or perhaps related to off-target interactions [17], [22] – a finding not supported by the present study. Transcutaneous-vagus nerve stimulation increases HRV [65] and have been found to increase mechanical and pressure pain thresholds and reduce mechanical pain sensitivity [56] in healthy males and to reduced evoked pain intensity and TSP in patients with chronic pelvic pain [66].
Increase HRV can be achieved by other pharmaceutical approaches, such as Scopolamine [67], [68] or Atropine [69], [70], or non-pharmacological, such as transcutaneous-vagus nerve stimulation [65] or deep breathing [71], and future studies could investigate if these have different effects on the central pain mechanisms investigated in the current study.
4.2 Pressure pain thresholds
Pain thresholds are commonly used to assess alterations in pain sensitivity following acute or chronic injury. However, pain thresholds exhibit high inter-individual variability, which is believed to be driven by factors such as genotype [72], sex [73], psychological state [74], and ANS activity [9]. Despite this, the intra-individual reliability of pressure pain thresholds has been documented as good-to-excellent in studies assessing the intra- and inter-session [50], [75] reliability. Clinically, patients with chronic pain conditions such as osteoarthritis [26], migraine [76], or fibromyalgia [27] show lower pressure pain thresholds compared to pain-free individuals. Therefore, understanding the variability related to pressure pain threshold testing is critical for future clinical use. In this context, the ANS has been suggested to be associated with experimental pain outcomes, and some of the variance found in pain threshold testing [9]. The current study administrated a β-blocker, evoking an increased HRV, but found the β-blocker to have no effect on pressure pain detection or tolerance thresholds compared with placebo. These results indicate that a small but significant increase in HRV does not alter pain sensitivity in pain-free male subjects per se. Notably, previous studies have demonstrated that intramuscular propranolol provides an immediate analgesic response [22] to pain from intramuscular injection of serotonin [21]. However, the intramuscular injection of propranolol in these studies may have resulted in a higher local concentration of propranolol, compared to the systemic (i.e. oral) administration used in the current study.
4.3 Central pain modulatory mechanisms
Temporal summation of pain assesses pain facilitation, while CPM and OA assess endogenous pain inhibition in humans [26]. For CPM, a functioning inhibitory system is commonly reported in healthy subjects, corresponding to a significant increase in the perceived intensity of a test stimulus during the delivery of a conditioning stimulus [77], similar to what was found in the current study. OA represents a disproportional reduction in perceived pain following a slight decrease in painful stimulus intensity in healthy subjects [30], [49], which the current study also demonstrated.
Administration of ketamine influences CPM but not OA [32]. Furthermore, differences in brain activity have been recorded during an OA and CPM paradigm [78], suggesting that the mechanisms underlying CPM and OA are different. A recent study found that increased HRV was associated with lower pain ratings during an offset analgesia paradigm [79], suggesting an association between OA and the ANS. Nahman-Averbuch et al. [42] found that ANS activity in woman was associated with an OA-effect whereas ANS activity in men was associated with a CPM-effect, indicating sex-dependent effects, which should be investigated in future studies. It could be assumed that measures of ANS activity are associated with CPM, since afferent baroreceptor signals have been implicated in the modulation of pain perception via medullary and mesencephalic neural circuitry, influencing descending pain inhibition [58], [59] and medullary transections, reducing diffuse noxious inhibitory control (the preclinical counterpart to CPM) in rats [80] – presenting promising avenues for future research in humans. Schweinhardt et al. [81] investigated 39 healthy males and studied the effect of propranolol on heat pain sensitivity and a found small decreased effect size for propranolol compared with placebo, which could explain that the peripheral contribution of propranolol is limited, which could be an explanation for why OA did not change in the current study.
Maekawa et al. [82], compared infusion of propranolol to saline and fond propranolol to lower heart rate at baseline and during CPT, which is similar to the IBI findings from the current study.
4.4 Limitations
The current study found an increase in HRV following propranolol administration but did not find this to be associated with differences in efficacy of any facilitatory or inhibitory pain mechanisms. Due to safety reasons, the current study administrated a low single-dose propranolol to healthy young males who showed normal heart rate and blood pressure. This could limit a potential effect of propranolol on central pain processing mechanisms, rendering differences between propranolol and the placebo undetectable. Contrasting this, similar doses, as used in the current study, are used by students for exam-related anxiety [83] and similar low doses have previously lowered pain ratings in patients with fibromyalgia and TMD [15]. Moreover, 40 mg represents the initial maximal recommend dosage for hypertension and tachycardia. Despite this, the current study did find effects on heart rate, blood pressure and HRV but no effect and central pain mechanism. It is unknown if more substantial parasympathomimetic effects would modulate pain processing mechanisms in healthy subjects.
The most significant ANS responsiveness aberrations related to pain have been observed in chronic pain patients suffering from, e.g. fibromyalgia [15] or TMD [16] and are generally related to a decrease of parasympathetic resting activity. Prolonged suppression of parasympathetic activity is thus not necessarily reproducible in an acute design as applied in the present study. Several previous studies support an antihyperalgesic [23], [84] and a potential analgesic [15], [16] effect of propranolol but the linkage between these effects is unclear. The present study did not employ an experimental model of evoked hyperalgesia such as intradermal capsaicin [85], burn-injury or L-menthol [86] evoked secondary hyperalgesia and thus cannot corroborate previous finding related to propranolol-induced antihyperalgesia.
5 Conclusion
The current study found that propranolol decreased heart rate, blood pressure and increased HRV but had no impact on pain sensitivity or pain modulatory status in healthy male subjects.
-
Authors’ statements
-
Research funding: The authors thank The Innovation Fund Denmark (j.no. 136-2014-5), The Shionogi Science Program and the TaNeDS Europe grant for providing the opportunity to conduct the study.
-
Conflict of interest: Masato Tsukamoto is an employee of Asahi Kasei Pharma Corporation.
-
Informed consent: All participants were given oral and written information and signed written informed consent prior to the initiation of the study.
-
Ethical approval: The study complied with the Helsinki Declaration, was approved by the local Ethical Committee (reference number: N-20120043), and registered at ClinicalTrials.gov (registration number: NCT02808611).
References
[1] Lebrec D, Nouel O, Corbic M, Benhamou JP. Propranolol – a medical treatment for portal hypertension? Lancet 1980;2:180–2.10.1016/S0140-6736(80)90063-XSuche in Google Scholar PubMed
[2] Steenen SA, van Wijk AJ, van der Heijden GJ, van Westrhenen R, de Lange J, de Jongh A. Propranolol for the treatment of anxiety disorders: systematic review and meta-analysis. J Psychopharmacol 2016;30:128–39.10.1177/0269881115612236Suche in Google Scholar PubMed PubMed Central
[3] Silberstein SD. Preventive migraine treatment. Neurol Clin 2009;27:429–43.10.1016/j.ncl.2008.11.007Suche in Google Scholar PubMed
[4] Tripathi D, Hayes PC. Beta-blockers in portal hypertension: new developments and controversies. Liver Int 2014;34:655–67.10.1111/liv.12360Suche in Google Scholar PubMed
[5] Bendixen KH, Terkelsen AJ, Baad-Hansen L, Cairns BE, Svensson P. Effect of propranolol on hypertonic saline-evoked masseter muscle pain and autonomic response in healthy women during rest and mental arithmetic task. J Orofac Pain 2013;27:243–55.10.11607/jop.1013Suche in Google Scholar PubMed
[6] Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 2010;141:122–31.10.1016/j.ijcard.2009.09.543Suche in Google Scholar PubMed
[7] Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol 2007;74:224–42.10.1016/j.biopsycho.2005.11.013Suche in Google Scholar PubMed
[8] Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 2012;36:747–56.10.1016/j.neubiorev.2011.11.009Suche in Google Scholar PubMed
[9] Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain 2013;18:1–14.10.1002/j.1532-2149.2013.00379.xSuche in Google Scholar PubMed
[10] Thayer JF, Sternberg EM. Neural concomitants of immunity – focus on the vagus nerve. Neuroimage 2009;47:908–10.10.1016/j.neuroimage.2009.05.058Suche in Google Scholar PubMed PubMed Central
[11] Ballegaard S, Bergmann N, Karpatschof B, Kristiansen J, Gyntelberg F, Arendt-Nielsen L, Bech P, Hjalmarson Å, Faber J. Association between pressure pain sensitivity and autonomic function as assessed by a tilt table test. Scand J Clin Lab Invest 2015;75:345–54.10.3109/00365513.2015.1028095Suche in Google Scholar PubMed
[12] Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016;157:7–29.10.1097/j.pain.0000000000000360Suche in Google Scholar PubMed
[13] Lerma C, Martinez A, Ruiz N, Vargas A, Infante O, Martinez-Lavin M. Nocturnal heart rate variability parameters as potential fibromyalgia biomarker: correlation with symptoms severity. Arthritis Res Ther 2011;13:R185.10.1186/ar3513Suche in Google Scholar PubMed PubMed Central
[14] Bossmann T, Brauner T, Wearing S, Horstmann T. Predictors of chronic pain following total knee replacement in females and males: an exploratory study. Pain Manag 2017;7:391–403.10.2217/pmt-2017-0023Suche in Google Scholar PubMed
[15] Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain 2009;10:542–52.10.1016/j.jpain.2008.12.006Suche in Google Scholar PubMed PubMed Central
[16] Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics 2010;20:239–48.10.1097/FPC.0b013e328337f9abSuche in Google Scholar PubMed PubMed Central
[17] Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, Angst MS, Clark DJ. Modulation of remifentanil-induced postinfusion hyperalgesia by the β-blocker propranolol in humans. Pain 2012;153:974–81.10.1016/j.pain.2012.01.014Suche in Google Scholar PubMed
[18] Hartung JE, Ciszek BP, Nackley AG. β2- and β3-adrenergic receptors drive COMT-dependent pain by increasing production of nitric oxide and cytokines. Pain 2014;155:1346–55.10.1016/j.pain.2014.04.011Suche in Google Scholar PubMed PubMed Central
[19] Kline RH, Exposto FG, O’Buckley SC, Westlund KN, Nackley AG. Catechol-O-methyltransferase inhibition alters pain and anxiety-related volitional behaviors through activation of β-adrenergic receptors in the rat. Neuroscience 2015;290: 561–9.10.1016/j.neuroscience.2015.01.064Suche in Google Scholar PubMed PubMed Central
[20] Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both β2- and β3-adrenergic receptors. Pain 2007;128:199–208.10.1016/j.pain.2006.09.022Suche in Google Scholar PubMed PubMed Central
[21] Ernberg M, Lundeberg T, Kopp S. Pain and allodynia/hyperalgesia induced by intramuscular injection of serotonin in patients with fibromyalgia and healthy individuals. Pain 2000;85:31–9.10.1016/S0304-3959(99)00233-XSuche in Google Scholar
[22] Ernberg M, Lundeberg T, Kopp S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain 2000;84:339–46.10.1016/S0304-3959(99)00221-3Suche in Google Scholar PubMed
[23] Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol 1999;81:1104–12.10.1152/jn.1999.81.3.1104Suche in Google Scholar PubMed
[24] Yalcin I, Choucair-Jaafar N, Benbouzid M, Tessier LH, Muller A, Hein L, Freund-Mercier MJ, Barrot M. β2-Adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol 2009;65:218–25.10.1002/ana.21542Suche in Google Scholar PubMed
[25] Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156 Suppl:S24–31.10.1097/01.j.pain.0000460343.46847.58Suche in Google Scholar PubMed
[26] Arendt-Nielsen L, Skou ST, Nielsen TA, Petersen KK. Altered central sensitization and pain modulation in the CNS in chronic joint pain. Curr Osteoporos Rep 2015;13:225–34.10.1007/s11914-015-0276-xSuche in Google Scholar PubMed
[27] Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606.10.1038/nrrheum.2010.107Suche in Google Scholar PubMed
[28] Olesen SS, Brock C, Krarup AL, Funch-Jensen P, Arendt-Nielsen L, Wilder-Smith OH, Drewes AM. Descending inhibitory pain modulation is impaired in patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2010;8:724–30.10.1016/j.cgh.2010.03.005Suche in Google Scholar PubMed
[29] Hermans L, Calders P, Van Oosterwijck J, Verschelde E, Bertel E, Meeus M. An overview of offset analgesia and the comparison with conditioned pain modulation: a systematic literature review. Pain Physician 2016;19:307–26.10.36076/ppj/2016.19.307Suche in Google Scholar
[30] Grill JD, Coghill RC. Transient analgesia evoked by noxious stimulus offset. J Neurophysiol 2002;87:2205–8.10.1152/jn.00730.2001Suche in Google Scholar PubMed
[31] Honigman L, Yarnitsky D, Sprecher E, Weissman-Fogel I. Psychophysical testing of spatial and temporal dimensions of endogenous analgesia: conditioned pain modulation and offset analgesia. Exp Brain Res 2013;228:493–501.10.1007/s00221-013-3580-7Suche in Google Scholar PubMed
[32] Niesters M, Dahan A, Swartjes M, Noppers I, Fillingim RB, Aarts L, Sarton EY. Effect of ketamine on endogenous pain modulation in healthy volunteers. Pain 2011;152:656–63.10.1016/j.pain.2010.12.015Suche in Google Scholar PubMed
[33] Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 2011;115:1063–71.10.1097/ALN.0b013e31822fd03aSuche in Google Scholar PubMed
[34] Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 2014;113:148–56.10.1093/bja/aeu056Suche in Google Scholar PubMed
[35] Weissman-Fogel I, Granovsky Y, Crispel Y, Ben-Nun A, Best LA, Yarnitsky D, Granot M. Enhanced presurgical pain temporal summation response predicts post-thoracotomy pain intensity during the acute postoperative phase. J Pain 2009;10:628–36.10.1016/j.jpain.2008.12.009Suche in Google Scholar PubMed
[36] Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;156:55–61.10.1016/j.pain.0000000000000022Suche in Google Scholar PubMed
[37] Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L. The role of preoperative radiologic severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;34:193–7.10.1097/AJP.0000000000000528Suche in Google Scholar PubMed
[38] Thuillez C, Richer C, Duhazé P, Bergougnan L, Giudicelli JF. Beta-adrenoceptor blocking effects and plasma levels of bornaprolol and propranolol in man. Eur J Clin Pharmacol 1985;29:405–11.10.1007/BF00613453Suche in Google Scholar PubMed
[39] Izumi M, Petersen KK, Laursen MB, Arendt-Nielsen L, Graven-Nielsen T. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain 2017;158:323–32.10.1097/j.pain.0000000000000764Suche in Google Scholar PubMed
[40] Gamelin FX, Berthoin S, Bosquet L. Validity of the polar S810 heart rate monitor to measure R-R intervals at rest. Med Sci Sports Exerc 2006;38:887–93.10.1249/01.mss.0000218135.79476.9cSuche in Google Scholar PubMed
[41] Andersen HH, Imai Y, Petersen KK, Koenig J, Elberling J, Arendt-Nielsen L. Conditioning pain stimulation does not affect itch induced by intra-epidermal histamine pricks but aggravates neurogenic inflammation in healthy volunteers. Somatosens Mot Res 2016;33:49–60.10.3109/08990220.2016.1173535Suche in Google Scholar PubMed
[42] Nahman-Averbuch H, Dayan L, Sprecher E, Hochberg U, Brill S, Yarnitsky D, Jacob G. Sex differences in the relationships between parasympathetic activity and pain modulation. Physiol Behav 2016;154:40–8.10.1016/j.physbeh.2015.11.004Suche in Google Scholar PubMed
[43] Nahman-Averbuch H, Dayan L, Sprecher E, Hochberg U, Brill S, Yarnitsky D, Jacob G. Pain modulation and autonomic function: the effect of clonidine. Pain Med 2016;17:1292–301.10.1093/pm/pnv102Suche in Google Scholar PubMed
[44] Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry. Pain 2015;156:2193–202.10.1097/j.pain.0000000000000294Suche in Google Scholar PubMed
[45] Manafi Khanian B, Arendt-Nielsen L, Kjær Petersen K, Samani A, Graven-Nielsen T. Interface pressure behavior during painful cuff algometry. Pain Med 2016;17:915–23.10.1093/pm/pnv063Suche in Google Scholar PubMed
[46] Petersen KK, Graven-Nielsen T, Simonsen O, Laursen MB, Arendt-Nielsen L. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain 2016;157:1400–6.10.1097/j.pain.0000000000000531Suche in Google Scholar PubMed
[47] Vaegter HB, Graven-Nielsen T. Pain modulatory phenotypes differentiate subgroups with different clinical and experimental pain sensitivity. Pain 2016;157:1480–8.10.1097/j.pain.0000000000000543Suche in Google Scholar PubMed
[48] Petersen KK, Arendt-Nielsen L, Finocchietti S, Hirata RP, Simonsen O, Laursen MB, Graven-Nielsen T. Age interactions on pain sensitization in patients with severe knee osteoarthritis and controls. Clin J Pain 2017;33:1081–7.10.1097/AJP.0000000000000495Suche in Google Scholar PubMed
[49] Ligato D, Petersen KK, Mørch CD, Arendt-Nielsen L. Offset analgesia: the role of peripheral and central mechanisms. Eur J Pain 2018;22:142–9.10.1002/ejp.1110Suche in Google Scholar PubMed
[50] Imai Y, Petersen KK, Mørch CD, Arendt Nielsen L. Comparing test–retest reliability and magnitude of conditioned pain modulation using different combinations of test and conditioning stimuli. Somatosens Mot Res 2016;33:169–77.10.1080/08990220.2016.1229178Suche in Google Scholar PubMed
[51] Biurrun Manresa JA, Fritsche R, Vuilleumier PH, Oehler C, Mørch CD, Arendt-Nielsen L, Andersen OK, Curatolo M. Is the conditioned pain modulation paradigm reliable? A test-retest assessment using the nociceptive withdrawal reflex. PLoS One 2014;9:e100241.10.1371/journal.pone.0100241Suche in Google Scholar PubMed PubMed Central
[52] Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014;155:158–67.10.1016/j.pain.2013.09.023Suche in Google Scholar PubMed
[53] Khasar SG, Green PG, Miao FJP, Levine JD. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur J Neurosci 2003;17:909–15.10.1046/j.1460-9568.2003.02503.xSuche in Google Scholar PubMed
[54] Khasar SG, Miao FJP, Jänig W, Levine JD. Modulation of bradykinin-induced mechanical hyperalgesia in the rat by activity in abdominal vagal afferents. Eur J Neurosci 1998;10:435–44.10.1046/j.1460-9568.1998.00030.xSuche in Google Scholar PubMed
[55] Ren K, Zhuo M, Randich A, Gebhart GF. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol 1993;69:1530–40.10.1152/jn.1993.69.5.1530Suche in Google Scholar PubMed
[56] Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception – an experimental study. Brain Stimul 2013;6: 202–9.10.1016/j.brs.2012.04.006Suche in Google Scholar PubMed
[57] Sedan O, Sprecher E, Yarnitsky D. Vagal stomach afferents inhibit somatic pain perception. Pain 2005;113:354–9.10.1016/j.pain.2004.11.012Suche in Google Scholar PubMed
[58] Ghione S. Hypertension-associated hypalgesia: evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension 1996;28:494–504.10.1161/01.HYP.28.3.494Suche in Google Scholar PubMed
[59] Thurston CL, Randich A. Effects of vagal afferent stimulation on ON and OFF cells in the rostroventral medulla: relationships to nociception and arterial blood pressure. J Neurophysiol 1992;67:180–96.10.1152/jn.1992.67.1.180Suche in Google Scholar PubMed
[60] Appelhans BM, Luecken LJ. Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol Psychol 2008;77:174–82.10.1016/j.biopsycho.2007.10.004Suche in Google Scholar PubMed
[61] Duschek S, Mück I, Reyes del Paso GA. Relationship between baroreceptor cardiac reflex sensitivity and pain experience in normotensive individuals. Int J Psychophysiol 2007;65:193–200.10.1016/j.ijpsycho.2007.03.012Suche in Google Scholar PubMed
[62] Reyes del Paso GA, Garrido S, Pulgar Á, Duschek S. Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. J Psychosom Res 2011;70:125–34.10.1016/j.jpsychores.2010.09.012Suche in Google Scholar PubMed
[63] Nahman-Averbuch H, Granovsky Y, Sprecher E, Steiner M, Tzuk-Shina T, Pud D, Yarnitsky D. Associations between autonomic dysfunction and pain in chemotherapy-induced polyneuropathy. Eur J Pain 2014;18:47–55.10.1002/j.1532-2149.2013.00349.xSuche in Google Scholar PubMed
[64] Boyer N, Signoret-Genest J, Artola A, Dallel R, Monconduit L. Propranolol treatment prevents chronic central sensitization induced by repeated dural stimulation. Pain 2017;158: 2025–34.10.1097/j.pain.0000000000001007Suche in Google Scholar PubMed
[65] Singh JP, Kandala J, John Camm A. Non-pharmacological modulation of the autonomic tone to treat heart failure. Eur Heart J 2014;35:77–85.10.1093/eurheartj/eht436Suche in Google Scholar PubMed
[66] Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, Li A, Kim J, Maeda Y, Park K, Wasan AD. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med 2012;13:777–89.10.1111/j.1526-4637.2012.01385.xSuche in Google Scholar PubMed PubMed Central
[67] Pedretti R, Colombo E, Braga SS, Carú B. Influence of transdermal scopolamine on cardiac sympathovagal interaction after acute myocardial infarction. Am J Cardiol 1993;72:384–92.10.1016/0002-9149(93)91127-4Suche in Google Scholar PubMed
[68] La Rovere MT, Mortara A, Pantaleo P, Maestri R, Cobelli F, Tavazzi L. Scopolamine improves autonomic balance in advanced congestive heart failure. Circulation 1994;90: 838–43.10.1161/01.CIR.90.2.838Suche in Google Scholar PubMed
[69] Perlstein I, Stepensky D, Krzyzanski W, Hoffman A. A signal transduction pharmacodynamic model of the kinetics of the parasympathomimetic activity of low-dose scopolamine and atropine in rats. J Pharm Sci 2002;91:2500–10.10.1002/jps.10243Suche in Google Scholar PubMed
[70] Ali-Melkkilä T, Kaila T, Antila K, Halkola L, Iisalo E. Effects of glycopyrrolate and atropine on heart rate variability. Acta Anaesthesiol Scand 1991;35:436–41.10.1111/j.1399-6576.1991.tb03324.xSuche in Google Scholar PubMed
[71] Shields JW. Heart rate variability with deep breathing as a clinical test of cardiovagal function. Cleve Clin J Med 2009;76(Suppl. 2):37–40.10.3949/ccjm.76.s2.08Suche in Google Scholar PubMed
[72] Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. Br J Anaesth 2013;111: 52–8.10.1093/bja/aet127Suche in Google Scholar PubMed PubMed Central
[73] Greenspan JD, Craft RM, Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132(Suppl):S26–45.10.1016/j.pain.2007.10.014Suche in Google Scholar PubMed PubMed Central
[74] Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother 2009;9:745–58.10.1586/ern.09.34Suche in Google Scholar PubMed PubMed Central
[75] Graven-Nielsen T, Izumi M, Petersen KK, Arendt-Nielsen L. User-independent assessment of conditioning pain modulation by cuff pressure algometry. Eur J Pain 2017;21:552–61.10.1002/ejp.958Suche in Google Scholar PubMed
[76] Ladda J, Straube A, Förderreuther S, Krause P, Eggert T. Quantitative sensory testing in cluster headache: increased sensory thresholds. Cephalalgia 2006;26:1043–50.10.1111/j.1468-2982.2006.01134.xSuche in Google Scholar PubMed
[77] Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith OH. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339.10.1016/j.ejpain.2010.02.004Suche in Google Scholar PubMed
[78] Nahman-Averbuch H, Martucci KT, Granovsky Y, Weissman-Fogel I, Yarnitsky D, Coghill RC. Distinct brain mechanisms support spatial vs temporal filtering of nociceptive information. Pain 2014;155:2491–501.10.1016/j.pain.2014.07.008Suche in Google Scholar PubMed PubMed Central
[79] Van Den Houte M, Van Oudenhove L, Bogaerts K, Van Diest I, Van den Bergh O. Endogenous pain modulation: association with resting heart rate variability and negative affectivity. Pain Med 2017:1–10. [Epub ahead of print].10.1093/pm/pnx165Suche in Google Scholar PubMed
[80] Bouhassira D, Bing Z, Le Bars D. Effects of lesions of locus coeruleus/subcoeruleus on diffuse noxious inhibitory controls in the rat. Brain Res 1992;571:140–4.10.1016/0006-8993(92)90520-JSuche in Google Scholar
[81] Schweinhardt P, Abulhasan YB, Koeva V, Balderi T, Kim DJ, Alhujairi M, Carli F. Effects of intravenous propranolol on heat pain sensitivity in healthy men. Eur J Pain 2013;17:704–13.10.1002/j.1532-2149.2012.00231.xSuche in Google Scholar PubMed
[82] Maekawa K, Kuboki T, Miyawaki T, Shimada M, Yamashita A, Clark GT. Effect of intravenous infusion of a beta-adrenergic blocking agent on the haemodynamic changes in human masseter muscle induced by cold-pressor stimulation. Arch Oral Biol 1999;44:475–83.10.1016/S0003-9969(99)00028-XSuche in Google Scholar
[83] Butt JH, Dalsgaard S, Torp-Pedersen C, Køber L, Gislason GH, Kruuse C, Fosbøl EL. Beta-blockers for exams identify students at high risk of psychiatric morbidity. J Child Adolesc Psychopharmacol 2016;27:266–73.10.1089/cap.2016.0079Suche in Google Scholar PubMed
[84] Safieh-Garabedian B, Poole S, Haddad JJ, Massaad CA, Jabbur SJ, Saadé NE. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology 2002;42:864–72.10.1016/S0028-3908(02)00028-XSuche in Google Scholar PubMed
[85] Andersen HH, Elberling J, Sharma N, Hauberg LE, Gazerani P, Arendt-Nielsen L. Histaminergic and non-histaminergic elicited itch is attenuated in capsaicin-evoked areas of allodynia and hyperalgesia: a healthy volunteer study. Eur J Pain 2017;21:1098–109.10.1002/ejp.1013Suche in Google Scholar PubMed
[86] Andersen HH, Gazerani P, Arendt-Nielsen L. High-concentration L-menthol exhibits counter-irritancy to neurogenic inflammation, thermal and mechanical hyperalgesia caused by trans-cinnamaldehyde. J Pain 2016;17:919–29.10.1016/j.jpain.2016.05.004Suche in Google Scholar PubMed
©2018 Scandinavian Association for the Study of Pain. Published by Walter de Gruyter GmbH, Berlin/Boston. All rights reserved.
Artikel in diesem Heft
- Frontmatter
- Editorial comment
- Diagnosis of carpal tunnel syndrome
- Body image concerns and distortions in people with persistent pain
- The prevalence of recurrent pain in childhood is high and increases with age
- Friends in pain: pain tolerance in a social network
- Clinical pain research
- Correlation of clinical grading, physical tests and nerve conduction study in carpal tunnel syndrome
- Spectroscopic differences in posterior insula in patients with chronic temporomandibular pain
- Deconstructing chronicity of musculoskeletal pain: intensity-duration relations, minimal dimensions and clusters of chronicity
- “When I feel the worst pain, I look like shit” – body image concerns in persistent pain
- The prevalence of neck-shoulder pain, back pain and psychological symptoms in association with daytime sleepiness – a prospective follow-up study of school children aged 10 to 15
- The neglected role of distress in pain management: qualitative research on a gastrointestinal ward
- Pain mapping of the anterior knee: injured athletes know best
- The role of pain in chronic pain patients’ perception of health-related quality of life: a cross-sectional SQRP study of 40,000 patients
- The DoloTest® in a specialized headache center among patients receiving psychological treatment. A pilot study
- Observational study
- Chronic pelvic pain – pain catastrophizing, pelvic pain and quality of life
- Survey of chronic pain in Chile – prevalence and treatment, impact on mood, daily activities and quality of life
- Patients’ pre-operative general and specific outcome expectations predict postoperative pain and function after total knee and total hip arthroplasties
- The peer effect on pain tolerance
- Original experimental
- The effects of propranolol on heart rate variability and quantitative, mechanistic, pain profiling: a randomized placebo-controlled crossover study
- Idiographic measurement of depressive thinking: development and preliminary validation of the Sentence Completion Test for Chronic Pain (SCP)
- Adding steroids to lidocaine in a therapeutic injection regimen for patients with abdominal pain due to anterior cutaneous nerve entrapment syndrome (ACNES): a single blinded randomized clinical trial
- The influence of isometric exercise on endogenous pain modulation: comparing exercise-induced hypoalgesia and offset analgesia in young, active adults
- Do pain-associated contexts increase pain sensitivity? An investigation using virtual reality
- Differences in Swedish and Australian medical student attitudes and beliefs about chronic pain, its management, and the way it is taught
- An experimental investigation of the relationships among race, prayer, and pain
- Educational case report
- Wireless peripheral nerve stimulation for complex regional pain syndrome type I of the upper extremity: a case illustration introducing a novel technology
Artikel in diesem Heft
- Frontmatter
- Editorial comment
- Diagnosis of carpal tunnel syndrome
- Body image concerns and distortions in people with persistent pain
- The prevalence of recurrent pain in childhood is high and increases with age
- Friends in pain: pain tolerance in a social network
- Clinical pain research
- Correlation of clinical grading, physical tests and nerve conduction study in carpal tunnel syndrome
- Spectroscopic differences in posterior insula in patients with chronic temporomandibular pain
- Deconstructing chronicity of musculoskeletal pain: intensity-duration relations, minimal dimensions and clusters of chronicity
- “When I feel the worst pain, I look like shit” – body image concerns in persistent pain
- The prevalence of neck-shoulder pain, back pain and psychological symptoms in association with daytime sleepiness – a prospective follow-up study of school children aged 10 to 15
- The neglected role of distress in pain management: qualitative research on a gastrointestinal ward
- Pain mapping of the anterior knee: injured athletes know best
- The role of pain in chronic pain patients’ perception of health-related quality of life: a cross-sectional SQRP study of 40,000 patients
- The DoloTest® in a specialized headache center among patients receiving psychological treatment. A pilot study
- Observational study
- Chronic pelvic pain – pain catastrophizing, pelvic pain and quality of life
- Survey of chronic pain in Chile – prevalence and treatment, impact on mood, daily activities and quality of life
- Patients’ pre-operative general and specific outcome expectations predict postoperative pain and function after total knee and total hip arthroplasties
- The peer effect on pain tolerance
- Original experimental
- The effects of propranolol on heart rate variability and quantitative, mechanistic, pain profiling: a randomized placebo-controlled crossover study
- Idiographic measurement of depressive thinking: development and preliminary validation of the Sentence Completion Test for Chronic Pain (SCP)
- Adding steroids to lidocaine in a therapeutic injection regimen for patients with abdominal pain due to anterior cutaneous nerve entrapment syndrome (ACNES): a single blinded randomized clinical trial
- The influence of isometric exercise on endogenous pain modulation: comparing exercise-induced hypoalgesia and offset analgesia in young, active adults
- Do pain-associated contexts increase pain sensitivity? An investigation using virtual reality
- Differences in Swedish and Australian medical student attitudes and beliefs about chronic pain, its management, and the way it is taught
- An experimental investigation of the relationships among race, prayer, and pain
- Educational case report
- Wireless peripheral nerve stimulation for complex regional pain syndrome type I of the upper extremity: a case illustration introducing a novel technology

