Abstract
Epoxy polymer with damage indicating ability was very usable for ships and bridges to detect the cracks at an early stage and to prevent corrosion. 2′, 7′-dichlorofluorescein (DCF), as a damage indicator, was used to report the mechanical damage of epoxy-amine polymer by a strong color change from a light yellow to bright red due to the molecular structure transition from the acid molecular form to the base ion form. The effect of water on damage indicator and damaged epoxy-amine polymer film was evaluated by an immersion test and the properties were characterized by ultraviolet-visible spectrophotometry (UV-Vis), scanning electronic microscopy (SEM), energy dispersive X-ray spectrometer (EDS), zeta potential and thermal gravimetric analysis (TGA). The results showed that DCF was an easy, stable and permanent indicator for epoxy-amine polymer and the water only had a slight influence on the indication stability of damaged epoxy polymer.
1 Introduction
High-performance epoxy polymers are increasingly used in composites and coatings when high strength and stiffness, low weight, water-resistance and environmental stability are required (1), (2), (3). The intrinsic brittleness and defects of these materials make them susceptible to failure by the accumulation of fatigue damage until a critical flaw size-crack is exceeded (1). Crack damage, even on small scales, can significantly compromise the integrity and functionality of epoxy polymeric materials. Damage to the protective polymer coatings on metal substrates initiates corrosion undercutting and other forms of environmental degradation (4), (5). Therefore, detecting cracks particularly microscopic cracks is very critical.
For living organisms, e.g. skin, when the wound occurs, it immediately senses and reports injured tissue by nerves signaling pain and dark red color of bleeding until the injury is healed (6). The intrinsic mechanism is difficult to completely imitate in the man-made epoxy polymeric materials, but it gives some enlightenment in designing self-indicating materials.
Self-indicating materials that mimic nature’s ability to indicate damage have been shown in some papers (7), (8), (9), (10), (11). For example, Makyla et al. (8) reported an eYFP fluorescent protein as a force-responsive molecular sensor to monitor stress distributions, damage propagation and indicate micro-damage in a polymeric material by an easy-to-observe signals-fluorescent change. When the polymer was under a load or after an impact, the fluorescence would change or disappear. The mechanism of this change is called molecule “turn-off”. However, these detections are restricted to internal material interfaces. Meanwhile, the accuracy of the damage position and the long-term stability of mechanophores also require further investigation. Another successful example for damage detection was to rely on a mechanochemical reaction (10), (11). The damage indicator was filled into the microcapsules. When the microcapsules were mechanical fractured, the indicator was released and subsequently reacted with the catalyst in the epoxy matrix. Then a strong color change was produced and used for reporting mechanical damage. However, most of the polymeric materials are in service on the outside and undergo severe environmental change, which could deteriorate the properties of epoxy materials (12), (13). One of the most common environmental conditions is hydrothermal aging, which takes place when material is exposed to a wet environment at moderate or elevated temperatures. Interactions between the water in the environment and the polymer backbone of the resin can result in various modifications of the resin's properties (14), (15). But the best of our knowledge, until now, limited reports are available about the effect of water on the color stability of 2′, 7′-dichlorofluorescein (DCF) for epoxy polymer, which has an important influence on the indication of permanent damage. Therefore, studying the effect of water on indicating stability of DCF for damaged epoxy polymer is very significant.
In this paper, DCF was selected as a damage indicator to report the mechanical damage in the epoxy polymer through color change, and glycidyl methacrylate (GMA) was used as a solvent to dissolve the DCF powder and transport the DCF to the crack plane through capillary pressure. The color changes of DCF with diethylenetriamine (DETA) and amine cured epoxy polymer were imaged by a digital camera and monitored by ultraviolet-visible spectrophotometry (UV-Vis). The effect of water on the damage report stability of DCF for epoxy polymer was studied by an immersion test and the properties of the epoxy films were characterized by scanning electronic microscopy (SEM), energy dispersive X-ray spectrometer (EDS), UV-Vis spectroscopy and zeta potential. The thermal behavior of color-changed epoxy polymer before and after being immersed in water was evaluated by thermal gravimetric analysis (TGA). The results demonstrate that the damage indication of DCF for epoxy-amine polymer is stable and permanent, which aids in reporting mechanical damage persistently and broadens the application of this method in the epoxy-amine industry.
2 Materials and methods
2.1 Materials
2′, 7′-dichlorofluorescein (DCF, purity >90 wt%), glycidyl methacrylate (GMA, AR), diethylenetriamine (DETA, AR) were purchased from Aladdin (Shanghai) Reagent Ltd (China). Diglycidyl ether of bisphenyl A (DGEBA, Brand EP-4100HF, high-purity) was kindly provided by the ADEKA Corporation of Japan. The epoxy equivalent weight was 182 g/eq. Ultrapure deionized water was generated using a Millipore Milli-Q plus system. The hollow glass fibers were supplied by the 3 m (China) Corporation. All chemicals were used as received without further purification.
2.2 Method: preparation of epoxy polymer and epoxy polymer with hollow glass fiber
Epoxy specimens were prepared by DGEBA epoxy resin and DETA curing agent with stoichiometric ratios of 1:1. The detailed experimental procedure was described as follows: 100 g DGEBA and 11.5 g DETA was mixed using a SR-500 planetary mixer (THINKY, Japan) at 1000 rpm for 10 min, then the mixture was degassed under a vacuum of −0.09 MPa for 20 min. Finally, the mixture was poured into the pre-treated stainless steel mould or poured into the mould embedded with hollow glass fibers and cured at 25°C for 24 h. The DCF/GMA solution was prepared by dissolving 0.004 g DCF powder in 10 ml GMA monomer under sonication for 30 min to form a clear yellow solution with the concentration of 1×10−3 mol/l. Afterwards, the prepared DCF/GMA solution was filled into the hollow glass fiber under capillary pressure.
2.3 Characterization
The color changes of DCF with DETA and epoxy-amine matrix were observed visually. Visible spectra of DCF/GMA solution and soaked epoxy samples were obtained by ultraviolet-visible spectrophotometry (UV-Vis, Shimadzu UV3150, Japan). Epoxy swelling was evaluated by immersing the specimen in the DCF/GMA solution (1×10−3 mol/l) and the weight change was recorded after soaking for 1 min, 10 min, 20 min and 30 min. The color stability of the soaked specimen was characterized by an immersion experiment. Typically, 0.05 g epoxy polymer film was immersed in 5 ml 1×10−3 mol/l DCF/GMA solution for 30 min. The excess DCF/GMA solution was absorbed by filter paper. After 24 h, the red epoxy film was soaked in 20 ml water. The zeta potential of the upper aqueous solution at 1 min, 5 min, 10 min, 20 min, 30 min, 60 min and 120 min was measured using a Marven Zeta PALS potential analyzer (USA). The surface morphology of epoxy films before and after being immersed in water was observed by scanning electron microscopy (SEM, FEI Helios Nanolab 600i), and the C and Cl elements were analyzed by EDS. The thermal behavior of epoxy-amine polymer colored by DCF before and after immersing in water was performed with a TGA SDT 600 (TA instrument, USA) at a heating rate of 10°C min−1 under nitrogen atmosphere.
3 Results and discussion
3.1 Damage indication of DCF for epoxy polymer
DCF was selected as the indicating agent due to its high and fast reactivity towards amine (10), (16). GMA, a low viscosity and nontoxic hydrophobic solvent with water solubility of 0.023 g/g at 20°C, was used for dissolving the DCF powder (17), (18). As depicted in Figure 1, GMA had a good solubility with DCF due to its high Hansen solubility parameter (19), (20). When a droplet of amine (i.e. DETA) was added into the DCF/GMA solution, a strong color change from light yellow to bright red could be seen immediately. Meanwhile, the clear solution became opaque and the absorbance peak exhibited a large red shift. The molecular structure of DCF was transformed from the acid molecule state to the base ion state after the addition of amine. Meanwhile, solid DCF ion agglomerates were precipitated from GMA solution due to a dramatic drop in solubility and chemical reaction between GMA and DETA (10). The color change of DCF/GMA solution before and after addition of DETA directly identifies that DCF is an easy and convenient damage indicator for the amine group.
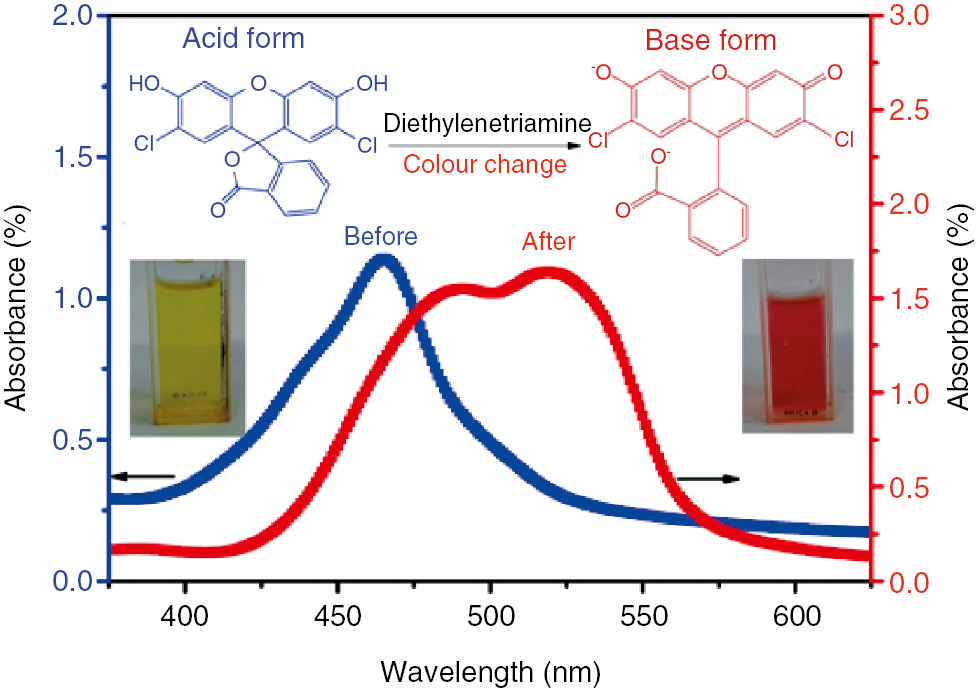
Visible spectra and color change of DCF/GMA solution before and after addition of DETA curing agent. The color change from light yellow to opaque red of the 1×10−3 mol/l DCF/GMA solution was generated by the addition of DETA curing agent.
To examine the ability of DCF to react with the epoxy-amine polymer, the cured epoxy films (50 μm) were soaked in DCF/GMA solution (molar concentration of 1×10−3 mol/l) for 1 min, 10 min, 20 min and 30 min (Figure 2). It can be seen that the color of epoxy film changed from colorless to red with the increase of the soaked time and a visual swelling occurred at about 30 min. The swelling degree was calculated by the weighting method and the mass changes were 0.50 wt%, 1.15 wt%, 3.53 wt% and 5.82 wt%, respectively. The curing degree of epoxy film determined by Fourier transform infrared (FTIR) spectroscopy was 97.38% (19), which indicated some residual amine group was retained in the epoxy matrix. Therefore, it is reasonable to deduce that the color-changing mechanism for epoxy-amine polymer is that DCF molecules react with the residual amine in the epoxy polymer and form red precipitates quickly inside the epoxy matrix. Because GMA has a moderate polarity force (δP) and hydrogen bonding (δH) (δPGMA=8.78, δHGMA=5.83) compared with epoxy polymer (δPepoxy=14.46, δHepoxy=5.99) (20), it has a good swelling ability to epoxy-amine matrix and transports DCF molecules into the cross-linked network. DCF molecules continuously diffuse toward the inside of the epoxy matrix and contact with the residual amine to form a large amount of red precipitates. Thus, the entire epoxy specimen turns red gradually.
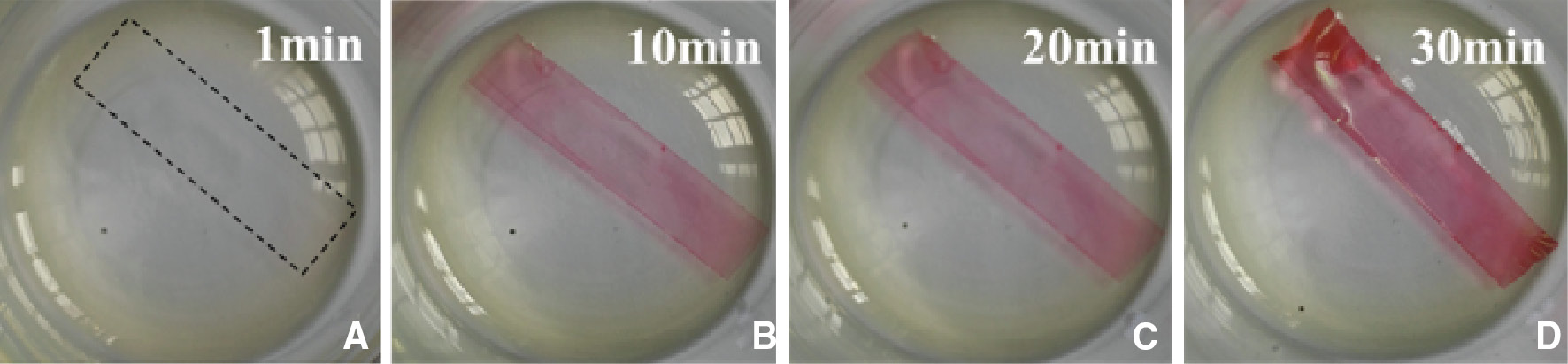
The swelling and color change of the epoxy film. (A) 1 min, (B) 10 min, (C) 20 min, (D) 30 min.
The transmittance spectra of epoxy film before and after soaking were also determined by UV-Vis spectrophotometry (Figure 3). It could be seen that there was no transmittance peak for the original epoxy specimen. When soaked in DCF/GMA solution for 30 min, a strong transmittance peak emerged, which further confirmed that DCF could be used as a damage indicator for epoxy-amine polymer.
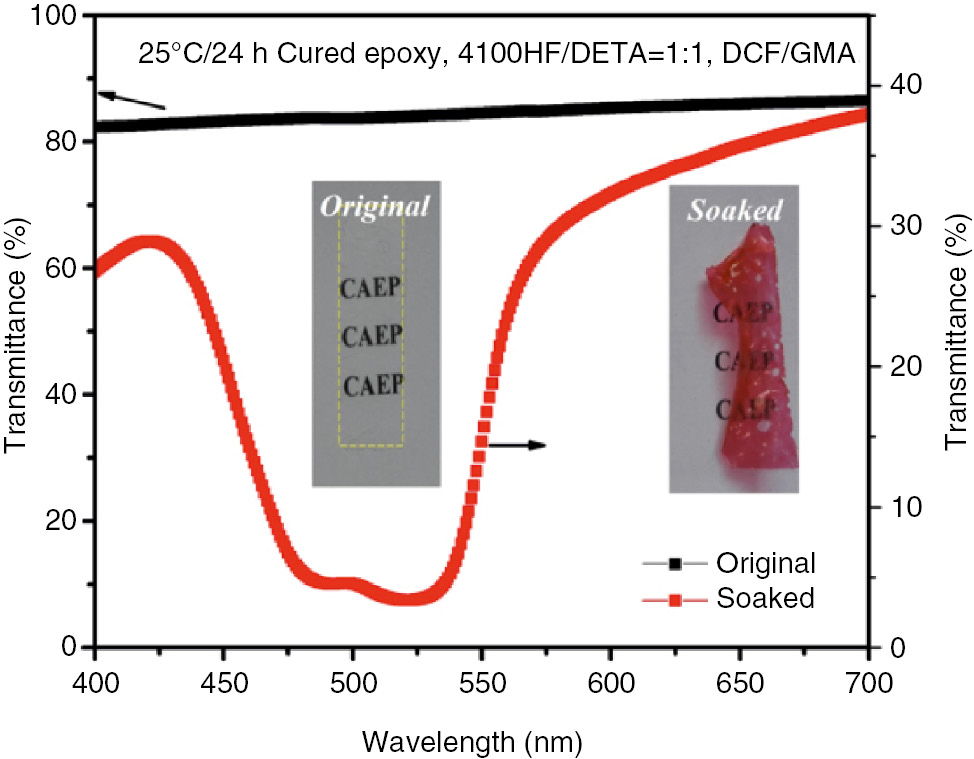
Visible spectra of the epoxy specimen before and after soaked in DCF/GMA solution.
The crack damage indication performance of DCF on the epoxy matrix was further evaluated by the micro-vascular fracturing method. Figure 4 gives the schematic diagram and the images of color change in the epoxy matrix before and after the micro-vascular was damaged. Firstly, a hollow glass fiber was embedded in the epoxy-amine polymer and cured into a transparent epoxy matrix (Figure 4A); secondly, the DCF/GMA solution was filled into the hollow glass fiber under capillary pressure, and a light yellow color was observed in the glass fiber (Figure 4B); thirdly, a load of 10 kN was applied to the epoxy matrix with hollow glass fiber, and the DCF/GMA solution released from the hollow glass fiber immediately and filled the cracks produced in the matrix rapidly (Figure 4C). The cracks and other regions contacting with the DCF/GMA solution started to become red. After 24 h, the damaged region became redder which indicated that the mechanical damage was generated (Figure 4D).

Schematic diagram (upper) and photographs (below) of color change of epoxy matrix after mechanical damage. (A) Epoxy matrix with hollow glass fiber, (B) epoxy matrix with hollow glass fiber filled with DCF/GMA solution, (C) color change of epoxy matrix after mechanical damage immediately, (D) color change of epoxy matrix after mechanical damage for 24 h.
3.2 The effect of water on damage indication
Epoxy polymer can be used as anti-corrosion coatings on ships, or the packaging materials on bridges, which is often threatened with severe environments, such as high or low temperature, rain or sunshine (2), (5), (10). Hence, the stability of damage indication is very important for the practical application of epoxy polymers. Figure 5 illustrates the effect of water on the damage indicator and damaged epoxy-amine polymer.

The effect of water on the color change of (A) DCF/GMA/DETA solution and (B) damaged epoxy-amine polymer.
In the experiment, a droplet of DCF/GMA/DETA solution (a1) and the damaged epoxy-amine polymer (b1) were added into two cups of water. Interestingly, the red color of a1 disappeared immediately after shaking for 30 s and the solution became a light green aqueous solution (a4). As shown in Figure 5B, the aqueous solution of b2 also became light green, but the color of the epoxy matrix immersed in water for 30 days (b3) was almost the same as that of the original epoxy matrix (b1). The results demonstrated that the water had some influence on the color stability of damaged epoxy polymer, but it did not result in the disappearance of the damage indication function.
In order to reveal the effect of water on the damage indicator and damaged epoxy-amine polymer, the characteristic absorbance spectra of a1, a4 solution and the upper aqueous solution of b2 were monitored by UV-Vis spectroscopy (Figure 6). As illustrated in Figure 6A, the absorbance peak of a4 became narrow when a1 solution was added in water. The possible reason is that DCF ions are easy to dissolve in water under base conditions (21), (22). Compared with the visible spectra of b2 and a4 (Figure 6B), identical absorbance peaks were presented which meant that the two solutions contained the same DCF ions. From the above experimental phenomenon of Figures 5 and 6, it is not difficult for us to think that the effect of water on DCF/GMA/DETA solution and damaged epoxy-amine polymer are different.
![Figure 6: Visible spectra of (A) DCF/GMA/DETA solution and a droplet of DCF/GMA/DETA solution added to water; (B) upper aqueous solution [Figure 5 (b2)] and a droplet of DCF/GMA/DETA solution added to water.](/document/doi/10.1515/epoly-2016-0135/asset/graphic/j_epoly-2016-0135_fig_006.jpg)
Visible spectra of (A) DCF/GMA/DETA solution and a droplet of DCF/GMA/DETA solution added to water; (B) upper aqueous solution [Figure 5 (b2)] and a droplet of DCF/GMA/DETA solution added to water.
To reveal the influence of water on the epoxy-amine polymer further, 0.05 g color-changed epoxy-amine polymer film (Figure 2D) was soaked in 20 ml water. The zeta potential of upper solution was measured at 1 min, 5 min, 10 min, 20 min, 30 min, 60 min and 120 min (Figure 7).
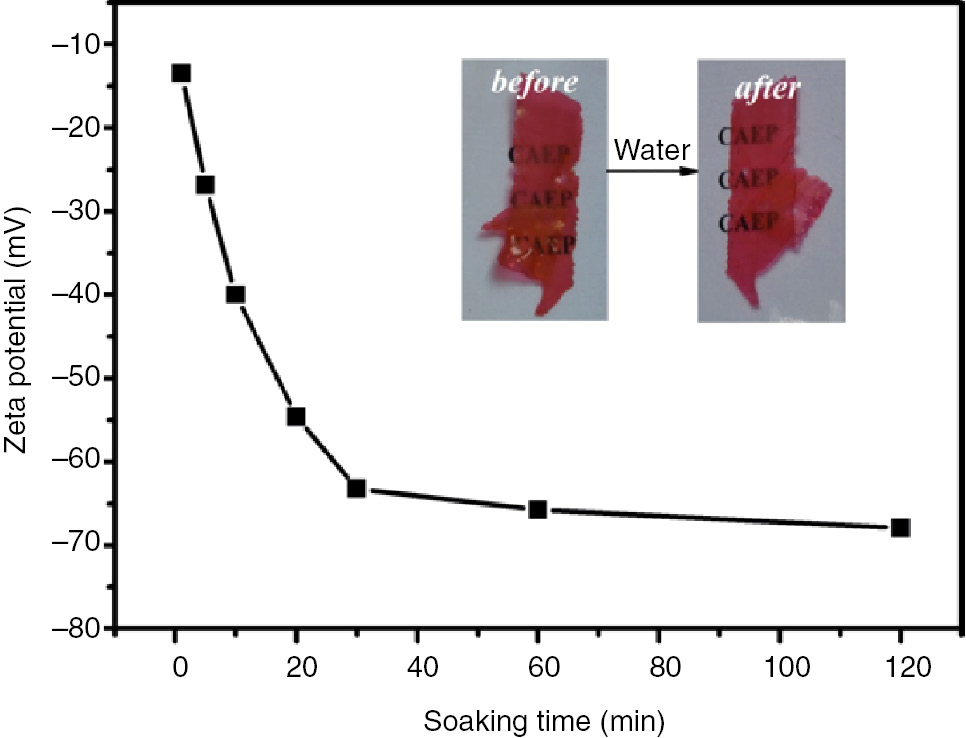
Zeta potential of the aqueous solution with different soaking time.
As shown in Figure 7, the zeta potential was negative, indicating that negative DCF ions were present in the water. With the increase of the immersing time, the absolute values of the zeta potential increased gradually, which meant some free DCF ions had entered in the water. Continuing to extend the soaking time, the zeta potential reached equilibrium, indicating the ionic concentration had achieved a balance and no free DCF ions were released in water again. Also, the color of immersed epoxy film remained, implying some DCF precipitates were present.
The surface morphology of epoxy films before and after being immersed in water was observed by SEM measurement. Element mapping of the sample surface was imaged (e.g. C and Cl) (Figure 8). It can be seen that C and Cl elements dispersed uniformly on the surface of original epoxy film (Figure 8B and C) and DCF precipitates appeared on the surface of the epoxy-amine polymer in nanoscale (Figure 8D). This phenomenon further confirms that DCF molecules can react with residual amine in the epoxy polymer and form red precipitates. However, when the epoxy film was immersed in water, the nanosize precipitates were reduced significantly on the surface of epoxy film but the Cl element still existed, confirming that DCF ions was present. The reasonable interpretation may be that GMA is hydrophobic and has a good swelling ability to epoxy polymer, which can transport more DCF molecules into the inner of epoxy matrix and form more red precipitates to indicate damage. While the water molecule has a poor penetration into the epoxy network with high crosslinking density (17), (23), it is unable to dissolve the DCF precipitates formed inside the epoxy matrix. Hence, the red color could be kept despite the DCF precipitates on the surface of epoxy film being dissolved by water. Therefore, it is reasonable to think that DCF has a persistent damage indicating ability no matter whether the damage process comes into contact with water or not.
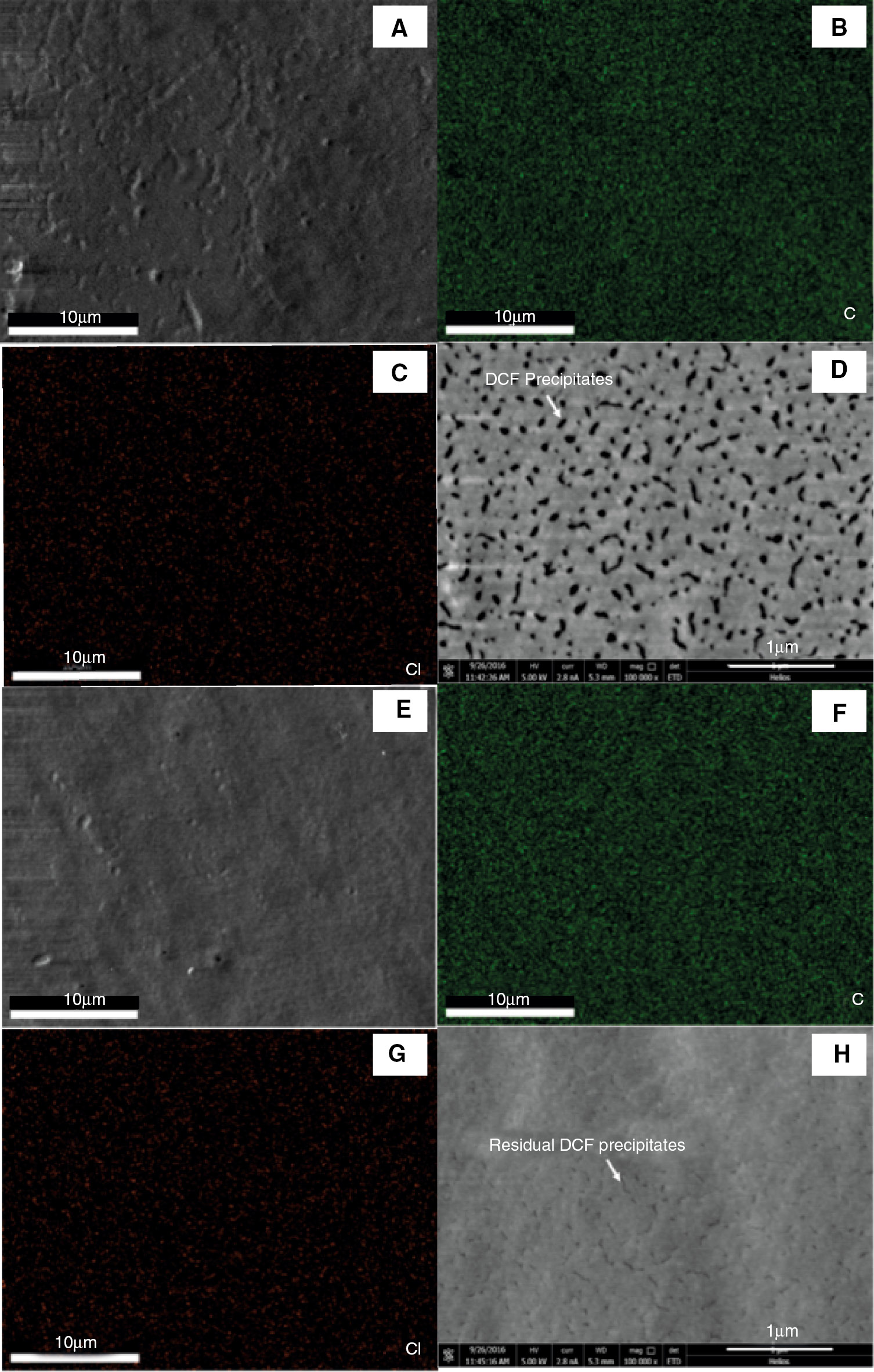
SEM micrographs and element mapping of damaged epoxy film before and after immersed in water. (A–D) SEM image, C and Cl mapping of epoxy film after soaked in DCF/GMA solution; (E–H) SEM image, C and Cl element mapping of epoxy film with DCF after immersed in water.
3.3 Thermal behavior of epoxy polymer before and after being immersed in water
The thermal behavior of the red soaked epoxy-amine polymer (Figure 2D) before and after being immersed in water was analyzed by TG, and the curves are presented in Figure 9. The results showed that the water had a slight effect on the degradation of epoxy adhesive. Epoxy adhesive before immersing in water exhibited two stages. The first, which occurred at 173.1°C was due to the volatilization of GMA; the second and the larger mass loss at 360.5°C, was attributed to the thermal degradation of the cured DGEBA and DCF precipitates. The literature describes exactly the same behavior for thermal degradation of epoxy adhesive (24). After being immersed in water for 30 days, the weight loss of epoxy adhesive at 241.9°C was 3.88 wt%, 1.81 wt% lower than the original sample, which indicated some GMA and DCF ions on the surface of epoxy film had been released into the water. Meanwhile, the maximum decomposed temperature shifted to 347.1°C, which was 13.4°C lower than the original specimen. The reason may be some water molecules diffusing into the voids on the surface of epoxy film leaved by DCF precipitates (12).

TGA curves of epoxy-amine polymer colored by DCF before and after immersing in water. (A) TG curves; (B) DTG curves (heating rate: 10°C/min, N2 flow: 50 ml/min).
4 Conclusions
2′, 7′-dichlorofluorescein (DCF), as a damage indicator to report the mechanical damage of epoxy polymer by a strong color change from a light yellow to bright red was demonstrated. The damage indication stability of epoxy film was evaluated using the immersion test, and characterized by UV-Vis spectroscopy, SEM, EDS, zeta potential and TG. The results identified that the water had a slight influence on the color change of the damaged epoxy polymer, but it could not result in the disappearance of the damage indication function. Therefore, DCF was an easy, stable and permanent indicator for epoxy-amine polymer.
Acknowledgments
The authors thank the support of the Natural Science Foundation of China (Grants: 51573172, 11405149, and 51401187), the Science and Technology Planning Project of Sichuan Province (SCXSDTR15001) and Director’s Funds of Materials Institute of China Academy of Engineering Physics (SJZ201506).
References
1. Wu DY, Meure S, Solomon D. Self-healing polymeric materials: a review of recent developments. Prog Polym Sci. 2008;33:479–522.10.1016/j.progpolymsci.2008.02.001Suche in Google Scholar
2. Hughes AE, Cole IS, Muster TH, Varley RJ. Designing green, self-healing coatings for metal protection. NPG Asia Mater. 2010;2(4):143–51.10.1038/asiamat.2010.136Suche in Google Scholar
3. Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR, White SR. Self-healing polymers and composites. Annu Rev Mater Res. 2010;40:179–211.10.1146/annurev-matsci-070909-104532Suche in Google Scholar
4. Diesendruck CE, Sottos NR, Moore JS, White SR. Biomimetic self-healing. Angew Chem Int Ed. 2015;54:2–22.10.1002/anie.201500484Suche in Google Scholar PubMed
5. Stoddart A. Polymers: colour in the cracks. Nat Rev Mater. 2016;1:16004.10.1038/natrevmats.2016.4Suche in Google Scholar
6. Nosonovsky M, Rohatgiin PK. Biomimetics in Materials Science. New York: Springer-Verlag, 2012, p. 103.10.1007/978-1-4614-0926-7Suche in Google Scholar
7. Sagara Y, Yamane S, Mitani M, Weder C, Kato T. Mechanoresponsive luminescent molecular assemblies: an emerging class of materials. Adv Mater. 2016;28(6):1073–95.10.1002/adma.201502589Suche in Google Scholar PubMed
8. Makyla K, Müller C, Lörcher S, Winkler T, Nussbaumer MG, Eder M, Bruns N. Fluorescent protein senses and reports mechanical damage in glass-fiber-reinforced polymer composites. Adv Mater. 2013;25:2701–6.10.1002/adma.201205226Suche in Google Scholar PubMed
9. Davis DA, Hamilton A, Yang J, Cremar LD, Gough DV, Potisek SL, Ong MT, Braun PV, Martinez TJ, White SR, Moore JS, Sottos NR. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature. 2009;459(7243):68–72.10.1038/nature07970Suche in Google Scholar PubMed
10. Odom SA, Jackson AC, Prokup AM, Chayanupatkul S, Sottos NR, White SR, Moore JS. Visual indication of mechanical damage using core-shell microcapsules. ACS Appl Mater Interfaces. 2011;3:4547–51.10.1021/am201048aSuche in Google Scholar PubMed
11. Li WL, Matthews CC, Yang K, Odarczenko MT, White SR, Sottos NR. Autonomous indication of mechanical damage in polymeric coatings. Adv Mater. 2016;28(11):2189–94.10.1002/adma.201505214Suche in Google Scholar PubMed
12. Zanni-Deffarges MP, Shanahan MER. Diffusion of water into an epoxy adhesive: comparison between bulk behavior and adhesive joints. Int J Adhes Adhes. 1995;15(3):137–42.10.1016/0143-7496(95)91624-FSuche in Google Scholar
13. Zhang F, Wang HP, Hicks C, Yang X, Carlson BE, Zhou Q. Experimental study of initial strengths and hygrothermal degradation of adhesive joints between thin aluminum and steel substrates. Int J Adhes Adhes. 2013;43:14–25.10.1016/j.ijadhadh.2013.01.001Suche in Google Scholar
14. Yagoubi JE, Lubineau G, Saghir S, Verdu J, Askari A. Thermomechanical and hygroelastic properties of an epoxy system under humid and cold-warm cycling conditions. Polym Degrad Stabil. 2014;99:146–55.10.1016/j.polymdegradstab.2013.11.011Suche in Google Scholar
15. Quino G, Yagoubi JE, Lubineau G. Characterizing the toughness of an epoxy resin after wet aging using compact tension specimens with non-uniform moisture content. Polym Degrad Stabil. 2014;109:319–26.10.1016/j.polymdegradstab.2014.08.005Suche in Google Scholar
16. Choi MG, Moon JO, Bae J, Lee JW, Chang SK. Dual signaling of hydrazine by selective deprotection of dichlorofluorescein and resorufin acetates. Org Biomol Chem. 2013;11:2961–5.10.1039/c3ob40091cSuche in Google Scholar PubMed
17. Meng LM, Yuan YC, Rong MZ, Zhang MQ. A dual mechanism single-component self-healing strategy for polymers. J Mater Chem. 2010;20:6030–8.10.1039/c0jm00268bSuche in Google Scholar
18. Zhu DY, Guo JW, Cao GS, Qiu WL, Rong MZ, Zhang MQ. Thermo-moldable self-healing commodity plastics with heat resisting and oxygen-insensitive healant capable of room temperature redox cationic polymerization. J Mater Chem A 2015;3:1858–62.10.1039/C4TA06381CSuche in Google Scholar
19. Guo YK, Chen L, Xu DG, Zhong JR, Yue GZ, Astruc D, Shuai MB, Zhao PX. A dual functional epoxy material with autonomous damage indication and self-healing. RSC Adv. 2016;6(69):65067–71.10.1039/C6RA13519FSuche in Google Scholar
20. Ma W, Zhang W, Zhao Y, Yu H, Wang S, Wang Y. Predictions of healing performance for solvent-promoted self-healing materials by using Hansen solubility parameters. Mater Lett. 2016;163:244–6.10.1016/j.matlet.2015.10.090Suche in Google Scholar
21. Yao H, Jockusch RA. Fluorescence and electronic action spectroscopy of mass-selected gas-phase fluorescein, 2′, 7′-dichlorofluorescein, and 2′, 7′-difluorofluorescein ions. J Phys Chem A. 2013;117(6):1351–9.10.1021/jp309767fSuche in Google Scholar PubMed
22. Kim HY, Im HG, Chang SK. Colorimetric and fluorogenic signaling of fluoride ions by thiophosphinated dichlorofluorescein. Dyes Pigments. 2015;112:170–5.10.1016/j.dyepig.2014.06.030Suche in Google Scholar
23. Baines FL, Billingham NC, Armes SP. Synthesis and solution properties of water-soluble hydrophilic-hydrophobic block copolymers. Macromolecules 1996;29(10):3416–20.10.1021/ma951699+Suche in Google Scholar
24. Rodrigues GGM, Paiva JMF, Carmo JB, Botaro VR. Recycling of carbon fibers inserted in composite of DGEBA epoxy matrix by thermal degradation. Polym Degrad Stabil. 2014;109:50–8.10.1016/j.polymdegradstab.2014.07.005Suche in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels
Artikel in diesem Heft
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels


