Abstract
The waterborne polyurethane (WPU) was synthesized with isophorone diisocyanate (IPDI), polyethyleneglycol-400 (PEG-400), polyethyleneglycol-2000 (PEG-2000), dimethylolpropionic acid (DMPA) and trimethylolpropane (TMP) in this work. And the hydroxyethyl methacrylate (HEMA), butyl acrylate (BA) and methyl methacrylate (MMA) followed to modify the WPU by physical mixing and chemical integration to obtain the polyurethane/polyacrylate (PU/PA) and crosslinked polyurethane-acrylate (LPUA), respectively. Fourier transform infrared (FTIR) spectroscopy confirmed the presence of functional groups of LPUA. Thermogravimetric analysis (TGA) illustrated that the heat resistance of LPUA was better than that of PU/PA and WPU. And the effect of acrylate used to modify WPU on LPUA is also discussed. Lastly, LPUA was combined with Basic Red 1# to prepare fluorescent composite which could be used to dye cotton fabric. Such properties of fluorescent composite such as washing color fastness, heat-resistance were studied.
1 Introduction
Polyurethane (PU) materials are known to offer high performance due to their toughness, abrasion resistance, mechanical flexibility and chemical resistance (1), (2), (3). But organic solvents such as acetone, tetrahydrofuran added in the synthesis process are harmful to the environment. Therefore, waterborne polyurethane (WPU) has become a major object of study in recent years. However, the weak water resistance and poor membrane performance of WPU has restricted its application to some extent. Because of the excellent toughness, compatibility and miscibility of polyacrylate (PA), a lot of research is conducted on the modification of WPU with PA (4), (5). Tuba Cakir Canak modified PU with 3,5-bis(perfluorobenzyl)oxy benzyl alcohol and 2-hydroxyethyl methacrylate, the heat-resistance and water resistance of the modified PU were improved (6). Yu-Hua Guo and his team applied n-butyl acrylate and styrene to PU, the mechanical property and water resistance of the products were enhanced (7). Chu-Yin Zhang used dihydroxybutyl-terminated polydimethylsiloxane to increase the water resistance of PU (8). In this article, butyl acrylate (BA) and methyl methacrylate (MMA) were employed to reform WPU by physical mixing and chemical integration, and the polyurethane/polyacrylate (PU/PA) and crosslinked polyurethane-acrylate (LPUA) were obtained, respectively. Meanwhile, the performance of LPUA was compared with that of PU/PA.
As for the unique color and special application of fluorescent dyestuff, it is significative to apply the fluorescent dyestuff to dyeing cotton fabric, because most fluorescent substances do not contain the reactive groups to react with the hydroxy group of cotton. In this article, the fluorescent dyestuff was combined with the modified WPU to prepare fluorescent composite which could act on cotton fabric relying on the adhesive property of the composite.
2 Experiment
2.1 Materials
Isophorone diisocyanate (IPDI), polyethyleneglycol-400 (PEG-400), polyethyleneglycol-2000 (PEG-2000) and trimethylolpropane (TMP), hydroxyethyl methacrylate (HEMA) were purchased from Aladdin Chemistry. Butyl acrylate (BA), MMA, sodium dodecyl sulfate (SDS), dibutyltin dilaurate (DBTDL), 1,4-butanediol (1,4-BDO) were obtained from the Shanghai Lengfeng Chemistry Co., Ltd (Shanghai, China). Dimethylolpropionic acid (DMPA), peroxidisulfate (K2S2O4), triethylamine (TEA) were bought from Sinopharm (Nanjing, China). Basic Red 1# was got from the Zhengzhou Jinhong Chemical Material Co., Ltd (Zhengzhou, China). All chemicals were used as received without further purification.
2.2 Synthesis of waterborne polyurethane (WPU)
PEG-400 (1 g, 2.5 mmol), PEG-2000 (4.05 g, 2.025 mmol) were poured into a four-neck flask and dehydrated for 2 h under 120°C. After that, IPDI (2.4 g, 10.80 mmol) was poured in the four-neck flask and stirred for 2 h with the protection of nitrogen when the temperature dropped to 80°C; the reaction temperature was maintained at 80°C. DMPA (0.25 g, 1.864 mmol), TMP (0.09 g, 0.672 mmol), 1,4-BDO (0.175 g, 1.942 mmol) and four drops of DBTDL were added in the four-neck flask and stirred fully. Residual-NCO was tested with dibutylamine with the defined sample taken from the flask per 25 min, and the reaction ended when the content of -NCO dropped to the theoretical value. The reaction was neutralized with TEA (0.18 g, 1.779 mmol) for 30 min when the temperature dropped to 40°C. Lastly, 20 ml EDA aqueous solution (0.5%) was added to the flask and stirred for 30 min, and WPU emulsion was obtained. During the whole preparation, the agitation speed was controlled at 250 rpm.
2.3 Synthesis of hybrid emulsion of polyurethane/polyacrylate (PU/PA)
Defined amount of MMA, BA were poured into the WPU emulsion made in Section 2.2 and the hybrid emulsion were stirred fully for 30 min. The hybrid emulsion was heated to 75°C, and K2S2O4 (0.1 g, 0.370 mmol) was added to the reacting mixture slowly, after that, the reaction ended 2 h later. PU/PA emulsion was obtained.
2.4 Synthesis of crosslinked polyurethane-acrylate emulsion (LPUA)
PEG-400 (1 g, 2.5 mmol), PEG-2000 (4.05 g, 2.025 mmol) were poured into a four-neck flask and dehydrated for 2 h under 120°C. After that, IPDI (2.4 g, 10.80 mmol) was poured into the four-neck flask and stirred for 2 h with the protection of nitrogen when the temperature dropped to 80°C; the reaction temperature was maintained at 80°C. DMPA (0.25 g, 1.864 mmol), TMP (0.09 g, 0.672 mmol), 1,4-BDO (0.175 g, 1.942 mmol) and four drops of DBTDL were added into the four-neck flask and stirred fully. Residual -NCO was tested with dibutylamine with the defined sample taken from the flask per 25 min, and the reaction was cooled to 75°C when the content of -NCO dropped to the theoretical value. HEMA (0.405 g, 3.112 mmol) was poured into the reaction, and the reaction was cooled to 40°C after the -NCO disappeared. TEA (0.18 g, 1.779 mmol) was used to neutralize the reaction, after that, 20 ml H2O was added to the flask and stirred fully for 30 min. MMA (0.2 g, 1.998 mmol) and BA (0.2 g, 1.560 mmol) were poured into the reaction and the emulsion was stirred for 20 min, then, the reaction was heated to 75°C and reacted for 2 h with the adding of K2S2O4 (0.1 g, 0.370 mmol). LPUA emulsion is obtained. Figure 1 gives the reaction process of LPUA. During the whole preparation, the agitation speed was controlled at 250 rpm.
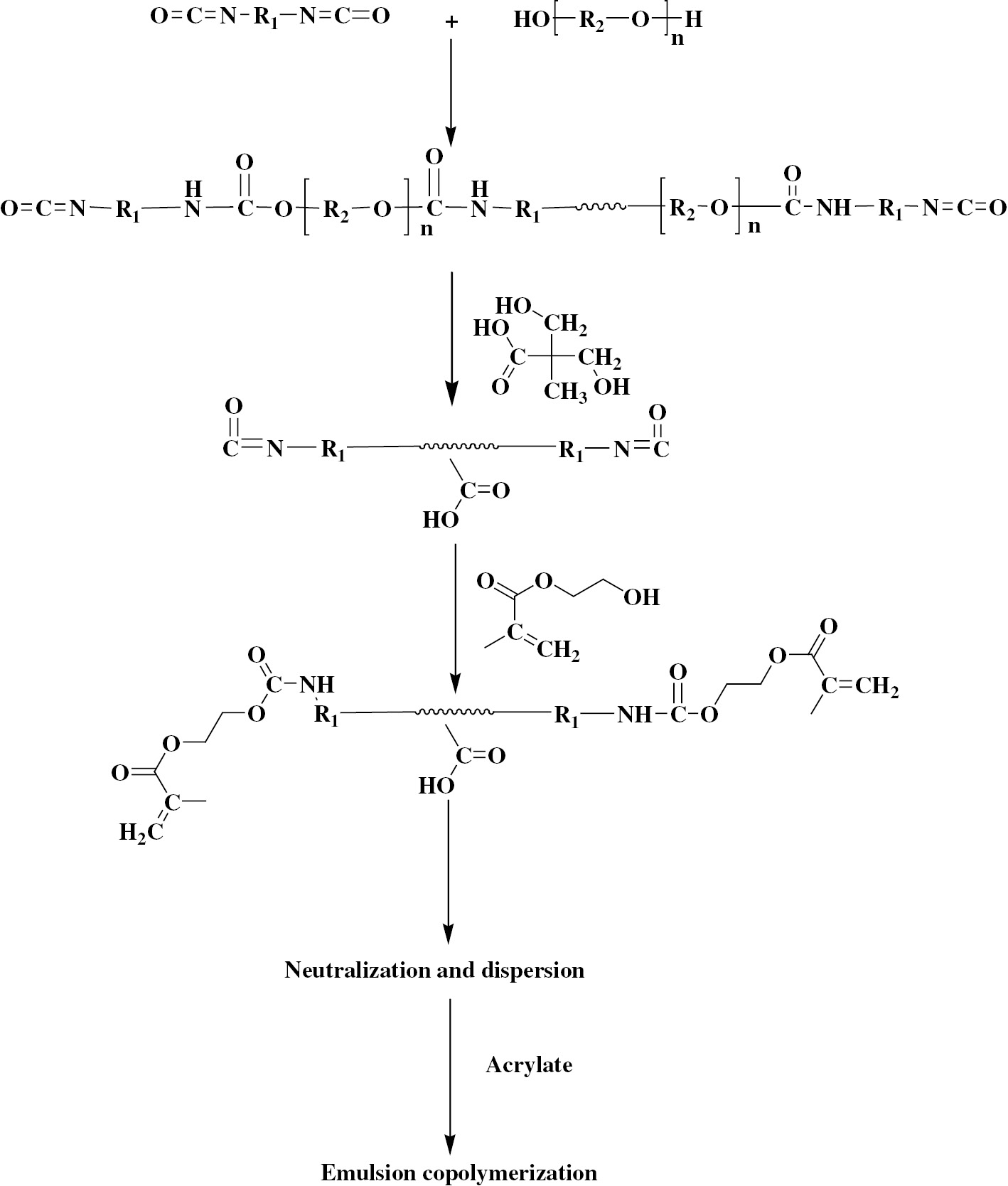
Synthesis of crosslinked polyurethane-acrylate emulsion (LPUA).
2.5 Synthesis of fluorescent composite
PEG-400 (1 g, 2.5 mmol) and PEG-2000 (4.05 g, 2.025 mmol) were poured into a four-neck flask and dehydrated for 2 h under 120°C. After that, IPDI (2.4 g, 10.80 mmol) was poured into the four-neck flask and stirred for 2 h with the protection of nitrogen when the temperature dropped to 80°C, the reaction temperature was maintained at 80°C. DMPA (0.25 g, 1.864 mmol), TMP (0.09 g, 0.672 mmol), 1,4-BDO (0.175 g, 1.942 mmol) and four drops of DBTDL were added into the four-neck flask and stirred fully. Residual -NCO was tested with dibutylamine with the defined sample taken from the flask per 25 min, and the reaction was cooled to 75°C when the content of -NCO dropped to the theoretical value. HEMA (0.405 g, 3.112 mmol) was poured into the reaction, and the reaction was cooled to 40°C after the disappearance of -NCO. TEA (0.18 g, 1.779 mmol), Basic Red 1# (0.5 g, 1.130 mmol) and SDS (0.2 g, 0.694 mmol) dissolved in 20 ml H2O were poured into the reaction, and the emulsion was stirred for 30 min, then, the reaction was heated to 75°C and reacted for 2 h with K2S2O4 (0.1 g, 0.370 mmol) added. During the whole preparation, the agitation speed was controlled at 250 rpm. The fluorescent composite emulsion was obtained as is illustrated in Figure 2.

Fluorescent composite emulsion.
2.6 Dyeing of cotton with dluorescent composite
Cotton fabric was dyed with the fluorescent composite made in Section 2.6, and the recipe of dyeing was as follows:
The bleached cotton was firstly steeped in the composite prepared before for 10 min at ambient temperature. Then the composite was heated to 80°C and lasted for 20 min. The dyeing liquor was stirred fully with a glass stick during the dyeing process, and the dyed cotton was dried in an oven at 60°C. Finally, the dyed cotton was washed twice with flowing water and dried at 40°C.
2.7 Measurement and characterization
The diagnostic functional groups of PA and LPUA membranes were confirmed with Fourier transform infrared spectroscopy [FTIR, BOEN 35921 (Germany), scan from 4000 cm−1 to 400 cm−1, resolution 4 cm−1] and the particle size of the emulsion was measured with the surface potential grain size instrument [Malvern Zetasizer ZS90 (UK)]. The mechanical property of the membrane made of the emulsion was tested with the tensile testing machine [(Tianjin, China) 100 mm/min], besides, the thermal property was also tested with thermogravimetric analysis [TGA Q500 (Beijing, China)]. In the case of the dyed cotton, washing color fastness, wrinkle recovery angle, stiffness and fluorescent effect were tested, respectively, with the gray scale for assessing staining (USA), wrinkle recovery tester (Changzhou, China), stiffness tester (Changzhou, China) and fluorescence detection spectrometer (USA). At last, the heat-resistant quality was measured with a high-temperature distillation apparatus (Changzhou, China).
3 Result and discussion
3.1 FTIR of PA and LPUA
FTIR analysis of PA and LPUA were illustrated in Figure 3. The spectra of LPUA has another two additional peaks of 3340 cm−1 and 1537 cm−1. The peaks of 3340 cm−1 and 1537 cm−1 were assigned to the vibration of the -NH of carbamate. The spectra of PA and LPUA all have the peak of 1720 cm−1 which was assigned to the vibration of carbonyl moiety -CO-. All the above indicated the success of modification of WPU.
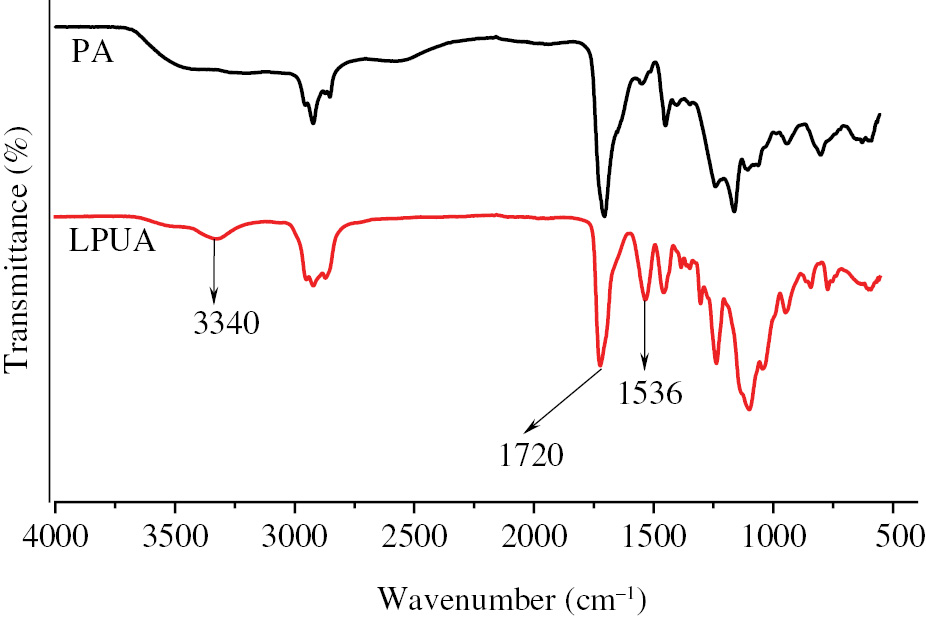
FTIR spectra of PA and LPUA.
3.2 Influence of acrylate on the particle size and stability of LPUA emulsion
The amount of acrylate (BA, MMA) has an obvious impact on the particle size of LPUA. Figure 4 illustrated the change of particle size of LPUA with different amounts of acrylate. With the increasing of the amount of acrylate, the particle size of LPUA was larger. Because the micelle was already formed before the adding of acrylate, and the amount of particle did not change with the adding of acrylate. The acrylate monomer was just wrapped by emulsion particle which led to the larger size of the emulsion particle. Besides, when the amount of acrylate was too large to be wrapped by the emulsion particle, different particles blended to form micelle which could wrap more acrylate.

Effect of acrylate on the particle size of LPUA.
In addition to the effect on particle size of LPUA, acrylate also has an impact on the appearance and stability of LPUA emulsion. The color of LPUA emulsion changed from light blue to milk white, the precipitate appeared in emulsion with the increase of acrylate and the more acrylate, the more precipitate. When the particle size became larger, the LPUA particle was more hydrophobic and more different particles blended, which led to poor stability of LPUA emulsion.
3.3 Influence of acrylate on water-resistance of LPUA membrane
The amount of acrylate (BA, MMA) has a great influence on the water-resistance of LPUA membrane. Figure 5 illustrated the change of water absorption of LPUA membrane with different amounts of acrylate. Water absorption dropped from 15.3% to 6.1% with the increase of acrylate in LPUA. There were a lot of carboxyl groups in LPUA and carboxyl group could associate with water, so, the LPUA membrane swelled when it absorbed water, and this led to poor water resistance. The surface energy of LPUA membrane modified with acrylate dropped a lot because of the ester groups in acrylate, as a result, water absorption of the LPUA membrane also decreased a lot.

Effect of acrylate on the particle size of LPUA.
3.4 Influence of acrylate on mechanical property of LPUA membrane
The amount of acrylate used to modify LPUA was 30% according to the analysis above. Table 1 illustrates the changes in tensile strength and elongation at the break of the LPUA membrane when the mass ratio between BA and MMA changed. The tensile strength increased from 10.2 MPa to 26.4 MPa and elongation at the break decreased from 823.5% to 311.2% when the mass ration between BA and MMA decreased from 6:0 to 0:6. The strength of the LPUA membrane promoted and elongation at break decreased when the amount of MMA increased because MMA was a hard monomer, the chain of LPUA was hard to rotate when MMA was added to the chain. On the contrary, BA was a soft monomer, so, the strength of the LPUA membrane reduced and elongation at the break increased. At last, the mass ratio between MMA and BA was confirmed to 2:1 according to the comprehensive analysis of tensile strength and elongation at break.
Influence of the mass ration between MMA and BA on tensile properties of LPUA membrane.
| M (BA):M(MMA) | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|
| 6:0 | 10.2 | 823.5 |
| 5:1 | 11.8 | 711.5 |
| 2:1 | 15.1 | 559.3 |
| 1:1 | 18.5 | 480.1 |
| 1:2 | 20.2 | 412.6 |
| 1:5 | 23.5 | 375.3 |
| 0:6 | 26.4 | 311.2 |
3.5 Thermogravimetric analysis (TGA) of LPUA membrane
Figure 6 illustrated the mass loss of WPU, PU/PA, LPUA membrane when the membranes were heated at the same heating rate. The mass of WPU, PU/PA, LPUA membrane began to reduce at 150°C, 200°C and 250°C, respectively, besides that, the rate of mass loss of LPUA membrane was lower than those of the WPU and PU/PA membranes. Polyacrylate was a material with excellent heat-resistant quality, so the heat-resistant quality of LPUA and PU/PA was better than that of WPU. LPUA has more chemical bonds than PU/PA and the molecular weight of LPUA was also larger than that of PU/PA. As a result, LPUA has better heat-resistant quality than that of PU/PA.

TGA of LPUA, WPU, PU/PA.
3.6 Washing color fastness of dyed cotton fabric
Washing color fastness of dyed cotton was tested according to GBT3921.1-1997. After the cotton fabric was washed and dried, the gray scale was used for assessing the heat-resistant quality of dyed cotton fabric, and it was confirmed to 4–5 which states the washing color fastness was good.
3.7 Wrinkle recovery angle and stiffness of dyed cotton fabric
Wrinkle recovery angles of dyed and undyed cotton fabric were obtained in 0, 5 and 15 min after wrinkle recovery tester stopped. Table 2 illustrated the rate of change of wrinkle recovery angle of dyed cotton fabric, which was smaller compared with that of undyed cotton fabric. And the rate of change diminished with the time extended.
Rate of change of wrinkle recovery angle of dyed cotton fabric.
| t (min) | Dyed cotton fabric (°) | Undyed cotton fabric (°) | Rate of change (%) |
|---|---|---|---|
| 0 | 64 | 61 | 4.9 |
| 5 | 75 | 73 | 2.7 |
| 15 | 80 | 79 | 1.2 |
According to the test of stiffness, the stiffness of dyed cotton fabric was 17.56 cm and undyed cotton was 18.17 cm, the rate of change of stiffness was 2.9%.
Wrinkle recovery angle and stiffness could be used to assess the tactile quality of cotton fabric, so, the small rate of change of wrinkle recovery angle and stiffness illustrated that the fluorescent composite synthesized above has little influence on the tactile quality of dyed cotton fabric.
3.8 K/S of dyed cotton fabric dealt with high temperature
Figure 7 displayed the K/S curve of dyed cotton fabric dealt with high temperature (150°C, 30 s) and the graph was also compared with the K/S curve graph of untreated dyed cotton fabric. From the figure, the hue saturation of untreated dyed cotton fabric was a little higher than that of the treated cotton fabric. Therefore, it could be concluded that the fluorescent coating has good heat-resistant quality.

K/S curves of dyed cotton fabric dealt with high temperature (150°C, 30 s) and untreated dyed cotton fabric.
3.9 Fluorescent performance of fluorescent composite
Figure 8 illustrated the emission spectrum of dyed cotton fabric when the exciting wavelength was 350 nm. In the spectrum, there were obvious emission peaks around 350 nm and 700 nm, respectively. The emission peak at 700 nm was the red hue of the fluorescent coating which would show itself as visible and the emission peaks at 350 nm indicated the fluorescence of the composite. The overlooking bands in the spectrum might indicate the visible red contains slight green (580 nm) and magenta (420 nm). The intensity of fluorescence was just the same as the red hue of the composite and as such it could be concluded that the composite has favorable fluorescent performance.
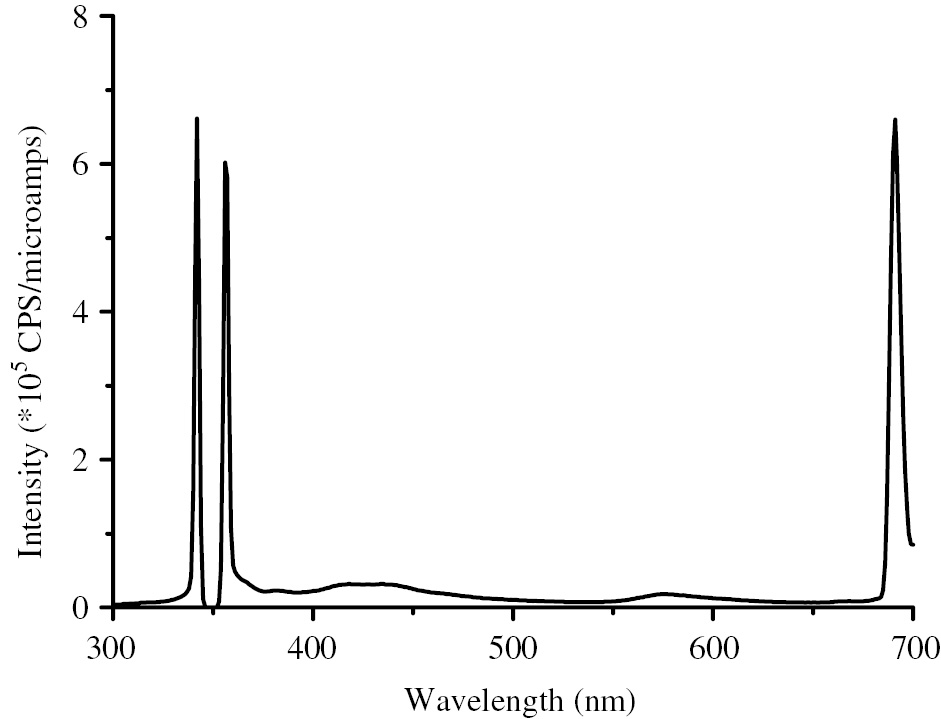
Emission spectrum of fluorescent composite with excitation ray at 350 nm.
4 Conclusions
In this study, WPU and PU/PA physical mixing emulsion and LPUA were obtained, and the structure of LPUA was confirmed with FTIR. The effect of acrylate on the performance of LPUA including particle size, emulsion stability, water-resistant property, mechanical property, heat-resistant property, was also discussed. As for the dyed cotton fabric, washing color fastness, wrinkle recovery angle and stiffness were tested, and all of the properties were compared with those of undyed cotton fabric. After that, the dyed cotton fabric was dealt with high temperature (150°C, 30 s) and the K/S value was measured to study the heat-resistant property of the fluorescent composite. Lastly, fluorescent performance was tested with a fluorescence detection spectrometer. With all of the testing items, it was concluded that the fluorescent composite synthesized here has many outstanding properties for industrial application.
References
1. Chen L, Chen S. Latex interpenetrating networks based on polyurethane, polyacrylate and epoxy resin. Prog Org Coat. 2004;49(3):252–8.10.1016/j.porgcoat.2003.10.010Search in Google Scholar
2. Xin H, Shen YD, Li XR. Novel cationic polyurethane fluorinated acrylic hybrid latexes: synthesis, characterization and properties. Colloid Surface A: Physicochem Eng Aspects. 2011;384(1–3):205–11.10.1016/j.colsurfa.2011.03.056Search in Google Scholar
3. Zhang Y, Anila A, Shi WF. Highly branched polyurethane acrylates and their waterborne UV curing composite. Prog Org Coat. 2011;71(3):295–301.10.1016/j.porgcoat.2011.03.022Search in Google Scholar
4. Lei L, Zhong L, Lin XQ, Li YY, Xia ZB. Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink. Chem Eng J. 2014;253(1):518–25.10.1016/j.cej.2014.05.044Search in Google Scholar
5. Wang XR, Shen YD, Lai XJ. Micromorphology and mechanism of polyurethane/polyacrylate membranes modified with epoxide group. Prog Org Coat. 2014;77(1):268–76.10.1016/j.porgcoat.2013.09.013Search in Google Scholar
6. Canak TC, Serhatli IE. Synthesis of fluorinated urethane acrylate based UV-curable composites. Prog Org Coat. 2013;76(2–3):388–99.10.1016/j.porgcoat.2012.10.024Search in Google Scholar
7. Guo YH, Li SC, Wang GS, Ma W, Huang Z. Waterborne polyurethane/poly(n-butyl acrylate-styrene) hybrid emulsions: particle formation, film properties, and application. Prog Org Coat. 2012;74(1):248–56.10.1016/j.porgcoat.2011.12.016Search in Google Scholar
8. Zhang CY, Zhang XY, Dai JB, Bai CY. Synthesis and properties of PDMS modified waterborne polyurethane-acrylichybrid emulsion by solvent-free method. Prog Org Coat. 2008; 63(2):238–44.10.1016/j.porgcoat.2008.05.011Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels

