Abstract
A new type of medicated polymeric composite consisting of acyclovir (ACY), polyvinylpyrrolidone K60 (PVP) and polyethylene glycol 6000 (PEG) with core-shell structure were prepared by a coaxial electrospinning process. The composites could enhance the dissolution of the poorly water-soluble drug. The shell layers were formed from a spinnable working fluid containing the filament-forming PVP and citric acid while the core parts were prepared from an un-spinnable co-dissolving solution composed of ACY, sodium hydrate and PEG. Scanning electron microscope and transmission electron microscope observations demonstrated that the composites had a homogeneous linear topography with a slippery surface, a diameter of 670±130 nm, and an obvious core-shell structure. X-ray diffraction (XRD) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy results demonstrated that the drug and citric acid contained in the core and shell parts were in an amorphous status. In vitro dissolution experiments exhibited that ACY was able to be free within 1 min, and the dissolution media were neutral due to acid-basic action within the core-shell structures. The medicated nanocomposites resulted from a combined usage of hydrophilic polymeric excipients PVP and PEG could provide a new solution to the problem associated with the dissolution of poorly water-soluble drugs.
1 Introduction
Numerous active pharmaceutical ingredients (API) suffer from poor bioavailability owing to their poor aqueous solubility and low dissolution rates (1). Developing new strategies for improving their soluble behaviors remains one of the most challenging aspects in pharmaceutical and the related fields. Polymer science and engineering have acted as the backbone to support the development of new types of formulations over the past several decades, particularly in the area of drug controlled release (2). Many novel drug delivery systems (DDS) rely on the physical and chemical properties of the polymer excipients with the API homogeneously distributed on the polymer matrices (3), which are often polymer-based composites.
Composite materials are broadening their ways from macroscale to micro-/nano scale, from no structural characteristics to structural and from the main usages for improving mechanical performance to a wide variety of different functional applications (4). For biomedical applications, drug-loaded polymer-based composites, a combination of amorphous API and nano DDS, have drawn increasing attention in providing new ways of improving the dissolution properties of poorly water-soluble drugs since the surge of nanotechnology in the 1990s (5). Among different types of techniques for generating polymer-based composites, electrospinning has proved its capability for producing this kind of material during the past 10 years, which are often in the form of non-woven fabrics consisting of interlinked nanofibers (6, 7).
For the fabrication of composite monolithic nanofibers from single fluid electrospinning, the working fluid is often a mixed liquid of the guest drug, the host polymer and sometimes some other additive components (8). Thus, the following conditions should be met for a successful preparation: 1) all the components can dissolve together without chemical reactions or coagulation; 2) the polymer possesses good filament-forming properties in the solvents; 3) the API has enough concentration to achieve the desired functional performance. These strict conditions have limited the development of novel drug-loaded nanocomposites, particularly for those which are insoluble in aqueous solutions and a series of typical organic solvents. Fortunately, the electrospinning processes themselves have developed from a single-fluid process to double-fluid (coaxial and side-by-side) and even multiple-fluid (tri-axial) processes (9). These advanced processes should provide opportunities for generating new medicated composites. Further, they should be able to fabricate the new types of composites comprising different types of polymeric excipients and with structural properties, regardless of a co-solvent for the drug and the filament-forming polymer.
Among the double- and multiple-fluid electrospinning processes, coaxial electrospinning is the most popular. In this process, two fluids are driven from a concentric spinneret and co-electrospun synchronously under the electric fields, with only one required to be spinnable for a whole process (10). The popularity of coaxial electrospinning should be attributed to the relatively easy implementation of the process, and most probably, the usefulness of core-shell structure that facilitates the design of different exterior and interior parts for achieving improved or even new functional performances (11). Needless to say, this process provides the possibility of tailoring components and compositions within the nanofibers spatially for a whole performance.
Insoluble APIs share over 40% of the medicated products on the market and 60% of the developing potential APIs have a poor solubility (12). Among them, there are many drugs that have a pH-dependent solubility. These drugs are soluble in particular acid or basic conditions although they have poor solubility in the neutral solutions and also a series of typical organic solvents. Based on this knowledge, here we report the feasibility of utilizing an acid-base neutralization reaction to prepare structural nanocomposites for poorly water-soluble drugs with pH-dependent solubility, which should have many potential applications in the pharmaceutical industry. In the experiment, acyclovir (ACY) was exploited as a model of a poorly water-soluble drug owing to its solubility in the basic condition. Two of the most common polymeric excipients in the pharmaceutical field, polyvinylpyrrolidone (PVP) and polyethylene glycol (PEG) were explored as the shell filament-forming matrix and the core drug carrier, respectively.
2 Materials and methods
2.1 Materials
ACY was bought from Huyu Biotechnology Co. Ltd. (Shanghai, China); PVP K60 (M=360,000) was purchased from BASF Co. Ltd. (Shanghai, China). PEG 6000 (M=6000), methylene blue, sodium hydrate, citric acid monohydrate, and anhydrous ethanol were obtained from a local Chemical Reagent Co. Ltd. (Sinopharm, Shanghai, China). All chemicals with analytical grade were used directly without further purification. Water was purified through double distillations just before use
2.2 Preparation of the medicated composites
The shell fluid was composed of 0.46 g of citric acid and 8.0 g of PVP K60 in 100 ml ethanol. The core solutions consisted of 10.0 g PEG 6000, 0.40 g sodium hydrate, and 4.0 g ACY in 100 ml ethanol aqueous solution (50:50, v:v). For observations of the coaxial process using a digital camera, 2 mg of methylene blue was added to a 10 ml polypropylene syringe holding the core fluids.
A voltage supply (ZGF60 kV/2 mA, Wuhan Huatian Corp., Hubei, China) and two types of syringe pumps (KDS100 and KDS200, Cole-Parmer®, IL, USA) were used in the experiments. A self-made coaxial spinneret (with an outside and inner diameter of 1.2 mm and 0.3 mm, respectively) was exploited to carry out both single fluid and coaxial processes. For optimization, the applied voltage and collector-to-spinneret distance were fixed at 14 kV and 15 cm, respectively. The other parameters can be found in Table 1.
Some implementation parameters and details of the resulted fibers.
| Electro-spinning | Flow rate (ml/h) | Morphologya | Drug concentrationb | Diameter (nm) | ||
|---|---|---|---|---|---|---|
| Shell | Core | |||||
| F1 | Single | 1.0 | 0 | Linear | 0 | 550±170 |
| F2 | 0 | 0.3 | – | – | – | |
| F3 | Coaxial | 1.0 | 0.3 | Linear | 9.4% | 740±110 |
| F4 | 1.0 | 0.5 | – | – | – | |
a“Linear” morphology means that the fibers have few spindles or beads on them. bThis is a calculated value (w/w) based on the drug concentration in the core fluid and the flow rates of the shell and core working fluids.
2.3 Characterization
2.3.1 Morphology and core-shell structure
The morphology of the nanofibers was evaluated by a JSM-5600LV field emission scanning electronic microscope (FESEM, JEOL, Tokyo, Japan). The samples were gold-coated under nitrogen before observation. The nanofiber size was measured in FESEM pictures using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The core-shell structures of fibers were observed using a JEM 2100F transmission electronic microscope (TEM, JEOL, Tokyo, Japan).
2.3.2 Physical form
X-ray diffraction (XRD) analysis was carried out on a Bruker X-ray Powder Diffractometer Bruker-AXS (Karlsruhe, Germany). Attenuated total reflectance (ATR) Fourier transform infrared (FTIR) analysis was implemented on a Perkin Elmer Spectrum 100 FTIR Spectrometer (Billerica, MA, USA).
2.3.3 Functional performance
In vitro dissolution experiment was implemented to detect the functional performance of structural composites (a paddle method, Chinese Pharmacopoeia, 2015 Ed.) on an RCZ-8A dissolution apparatus (Tianjin University Radio Factory, China). The nanofibers F3 (213 mg) and 20 mg of the raw ACY particles was put into 900 ml of physiological saline (PS) at 37±1 °C, 50 rpm, and sink conditions of C<0.2 Cs. At pre-determined time points, 5.0 ml dissolution media were pipetted and replaced using 5.0 ml fresh PS. The sample solutions were detected using a UV-2102PC spectrophotometer (Unico Instrument Co. Ltd., China).
3 Results and discussion
3.1 The preparation of medicated polymeric nanocomposites
Two working solutions were prepared with the shell fluid (acid solution) having the spinnability and the unspinnable core fluid (basic solution) containing the drug. Later, coaxial processes were conducted with the spinnable solution as a shell fluid to guide the core solution for the formation of a core-shell nanostructure. The fast drying processes resulting from the high-voltage electrostatic field were able to solidify the structural composites all at once with the drug encapsulated in the core parts of nanofibers.
A digital picture of the electrospinning system is exhibited in Figure 1A, in which the inset shows the self-made spinneret. Figure 1B tells the connection of voltage supply with the spinneret through a metal line. Figure 1C is a typical coaxial process when the nanofibers F3 were prepared. When the core fluid was turned off, a single electrospinning process of the shell fluid was realized. Shown in Figure 1D is the Taylor cone of shell solutions consisting of PVP and citric acid, which was transparent. In comparison, when the shell fluid was closed, an electrospraying process happened due to the core fluid lacking sufficient viscoelasticity. Its Taylor cone is shown in Figure 1E and the inset gives a digital photo of the atomization process. The single fluid electrospraying process could not result in any solid products. With the indication of methylene blue and under the conditions for the preparation of nanofibers F3, the compound Taylor cone of the shell and core fluid was exhibited in Figure 1F, in which the blue core solution was well encapsulated by the transparent shell fluid in a conical shape.
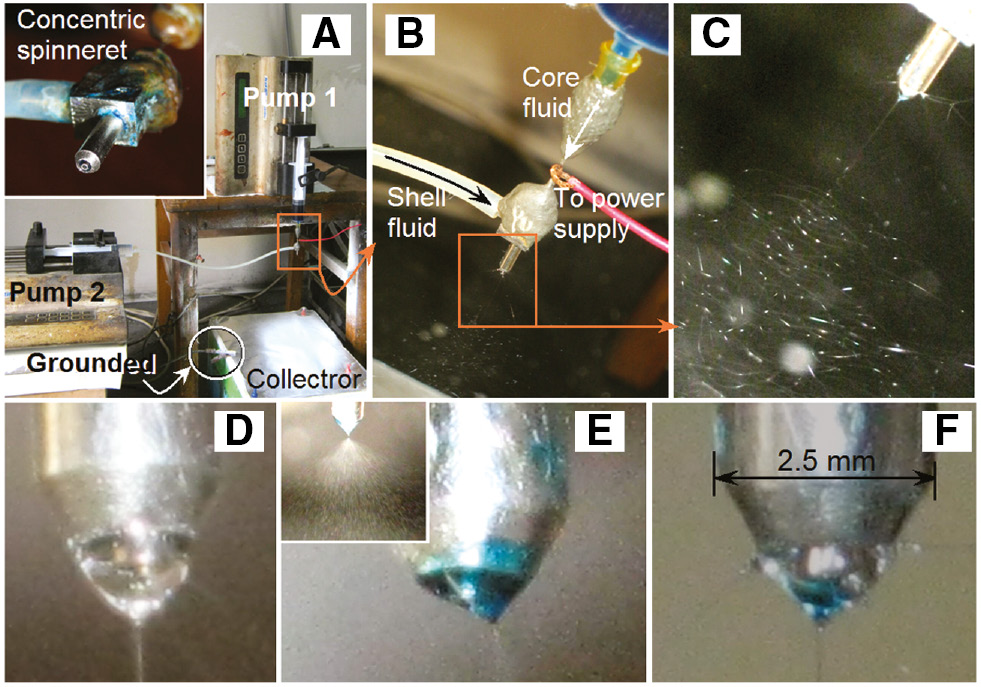
Preparation of the medicated polymeric nanocomposites: (A) the apparatus arrangement, the inset shows the coaxial spinneret; (B) connection between the spinneret and the voltage supply; (C) a typical coaxial electrospinning process for the fabrication of nanofibers F3; (D) Taylor cone of only shell fluid for fabricating nanofibers F1; (E) Taylor cone of only the core solution; (F) a complex Taylor cone for the preparation of fibers F3.
3.2 Morphology and structure of the medicated composites
Shown in Figure 2A is the morphology of nanofiber F1 resulting from the spinnable shell solution solely. Its inset shows an enlarged image of the fibers. From these observations, it is obvious that fibers F1 had linear structures, smooth surfaces and a diameter of 550±170 nm.

Morphology of the medicated composites. (A) Nanofibers F1; (B) nanofibers F4; (C) structural composites F3; (D) TEM image of fiber F3.
Most recently, modified coaxial and tri-axial electrospinning processes have been reported in the literature, which is characterized by the unspinnable outer layer solutions, including pure solvent, electrolyte solutions, and dilute polymer solutions (13). However, the mainstream of this advanced nanotechnology is still the standard one, in which the shell fluid has good spinnability while the core fluid may be or may not be spinnable (14). When an un-spinnable liquid was used as a core fluid, the flow rate ratio was an important parameter for implementing a successful process. When a core-to-shell flow rate ratio of 0.5 was used, the resultant nanofibers F4 have a poor morphology such as beads and clumps on the fibers (Figure 2B). An even larger ratio of 1 made the coaxial process fail to create solidified products. When a ratio of 0.3 was exploited, the resultant fibers F3 have linear morphology, smooth surface, uniform structure (Figure 2C and its inset) and a diameter of 740±110 nm.
Figure 2D shows a TEM image of the core-shell composite nanofiber F3. It had a distinct core-shell structure, with the core part had a diameter of about 420 nm and the shell layer had a thickness of about 150 nm. No nanoparticles can be discerned from both the core part and also the shell layer, suggesting both of them were composites with a homogeneous structure (15).
3.3 Physical forms and compatibility of components
At room temperature, both ACY and citric acid are white powders, and PEG 6000 is a flaky material. Figure 3 shows the XRD patterns of the crude materials and the prepared medicated composites, with all the raw materials except PVP showing peaks indicative of crystallinity. The crude PVP has a diffuse background pattern with two diffraction halos, giving a hint that it is an amorphous polymer. Similarly, the nanofibers F1 show no characteristic reflections of the crude citric acid and instead comprises diffuse halos, suggesting that all the citric acid was converted into amorphous forms during the electrospinning process. The composites F3 show a pattern of superposition of nanofibers F1 and PEG, without the characteristic peaks of ACY. This indicates that ACY was amorphously encapsulated by PEG and fibers F3 is a new type of structural composites comprising ACY-loaded PEG and citric-loaded PVP.
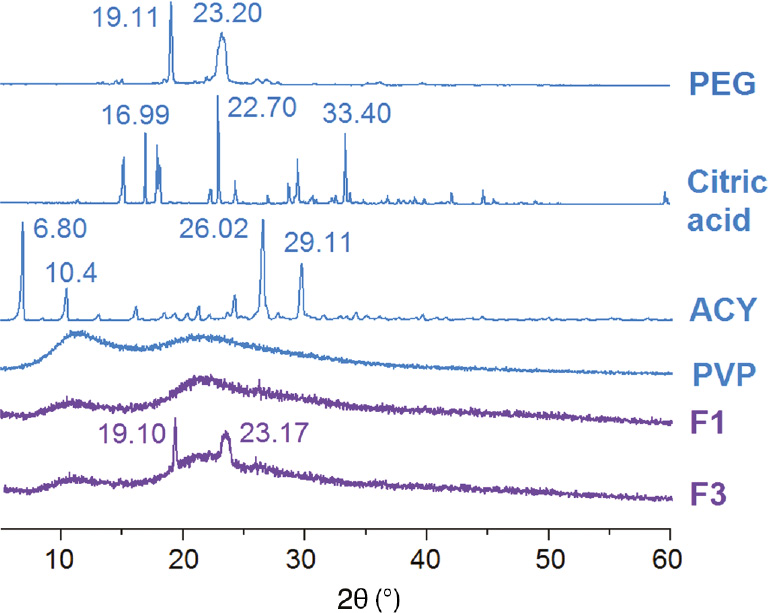
XRD curves of the raw materials (PVP, PEG, citric acid monohydrate and ACY), the nanofibers F1 and the medicated nanocomposites F3.
The molecular structures of the raw materials (PVP, PEG, citric acid and ACY), the ATR-FTIR spectra of them and also the two composites (F1 and F3) are shown in Figure 4. ACY has the characteristic peaks of 1712 and 1632 cm−1 resulted from the vibrations of -C=O groups. Similarly, citric acid has the characteristic peaks at 1688, 1724 and 1756 cm−1, representing the stretching vibrations of -C=O groups in its crystals in a different state. The characteristic peak of PVP at 1666 cm−1 and peak at 1101 cm−1 in PEG are assigned to the -C=O and C-O stretching, respectively.

ATR-FTIR spectra of the crude materials (PEG, citric acid monohydrate and ACY), the nanofibers F1, the medicated nanocomposites F3 and the molecular structures of raw materials.
In the spectra of nanofibers F1, the characteristic peaks of citric acid disappeared except a shoulder at the bottom of 1657 cm−1 (as indicated by “A” in Figure 4). In the fingerprinting regions of citric acid spectra, almost all peaks have shifted, decreased in intensity, or completely disappeared. Whereas the characteristic peak of PVP at 1666 cm−1 has a slight shift to lower wavenumber of 1657 cm−1. These phenomena indicated that hydrogen bonds were formed between PVP and citric acid, in which citric acid molecules act as proton donors and PVP molecules act as proton receptors.
Concerning medicated composites F3, it seems a superposition of the spectra of nanofibers F1 and PEG, as indicated by the shoulder (“B” in Figure 4) and the characteristic peak at 1105 cm−1. The characteristic peaks of ACY could not be discerned from the spectra of nanofibers F3, suggesting that hydrogen bonds occurred between PEG and ACY within the core parts.
3.4 In vitro dissolution experiments
The in vitro dissolution results are shown in Figure 5. The medicated composites F3 could release all the contained ACY in 1 min, whereas the ACY crude particles dissolved slowly, releasing only 31.4% (wt) in 1 h. The extremely fast release of ACY from composites F3 should benefit from the following facts: 1) both PVP and PEG are hydrophilic polymers that are often used as carriers for poorly water-soluble drugs (16); 2) the unique physical properties of the nanocomposites such as huge surface, small diameter, web structure of the assembled nanofibers; and 3) the amorphous physical form of ACY in the core of medicated composites, meaning no need to overcome crystal lattice energy for its dissolution.
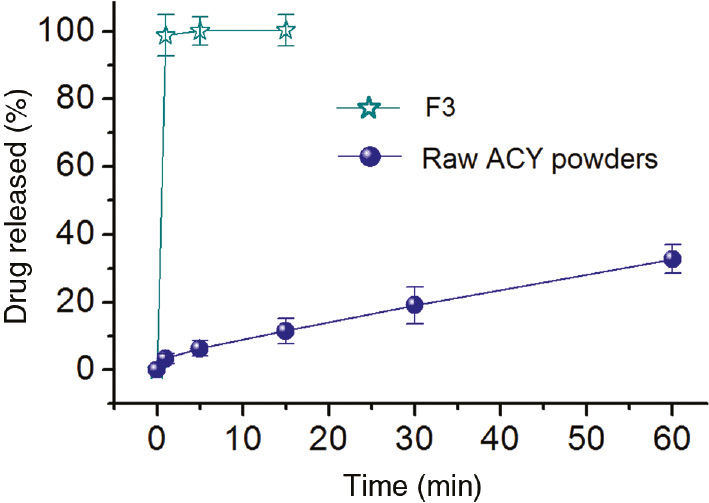
In vitro release profiles (n=6).
The content of citric acid in the shell is (0.46/210.4)×1000 (210.4 is the molecular weight of the citric acid monohydrate)=2.23 mmol, and the content of base in the core is (0.4/40)×1000 (210.4 is the molecular weight of sodium hydrate)=10 mmol. The core-to-shell flow rate ratio is 0.3 for preparing medicated nanocomposites F3, thus, the formed salts of the acid-basic reaction should consist of 2/3 of the sodium dihydrogen citric and 1/3 of the citric acid di-sodium. This was demonstrated by a measured pH value of 6.9 from the dissolution media using a PHBJ-260 pH-meter (Jingke Industrial Co. Ltd., Shanghai, China). By the way, the total ACY released from the fibers F3 was 19.83±0.41 mg, almost equivalent to the calculated value, suggesting little drug loss during the composite preparation process.
4 Conclusions
A new type of medicated nanocomposites in the form of core-shell fibers was created using coaxial electrospinning. A combined usage of PVP and PEG as polymeric matrices have ensured both a successful coaxial process and high functional performance of the structural composites in enhancing ACY dissolution. SEM and TEM results demonstrated that the prepared composites had a uniform linear morphology, a smooth surface, a diameter of 670±130 nm and a distinct core-shell structure. XRD and ATR-FTIR results verified that the structural composites comprised PVP and citric acid in the shell and amorphous ACY, PEG and sodium hydrate in the core. ACY could be freed within 1 min when the composites encountered water. The dissolution media were neutral due to acid-basic action. The combined usage of different kinds of polymeric excipients should greatly expand the applications of coaxial electrospinning in designing and fabricating new structural composites for potential application in biomedical fields.
Acknowledgments
The following financial support is appreciated: the Natural Science Foundation of China (Nos. 51373101 and 51403128) and the Training Project for Excellent Young and Middle-aged Backbone teachers of Higher Schools in Guangxi province.
References
1. Démuth B, Farkas A, Pataki H, Balogh A, Szabó B, Borbás E, Vigh T, Kiserdei É, Farkas B, Mensch J, Verreck G, an Assche I, Marosi G, Nagy ZK. Detailed stability investigation of amorphous solid dispersions prepared by single-needle and high speed electrospinning. Int J Pharm. 2016;498(1–2):234–44.10.1016/j.ijpharm.2015.12.029Search in Google Scholar PubMed
2. Gandhi KJ, Deshmane SV, Biyani KR. Polymers in pharmaceutical drug delivery system: a review. Int J Pharm Sci Rev Res. 2012;14(2):57–66.Search in Google Scholar
3. Vasconcelos T, Sarmento B, Costa P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov Today. 2007;12(23–24):1068–75.10.1016/j.drudis.2007.09.005Search in Google Scholar PubMed
4. Phong NT, Gabr MH, Okubo K, Chuong B, Fujii T. Improventment in the mechanical performances of carbon fiber/epoxy composite with addition of nano-(polyvinyl alcohol) fibers. Compos Struct. 2013;99:380–7.10.1016/j.compstruct.2012.12.018Search in Google Scholar
5. Farokhzad OC. Nanotechnology for drug delivery: the perfect partnership. Expert Opin Drug Del. 2008;5(9):927–9.10.1517/17425247.5.9.927Search in Google Scholar PubMed
6. Wang X, Li XY, Li Y, Zou H, Yu DG, Cai JS. Electrospun acetaminophen-loaded cellulose acetate nanofibers fabricated using an epoxy-coated spinneret. E-polymers. 2015;15(5):311–5.10.1515/epoly-2015-0088Search in Google Scholar
7. Wen HF, Yang C, Yu DG, Li XY, Zhang DF. Electrospun zein nanoribbons for treatment of lead-contained wastewater. Chem Eng J. 2016;290(4):263–72.10.1016/j.cej.2016.01.055Search in Google Scholar
8. Balogh A, Cselkó R, Démuth B, Verreck G, Mensch J, Marosi G, Nagy ZK. Alternating current electrospinning for preparation of fibrous drug delivery systems. Int J Pharm. 2015;495(1):75–80.10.1016/j.ijpharm.2015.08.069Search in Google Scholar PubMed
9. Yu DG, Li XY, Wang X, Yang JH, Bligh SWA, Williams GR. Nanofibers fabricated using triaxial electrospinning as zero order drug delivery systems. ACS Appl Mater Interfaces. 2015;7(33):18891–7.10.1021/acsami.5b06007Search in Google Scholar PubMed
10. Qu H, Wei S, Guo Z. Coaxial electrospun nanostructures and their applications. J Mater Chem A. 2013;1(38):11513–28.10.1039/c3ta12390aSearch in Google Scholar
11. Agarwal S, Greiner A, Wendorff JH. Functional materials by electrospinning. Prog Polym Sci. 2013;38(6):963–91.10.1016/j.progpolymsci.2013.02.001Search in Google Scholar
12. Le-Ngoc Vo C, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813.10.1016/j.ejpb.2013.09.007Search in Google Scholar PubMed
13. Yang C, Yu DG, Pan D, Liu XK, Wang X, Bligh SWA, Williams GR. Electrospun pH-sensitive core-shell polymer nanocomposites fabricated using a tri-axial processes. Acta Biomater. 2016,35:77–86.10.1016/j.actbio.2016.02.029Search in Google Scholar PubMed
14. Moghe AK, Gupta BS. Co-axial electrospinning for nanofiber structures: preparation and applications. Polym Rev. 2008;48(2):353–77.10.1080/15583720802022257Search in Google Scholar
15. Yu DG, Gao LD, White K, Brandford-White C, Lu WY, Zhu LM. Multicomponent amorphous nanofibers electrospun from hot aqueous solutions of a poorly soluble drug. Pharm Res. 2010;27(11):2466–77.10.1007/s11095-010-0239-ySearch in Google Scholar PubMed
16. Singh SK, Som S, Shankhwar U. Formulation and optimization of solid dispersion of Clopidogrel with PEG 6000. J Appl Pharm Sci. 2011;1(8):217–26.Search in Google Scholar
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels
Articles in the same Issue
- Frontmatter
- In this Issue
- Editorial
- Innovations in polymers and composite materials
- Full length articles
- Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites
- Synthesis and properties of well-defined carbazole-containing fluorescent star polymers of different arms
- The effect of high-current pulsed electron beam modification on the surface wetting property of polyamide 6
- Synthesis and application of waterborne polyurethane fluorescent composite
- Medicated structural PVP/PEG composites fabricated using coaxial electrospinning
- Research of the thermal aging mechanism of polycarbonate and polyester film
- Damage indication of 2′, 7′-dichlorofluorescein for epoxy polymer and the effect of water on its damage indicating ability
- Synthesis and characterization of thermosensitive and polarity-sensitive fluorescent PNIPAM-coated gold nanoparticles
- Comparative study of crystallization and lamellae orientation of isotactic polypropylene by rapid heat cycle molding and conventional injection molding
- Determination of deformation of a highly oriented polymer under three-point bending using finite element analysis
- Kinetic studies on the cure reaction of hydroxyl-terminated polybutadiene based polyurethane with variable catalysts by differential scanning calorimetry
- Preparation and swelling properties of poly(acrylic acid-co-acrylamide) composite hydrogels

