Abstract
This study demonstrates that different modification pulse voltages affect the wetting property of the surface of polyamide 6 (PA6) with a certain regularity. Broadly, the hydrophilic property of PA6’s surface increases with increasing pulsed voltage. Based on scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) analysis, this paper discusses the mechanism by which high current pulsed electron beam (HCPEB) etching modification influences the surface wettability of PA6. Within a certain range below 28 kV, this effect is caused by an increase of in surface roughness due to HCPEB bombardment of the surface. Within a certain range above 28 kV, HCPEB changes the surface morphology, resulting in changes to the wetting property. Furthermore, by using various pulsed voltages to modify the PA6 surface, this study investigated the ability of the Wenzel model to explain changes in the water contact angle and wetting property of PA6’s surface.
1 Introduction
Wetting, a common factor in nature, engineering and daily life, to a great extent determines the realizability and usability of material preparation (1). In the last two decades, synthetic light-stimuli-responsive polymers have attracted increasing attention, based on their wide variety of applications. These photo-responsive polymers are able to alter their properties in a reversible way, including their wetting behaviors, which change upon irradiation with light of a defined wavelength. These changes are often based on chromophoric groups of organic dyes, such as azobenzene, spiropyran, or salicylideneaniline, which undergo a reversible isomerization upon exposure to ultraviolet (UV) light or to visible light (2), (3), (4), (5). For example, UV exposure makes the surface of positron emission tomography (PET) hydrophilic, leading to a more uniform poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT: PSS) film distribution. In examining the PEDOT: PSS films deposited on UV-treated PET, both the strength of adhesion to the substrate and the stability of resistance after 20,000 cycles of bending were determined to be greater than in indium-tin-oxide and PEDOT: PSS deposited on untreated PET (6). Factors that influence a material’s surface wettability include the microstructure, roughness, and chemical composition of the material’s surface, ambient temperature; and, properties of the liquid used (7).
Polyamide 6 (PA6) is the most common type of engineering plastic in the polyamide family. Its polar amide group can form hydrogen bonds, which have great molecular force. The molecular chains are arranged in neat rows. In addition, a large number of amide groups exist, with a carboxyl group or amino group serving as the molecule’s terminal; this structure creates a kind of crystalline polymer with strong polarity, high intermolecular force, and certain reactivity. Compared with other engineering plastics, PA6 has many advantages, including superior wear performance, self-lubrication, good vibration absorption, noise reduction, good rigidity, high strength, good chemical stability, wide temperature range, and ease of processing (8). These properties make it an ideal friction material that has been widely applied in sliders, bushings, gears, bearings, and turbines (9).
High current pulsed electron beam (HCPEB) technology is a new and efficient surface treatment technology that not only can effectively improve the surface structure and performance of materials, but also can serve as a suitable radiation source to carry out related scientific research on radiation damage and the formation and evolution of defect clusters (10). In a materials treatment process involving pulsed electron beams, high energy (108–109 W/cm2) acts on the material surface instantly, causing the material’s surface to melt. At the same time, rapid cooling can be achieved because of the substrate’s thermal conductivity, and a shock wave can be generated to facilitate the formation of compressive stress on the surface. Furthermore, the electron beam treatment is conducted in a vacuum, which can reduce the effects of oxidation so as to produce a pure surface-treated layer (11). Surface modification technologies in the literature include chemical modification, plasma etching modification, laser etching modification, and electron beam etching modification. However, the literature has not explored high-polymer materials that can apply high-current electron beam etching modification.
To fill this gap in the research, this study applies HCPEB to bombard PA6 and conduct surface etching modification. By testing changes in the PA6 surface water contact angle under different pulse voltages, this study assesses the influence of different pulse voltages on the wettability of the PA6 surface. Meanwhile, scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) analysis are applied to analyze the morphology and elemental composition of the PA6 surface before and after modification, and to discuss the effect mechanism of high-current pulsed electron beams on PA6 surface wettability, thereby contributing to further research in this area.
2 Experimental materials and equipment
Materials used in this experiment include PA6 [composition: Polyamide matrix 100 wt%+glass fiber 15 wt%+styrene butadiene styrene block copolymer (SBS) 20 wt%] with dimensions of 15 mm×10 mm×5 mm (length×width×height). An ultrasonic cleaning machine was used to clean the specimen, which was then dehydrated with pure anhydrous ethanol prior to use. An RITM-2M type of high-current pulsed electron beam, made in Russia, was used to bombard the PA6 specimen, with the following test parameters: pulse voltage 26, 28, 30, 32, and 34 kV; target distance: 80 mm; constant bombardment times: 20 times. The PA6’s roughness was measured with a multi-point acquisition test on a TR-200 type roughmeter (China) before and after modification. A DropMeter A-100p-type (Japan) contact angle analyzer was applied to measure the contact angle of the PA6 surface before and after HCPEB modification. Under the same pulsed voltage parameters, the surface of the bombarded specimen was conducted with four times of multi-point measurement in order to yield an average value. All measurements were carried out at room temperature. Surface elemental composition before and after modification was analyzed through XPS using an ESCALAB 250XI produced by Thermo Scientific (USA). The surface morphology of PA6 before and after modification was observed and analyzed through SEM using a JSM-6460LV microscope (Japan).
3 Experimental results and analysis
3.1 Influence rule of HCPEB on the PA6 surface water contact angle before and after modification
In this experiment, the surface of the PA6 specimen was bombarded 20 times, in order to achieve better results and without affecting the properties of the substrate material. Figures 1 and 2 show the variations in the PA6 surface water contact angle, along with the pulsed voltage, under conditions of constant bombardment with a beam spot diameter of 80 mm.
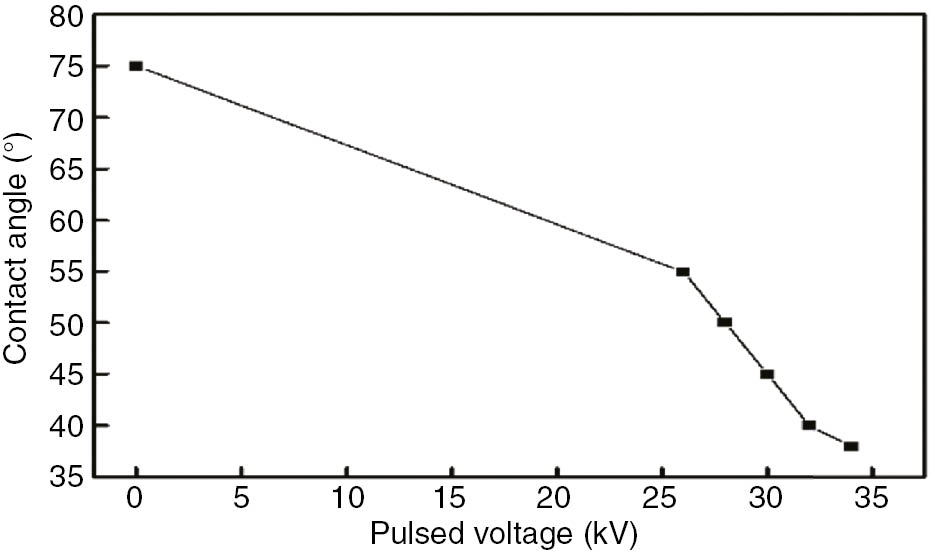
Variation of PA6 surface water contact angle along with pulsed voltage.
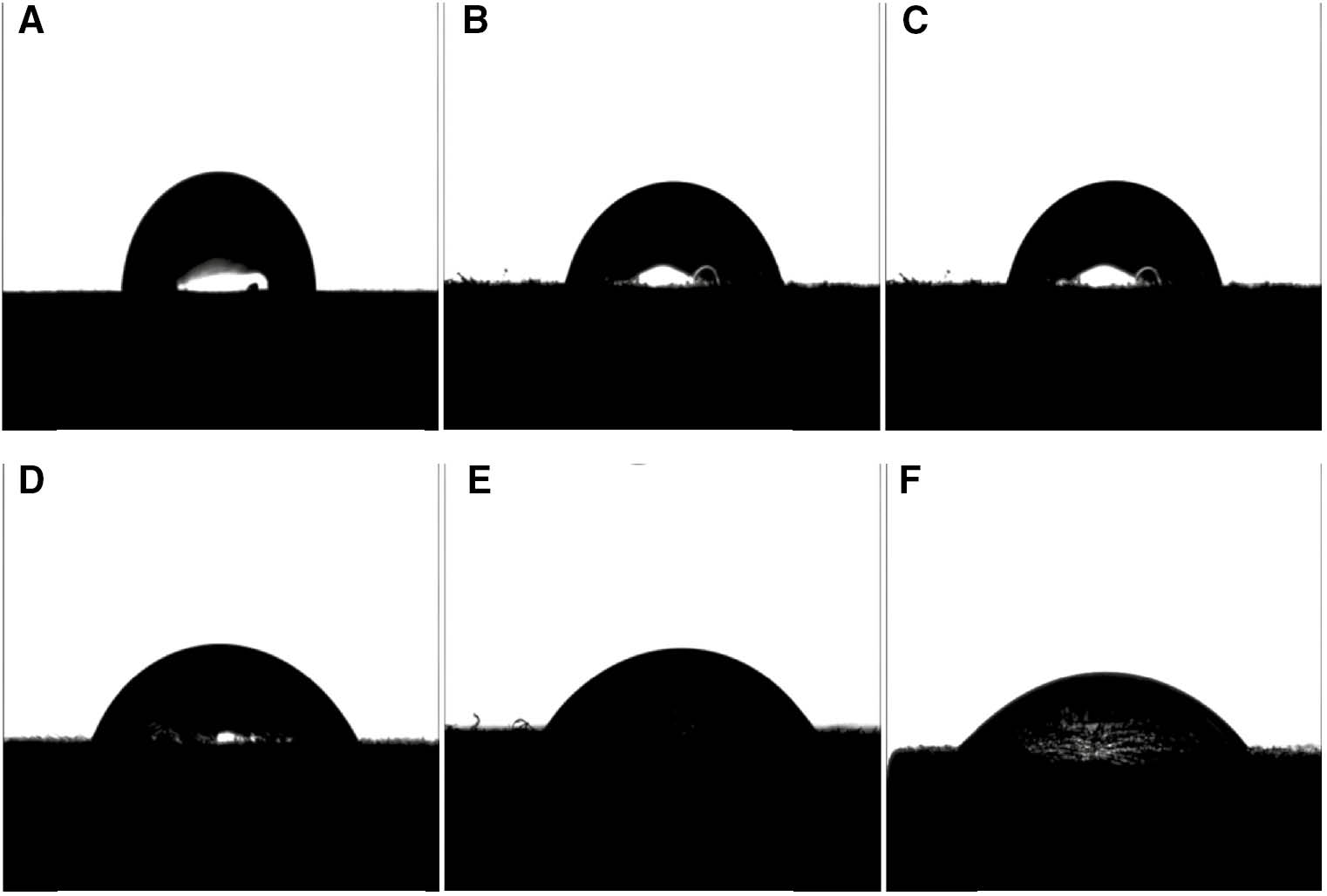
PA6 surface water contact angle before and after modification with different pulsed voltages.
(A) Untreated, 75°, (B) 26 kV, 55°, (C) 28 kV, 50°, (D) 30 kV, 45° (E) 32 kV, 40°, and (F) 34 kV, 38°.
As Figures 1 and 2 show, PA6’s surface water contact angle without bombardment treatment by HCPEB is 75°, indicating that the original PA6 surface is hydrophilic. After bombarding the PA6 surface with 26 kV of high-current pulsed electron beam, the contact angle became 55°, showing increased hydrophilicity. After that, the water contact angle decreased with increases in pulsed voltage, indicating that the hydrophilicity of the PA6 surface increases along with increasing pulse voltage. Therefore, the HCPEB voltage with which the PA6 surface is bombarded has an important effect on the PA6 material’s surface wetting properties. This suggests that surface modification with HCPEB is an effective way to increase the hydrophilicity of polymer materials.
3.2 Impact mechanism of HCPEB modification on PA6 surface wettability
3.2.1 Influence of surface elemental composition changes on surface wettability after HCPEB bombardment
Increases in non-polar functional groups like C-C and C-H can cause an increase in surface hydrophobicity; in contrast, increases in polar oxygen functional groups like C-O and C=O can cause an increase in surface hydrophilicity (12). This study applied XPS analysis to analyze the changes in surface elemental composition in PA6 before and after HCPEB modification (pulsed voltage, 34 kV). Figure 3 shows the results.
![Figure 3: PA6 surface XPS spectrogram before and after HCPEB modification [(A) Untreated, (B) C1s Spectra: 34 kV, (C) Untreated, and (D) O1s Spectra: 34 kV].](/document/doi/10.1515/epoly-2016-0078/asset/graphic/j_epoly-2016-0078_fig_003.jpg)
PA6 surface XPS spectrogram before and after HCPEB modification [(A) Untreated, (B) C1s Spectra: 34 kV, (C) Untreated, and (D) O1s Spectra: 34 kV].
Figure 3 indicates that the C1s peak on the PA6 surface is mainly composed of three sub-peaks, namely C1 (284.6 eV), C2 (285.2 eV), and C3 (287.7 eV), which correspond to C-H and C-C, C-O, and C=O, respectively. On the other hand, the O1s peak is mainly composed of two sub-peaks, namely O1 (531. 2 eV) and O2 (532.6 eV), which correspond to C=O and C-O, respectively. The results demonstrate that the proportion of the three sub-peaks in the C1s peak before modification was 47.57% C1, 43.24% C2, and 9.18% C3. The proportion of the two sub-peaks in the O1s peak was 50.84% O1 and 49.16% O2. After modification, the proportion of the three sub-peaks in the C1s peak was 45.94% C1, 43.98% C2, and 10.08% C3, while the proportion of the two sub-peaks in the O1s peak was 52.26% O1 and 47.74% O2. After HCPEB bombardment, the relative amount of carbon on the PA6 surface decreased slightly, but the relative amount of oxygen increased; the O/C ratio increased from 25.1% before modification to 42.8% after modification, indicating that HCPEB modification can increase the content of oxygen-containing polar groups on the PA6 surface. Therefore, we can chart the proportion of carbon and oxygen elements with different valence states on the PA6 surface, as shown in Table 1.
Element percentages on PA6 surface before and after HCPEB modification.
| PA6 | C1s/% | O1s/% | |||
|---|---|---|---|---|---|
| C1 | C2 | C3 | O1 | O2 | |
| Chemical bond | C-C, C-H | C-O | C=O | O=C | O-C |
| Untreated | 38.05 | 34.59 | 7.34 | 10.16 | 9.38 |
| Treated | 32.15 | 30.78 | 7.05 | 15.67 | 14.32 |
Table 1 shows that the carbon element on the PA6 surface decreases slightly after HCPEB modification, because of the decrease in carbon in C-C and C-H valence bonds from the original 38.05%–32.15%. The corresponding increase in oxygen on the surface is caused by increased oxygen in O=C and O-C valence bonds. However, Table 1 also shows that the elements’ percentage changes on the surface are relatively small before and after HCPEB modification. This suggests that although using HCPEB to bombard PA6 can increase hydrophilicity, the change in PA6’s wetting property is not caused primarily by the changes in elemental composition on the surface.
3.2.2 The influence of microstructure changes on wettability after HCPEB modification
In addition to its association with the surface’s elemental composition, the material surface’s wettability is also closely tied to the surface microstructure and roughness, ambient temperature, and the properties of the liquid. This study maintained the ambient temperature at a constant level, and the liquid used was consistently water; therefore, it was possible to isolate the influence of the surface’s microstructure and roughness on PA6’s wettability. When a solid surface is hydrophobic, high roughness will make it more hydrophobic; similarly, when a solid surface is hydrophilic, high roughness will make it more hydrophilic (13).
Figure 4 shows the roughness change curve after etching modification with different pulsed voltages on the surface; as the graph indicates, the roughness increases with increasing pulse voltage before stabilizing in a certain range. A comparison with Figure 1 reveals that the increased roughness increases the hydrophilicity of the PA6’s surface. When the pulse voltage exceeds 28 kV, the roughness tends to remain at a constant value, while the water contact angle is further reduced.
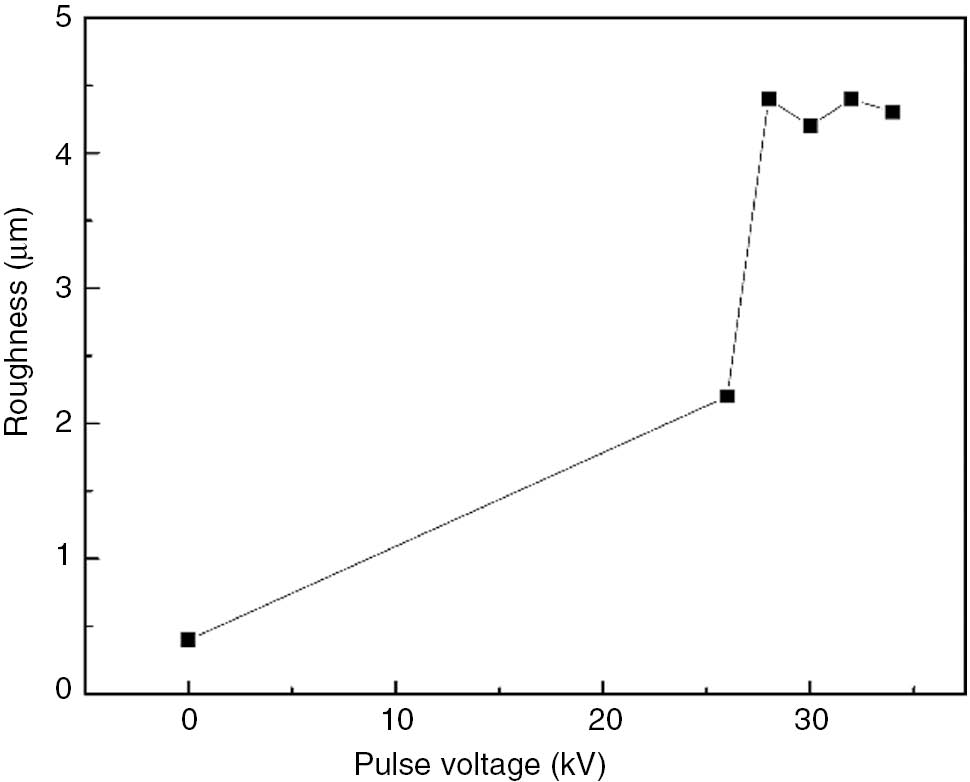
Roughness changes of PA6 surface.
Figure 5 displays the SEM photographs of the PA6 surface after etching modification with different pulsed voltages. When the pulsed voltage measures 26 kV, a small quantity of dimple-like structures appear on the surface, the glass fiber is interrupted, the roughness is enhanced, and the surface’s hydrophilic property improves. When the pulsed voltage exceeds 28 kV, the roughness on the PA6’s surface changes only slightly, but the hydrophilic property improves because of the increased amount of dimple-like structures. This result can be explained by the Wenzel theory.
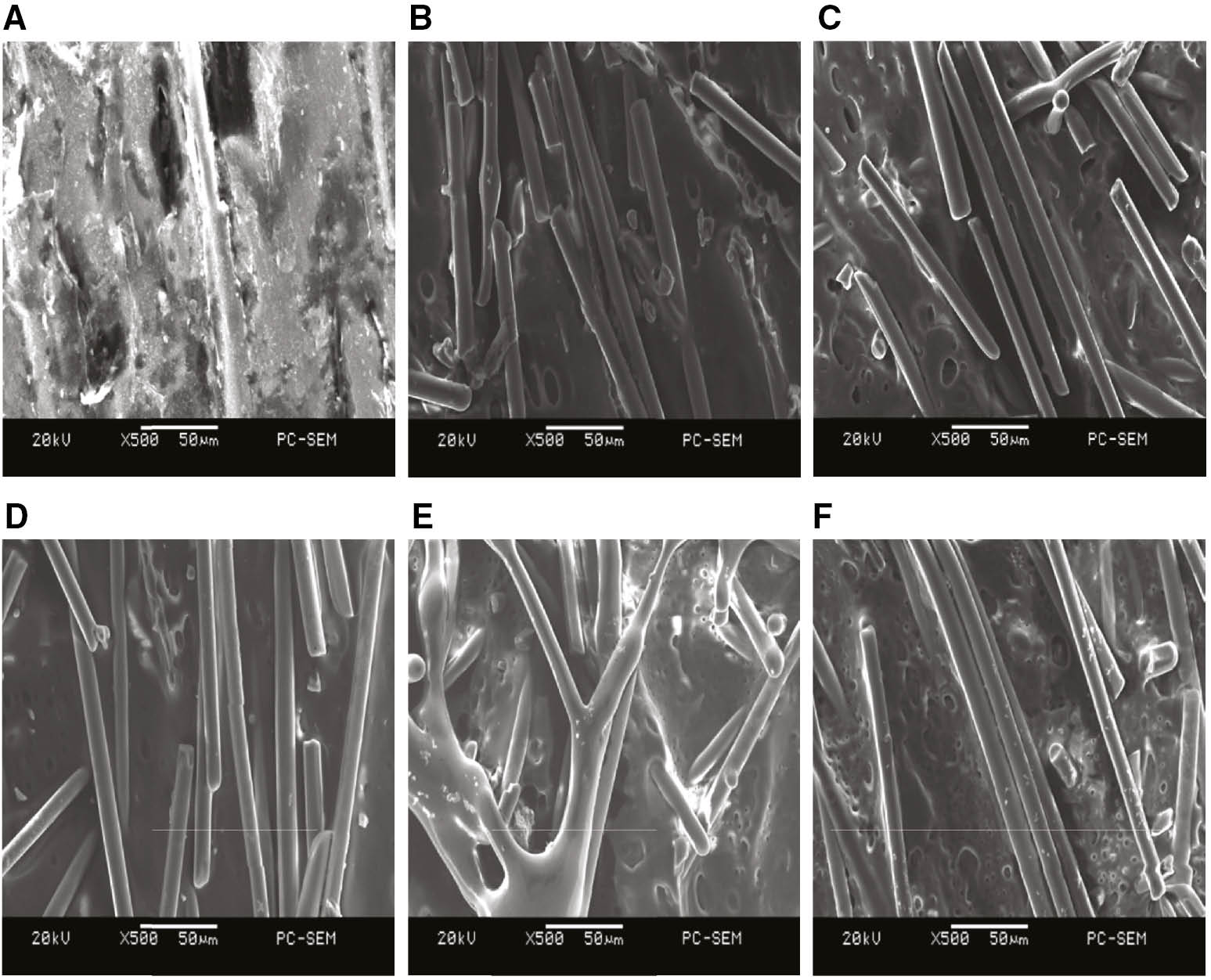
SEM photographs of PA6 surface after modification with different pulsed voltages.
(A) Untreated, (B) 26 kV, (C) 28 kV, (D) 30 kV, (E) 32 kV, and (F) 34 kV.
Wenzel (14) proposed that the presence of a rough surface makes the actual solid-liquid contact area greater than the apparent contact area, geometrically enhancing the hydrophobic or hydrophilic property of the surface. Assuming that liquid always fills groove structures on a surface, the apparent contact angle θw on a rough surface and the eigen contact angle θ on a smooth, flat surface have the following relationship:
where r is the ratio between the actual solid-liquid contact area Sa and the apparent contact area Sb, also known as roughness factor. This equation is called the Wenzel equation. As r is always >1, values of θ>90° indicate a hydrophobic surface; as θw increases along with increasing values of r, the surface becomes more hydrophobic. When θ<90, this indicates a hydrophilic surface; θw decreases with increasing values of r, and the surface becomes more hydrophilic.
Based on the dimple-like structures produced on PA6’s surface after etching modification with different pulsed voltages, as shown in Figure 5, Wenzel’s model was applied to this test in Figure 6. Figure 6 represents the rough microstructure surface of the cone, where the diameter of the retuse cone is indicated as R, the depth is h, and the retuse bodies pacing is a and b. These variables introduce the following surface characteristic values: α=a/R (transverse diameter ratio), β=b/R (longitudinal diameter ratio), γ=h/R (depth to diameter ratio), such that r can be expressed as follows:
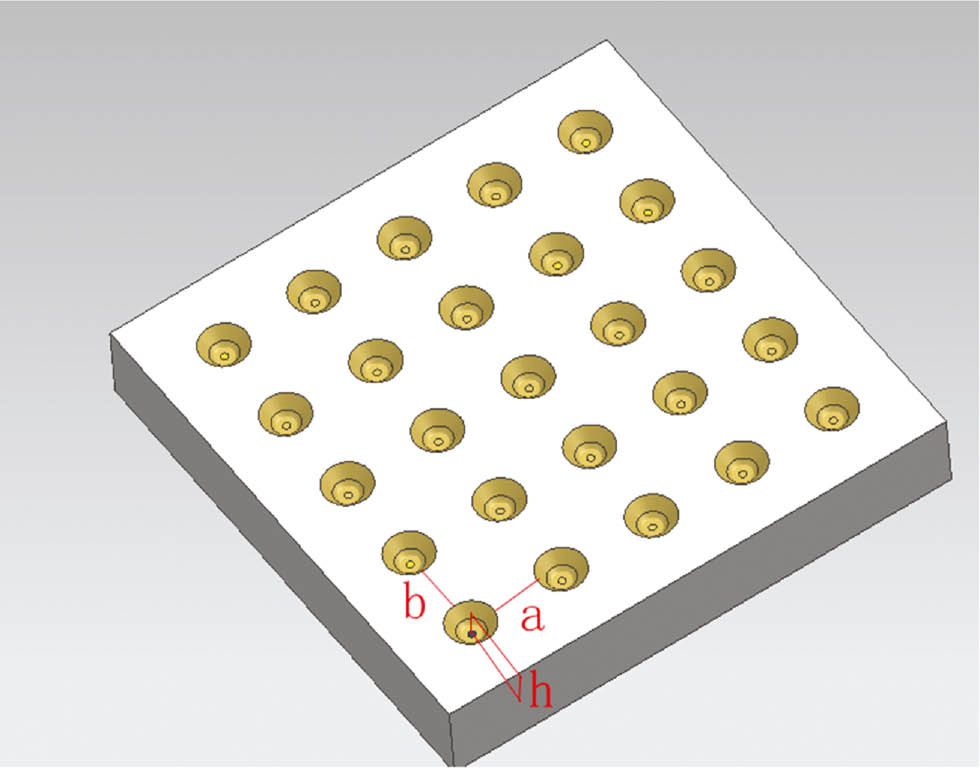
Wenzel model sketch with changes in pulsed voltage.
Substituting r into equation [1] Wenzel model yields the following:
When α and β are constant in equation [3], increasing values of γ make a hydrophobic material more hydrophobic, and a hydrophilic material more hydrophilic. When γ remains the same, α and β are reduced; in this situation too, a hydrophobic material becomes more hydrophobic, and a hydrophilic material becomes more hydrophilic.
The SEM photographs in Figure 5 show the number of dimple-like structures gradually increasing along with the increase in pulsed voltage; in other words, α and β in equation [3] are reduced, resulting in reductions in the water contact angle. Therefore, under certain conditions of pulsed voltage range modification, it is feasible to apply Wenzel’s theory to explain the water contact angle and wettability of PA6’s surface.
4 Conclusions
This paper applied HCPEB to conduct etching modification research on the surface of PA6. The results shed light on the effects of etching modification on the wetting property of PA6’s surface, as well as the corresponding impact mechanism. The results also show that the hydrophilic property of PA6’s surface increases along with increasing pulsed voltage. When the pulsed voltage is 34 kV, hydrophilicity reaches its maximum. This finding is due to the increased surface roughness produced by applying HCPEB bombardment to the surface. The impact mechanism can also be explained by Wenzel’s model.
Acknowledgments
This work is financially supported by the Chongqing Science and Technology Natural Science Fund Project: cstc2013-yykfc6004.
References
1. Dong L, Yin XH, Meng PP, Wang SG, Feng WJ. Research progress of wetting behavior and its applications in engineering. Acad J Shenyang Normal Uni (Nat Sci Edn). 2012;30(1):48–51.Search in Google Scholar
2. Möller S, Pliquett U, Hoffmann C. Synthesis of molecular photoswitches based on azobenzene with an organosilane anchor. RSC Adv. 2012;2(11):4792.10.1039/c2ra20151hSearch in Google Scholar
3. Fries K, Samanta S, Orski S, Locklin J. Reversible colorimetric ion sensors based on surface initiated polymerization of photochromic polymers. Chem Commun (Camb). 2008;(47):6288.10.1039/b818042cSearch in Google Scholar PubMed
4. Rosario R, Gust D, Hayes M, Jahnke F, Springer J, Garcia AA. Photon-modulated wettability changes on spiropyran-coated surfaces. Langmuir. 2002;18(21):8062–9.10.1021/la025963lSearch in Google Scholar
5. Kessler D, Choi J, Schattling P, Jochum FD, Pyun J, Char K, Theato P, Char K, Theato P. Reactive surface coatings based on polysilsesquioxanes: universal method toward light- responsive surfaces. ACS Appl Mater Interfaces. 2011;3(2): 124–8.10.1021/am1010892Search in Google Scholar PubMed
6. Mariya A, Nikolay K, Valentin V, Slavka T, Silvia S. Material alternative to ITO for transparent conductive electrode in flexible display and photovoltaic devices. Microelectron Eng. 2015;145(1):112–6.10.1016/j.mee.2015.03.053Search in Google Scholar
7. Wu JH, Zhang GL, Wang XD. Effects of low energy ion beam surface modification on polycarbonate (PC) wetting property. J Vac Sci Technol. 2008;28(4):370–3.Search in Google Scholar
8. Translated by Shi ZP. Polyurethane resin handbook. Beijing: China Petrochemical Press; 1994. pp. 21–23.Search in Google Scholar
9. Wang HZ, Gao XM. Polyamide situation and prospects in both at home and Abroad. Chemical Production and Technology; 2008. pp. 38–40.Search in Google Scholar
10. Liu JL, Zou ZR, Su BR. High energy beam heat treatment. Beijing: Mechanical Industry Press; 1997. pp. 320–321, 349–351.Search in Google Scholar
11. Proskurovsky DI, Rotshtein VP, Ozur GE, Markov AB, Nazarov DS. Pulsed electron-beam technology for surface modification of metallic materials. J Vac Sci Technol A. 1998;16(4):2480–8.10.1116/1.581369Search in Google Scholar
12. Rytlewskia P, Mróz W, Zenkiewicz M. Laser induced surface modification of polylactide. J Mater Process Technol. 2012;212(8):1700–4.10.1016/j.jmatprotec.2012.03.019Search in Google Scholar
13. Li XB, Liu Y. Microstructure surface contact angle model and its wettability. Mater Rev. 2009;24:101–3.Search in Google Scholar
14. Wenzel RN. Surface roughness and contact angle. J Phys Colloid Chem. 1949;53(9):1466–7.10.1021/j150474a015Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels

