Abstract
A V-doped titania catalyst was synthesized on titanium substrate by micro-arc oxidation and then it was carried out by heat treatment in nitrogen atmosphere. The surface characteristics of the synthesized titania coatings were investigated by SEM, EDS, XPS and XRD. The synthesized TiO2 coating doped with V (in form of V2O3 and V2O5) showed a large amount of “cellular” bulges with more microscopic defects. The anatase phase of the TiO2 coatings after heat treatment tended to be transformed into rutile phase. As a result, the corrosion resistance and photocatalytic performance of TiO2 coating doped with V after heat treatment had been enhanced.
1 Introduction
In recent years, photocatalyst materials have attracted significant interest among researchers for application in environmental remediation due to their outstanding photocatalytic performance towards organic pollutants degradation [1, 2, 3]. It is well known that metal oxide photocatalysis is a very promising approach to solve the pollution problem. Among various metal oxide, titanium oxide (TiO2) has become one of the most popular photocatalysis for its chemically inert and photocatalytically stable, and so on. The preparation of photocatalytic TiO2 coatings on the substrates has been the main research direction currently. A number of commonly surface techniques have been developed to synthesize titanium dioxide, such as sol–gel, chemical vapor deposition, cold spray, spray pyrolysis, and anodizing [4, 5]. Micro arc oxidation (MAO) is one of the most promising and environmentally friendly technology to prepare TiO2coatings, which could be carried out by ultrasonic catalysis, light-ound and light-lectric combined catalysis. It has recently gained much attention as useful photcatalysis for its simple manufacturing process, high growth rate and adhesion, porous microstructure, excellent anti vibration performance [6, 7, 8, 9]. Since the band gap energy (Eg) of titania is relatively wide, considerable efforts have been extended to broaden the absorption edge of TiO2 toward the visible part of the spectrum in the last three decades, and so it is expected to be a new method for the preparation of high performance TiO2 photocatalytic coating. The TiO2 coatings doped with metallic or nonmetallic elements (such as Ag, V, W, B, N and S) to enhance the photocatalytic properties have been reported by some references [10, 11, 12, 13]. Furthermore, heat treatment is another method for improving the photocatalytic properties of the coatings and also beneficial for increasing the coating adhesion strength, which is used in TiO2 thin film or powder [14, 15, 16]. However, heat treatment to influence the microstructure and photocatalytic performance of TiO2 coatings has been little reported.
In this paper, the TiO2 coatings formed by MAO were prepared on pure titanium in the electrolytes with or without NaVO3 addition. Then, the obtained coatings were carried out by heat treatment. Finally, the microstructure and photocatalytic performance of the TiO2 coatings with or without V doping before and after heat treatment had been comparatively studied. Meanwhile, the mechanical, electrochemical and photocatalytic performances of TiO2 coatings were also systematicly studied.
2 Experimental
2.1 Preparation of MAO coatings
Pure titanium (Φ16mm×5mm) was mechanically polished to an average surface roughness of Ra ≈ 28.6 nm, followed by ultrasonic cleaning in acetone for 10 min. To fabricate TiO2 ceramic coatings, the constant voltage mode was selected and 450 V was predefined. The MAO parameters were as follows: frequency 500 Hz, duty cycle 6% and time 5 min. The solution temperature was kept below 35∘C during the MAO process. Three electrolytes, 10 g/L Na2SiO3, 20 g/L Na2SiO3, 20 g/L Na2SiO3 + NaVO3, were used for the MAO deposition. As a result, the three TiO2 coatings with the thickness of about 8 μm were prepared. In the further text, we would use the terms “MAO-1, MAO-2,MAO-3” to denote the three TiO2 coatings. Then the three obtained TiO2 coatings were carried out by heat treatment using tube furnace (KSY, Beijing Kewei Instrument Co. Ltd. Yongxing, China), using “MAO-1+HT, MAO-2+HT, MAO-3+HT” to denote the three TiO2 coatings after heat treatment. Due to the relatively small size, the three samples wrapped with tin foil were loaded in porcelain boats during the heat treatment process. The annealing temperature for heat treatment were set at 400∘C in N2, at which the specimens were kept for 60 min. In the final step, the samples were left in the furnace to cool naturally.
2.2 Coating characterization
Field-emission scanning electron microscopy with energy dispersive X-ray spectrometer (EDS) (FE-SEM, S-4800, Hitachi, Japan) were performed for morphological characterization of the TiO2 coatings. X-ray diffraction meter with Cu Kα radiation was used to study the phases of the obtained TiO2 coatings. The x-ray generator was operated at 40 kV and 40 mA. X-ray photoelectron spectroscopy (XPS, Axis ultraDLD, Japan) with Al (mono) Kα irradiation at pass energy of 160 eV was used to characterize the chemical bonds of the coatings. The binding energies were referenced to the C 1s line at 285.0 eV.
2.3 Property testing
The corrosion resistance of the coatings was tested by the electrochemical system (PGSTAT302, OTL, Holland) under room temperature using electrochemical potentiodynamic polarization in 5 wt.% NaCl solution. During the process of electrochemical test, a three electrode cell with the coated specimens as working electrode, the platinum sheet as auxiliary electrode, the saturated calomel electrode as the reference electrode was used. The working electrode was covered with a holder with a circular window having a surface area of 0.785 cm2 and was exposed to the electrolytic solution. Before each measurement, the open circuit potential (OCP) of samples was monitored for 5 min to stabilize the surface condition. The scanning speed was 1 mV/s. A 64 bit system -zview software was used to fit and calculate the characterization parameters of electrochemical corrosion. The photocatalytic activities of the prepared samples were measured by determining the degradation of methylene blue (MB) solution, and a 8 W ultraviolet lamp was used as the light source of simulated sunlight. The distance between the samples and the lamp was 5 cm. The concentration of the MB solution was 8 mg/L and the test sample was fully immersed in 10 ml of MB solution. The decomposition rates of MB were monitored by measuring the absorbance of MB solution at 664 nm using a UV 723 spectrophotometer periodically every 1 h for a total time of 5 h. The coated samples were tested for the average value.
3 Results and discussion
3.1 Coating characteristics
Figure 1 shows the surface morphologies of TiO2 coatings prepared in three different electrolytes on pure Ti substrates. It was found that the surface morphologies of TiO2 coatings prepared in 10 g/L or 20 g/L Na2SiO3 solutions were compact and smooth, which was not clearly changed before and after heat treatment under 400∘C for 1 h. After adding 5 g/L NaVO3 into 20 g/L Na2SiO3 base solution, energy applied to the sample under the same electrical parameter output conditions had been enhanced and micro arc phenomenon could be strenghthened during the MAO process. So high energy resulted in more spattering of molten droplets and more holes observed in the ceramic coating. As a result, some “cellular” bulge and micropores were found on this TiO2 coating. Compared with the TiO2 coating prepared in 10 g/L or 20 g/L Na2SiO3 solutions, the TiO2 coating prepared in 20 g/L Na2SiO3 +5 g/L NaVO3 solutions was more coarse obviously. Furthermore, the surface defects on the TiO2 coating doped with V were increased after heat treatment. These “defects” microstructure might be beneficial for increasing the adsorption of organic matter on the coating surface and improving its photocatalytic performance due to the increase of surface area.
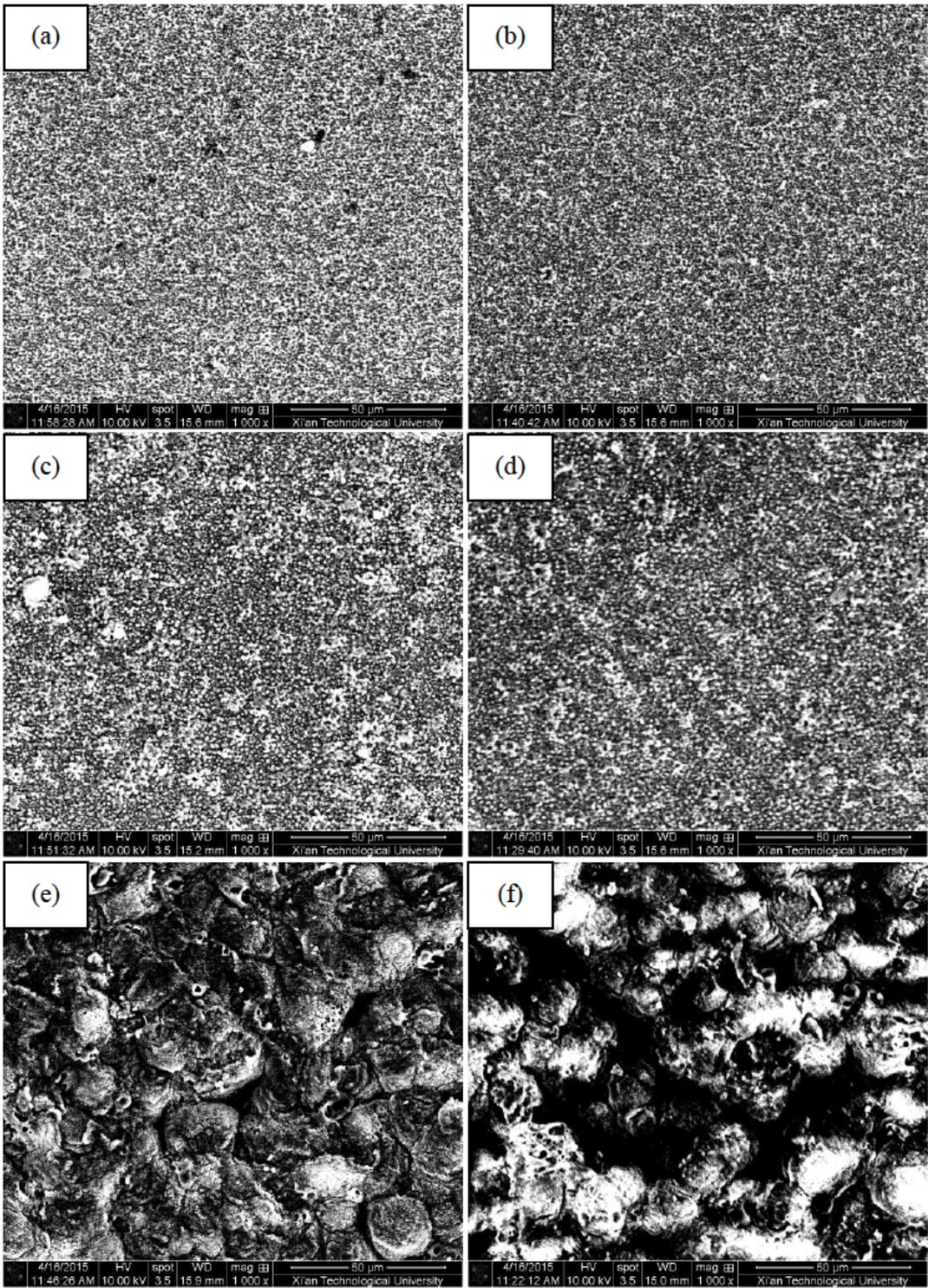
Surface morphologies of MAO coatings before and after heat treatment (a) MAO-1, (b) MAO-1+HT, (c) MAO-2, (d) MAO-2+HT, (e) MAO-3, (f) MAO-3+HT
The EDS analysis of the TiO2 coatings in three different electrolytes was shown in Table 1. It was found that the elementary compositions of the TiO2 coatings formed in the same electrolyte had no obvious change before and after heat treatment. The content of Si element was increased significantly, as the TiO2 coating was prepared in an increased concentration of Na2SiO3. Meanwhile, the content of Ti element significantly reduced and O content was not significantly changed. So, it was obtained that Si element from the electrolyte was strongly involved in the formation process of TiO2 coating. The introduction of V into the base Na2SiO3 solution resulted in a sharp micro arc discharge phenomenon. As a result, V and Si elements from the electrolyte strongly participated in the formation process of TiO2 coating, which resulted in the decreased content of O and Ti elements in the coating. So it was obtained that the degree of solute ions participating in the TiO2 coatings could be affected by each other.
EDS results of MAO coatings prepared on Ti substrates before and after heat treatment
| Coatings | O | Ti | Si | Na | C | V |
|---|---|---|---|---|---|---|
| MAO-1 | 71.56 | 16.05 | 11.91 | 0.39 | 0.09 | |
| MAO -2 | 71.66 | 10.67 | 17.26 | 0.34 | 0.07 | |
| MAO-3 | 65.81 | 10.11 | 20.01 | 1.12 | 0.12 | 1.29 |
| MAO-1+HT | 71.22 | 16.47 | 11.80 | 0.50 | ||
| MAO-2+HT | 70.79 | 11.16 | 17.56 | 0.43 | 0.06 | |
| MAO-3+HT | 64.55 | 10.15 | 20.53 | 1.26 | 0.06 | 1.59 |
In order to study the elemental composition of the TiO2 coating doped with V after heat treatment, the XPS analysis has been employed. The XPS survey spectra of the prepared coating is shown in Figure 2 (a). It was noted that the dominant elements were O,Ti, Si, V and C. The high concentration of C was commonly in the surface XPS scan, which was attributable to environmental contamination. The concentration of Ti and O elements were 11.28 at.% and 63.67 at.%, respectively. The V2p peak of the TiO2 coating had been found and the atomic percentage of the V element in this coating was 1.85 at.%, which indicated that the V element from the solution had been doped into the TiO2 coating. Furthermore, a large amount of Si element (about 18.36 at.%) from the base solution had been obviously detected, which illustrated that the solute element had been strongly involved in the formation process of TiO2 coatings. This was consistent with some literatures [17, 18]. The Ti2p3/2 peak of TiO2 coating was shown on Figure 2 (b), and theTi2p3/2 peak of 558.41 eV indicated the existence of TiO2. So it could be obtained that the titanium oxide was formed in this TiO2 coating. The V2p peak of TiO2 coating was shown on Figure 2 (c). The V2p3/2 peak of 516.98 eV and V2p1/2 peak of 524.58 eV corresponded to V2O3 and V2O5, respectively. Therefore, it was confirmed by XPS analysis that V element from the solution was incorporated into in the oxide film through the electrochemical oxidation process. According to M.R. Bayati et al. [19], the absorption edge of the TiO2 coating doped with V (V2O5) shifted toward the visible wavelengths and the photocatalytic performance of this composited TiO2 coating could be enhanced. Thus it could be speculated that the photocatalytic performance of the obtained TiO2 coating doped with V might be changed compared with the TiO2 coating without V doping [20].
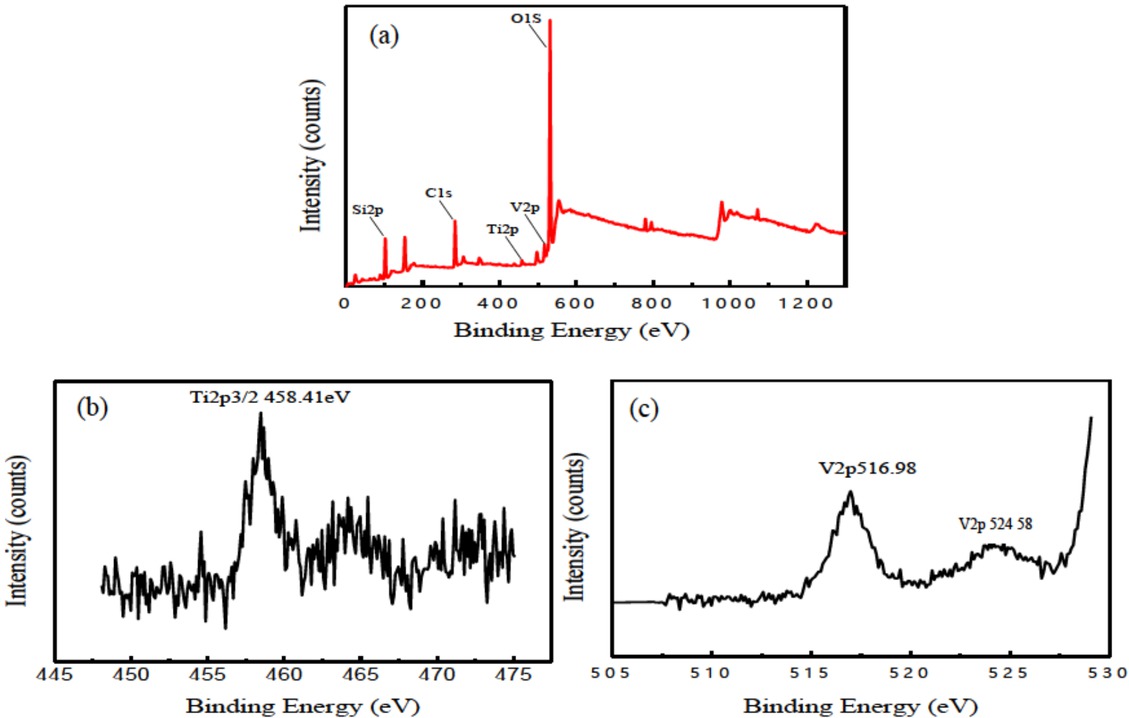
(a) XPS survey spectra, (b) typical Ti2p3/2 and (c) V2p high-resolution XPS spectrum of MAO coating doped with V on Ti substrate after heat treatment
The XRD patterns of the oxidized samples prepared by different MAO technology are presented in Figure 3. The proportion of anatase and rutile phase of different TiO2 coatings is shown in Table 2. XRD analysis revealed that the surfaces of the coated samples were covered by Ti and TiO2. The appearance of Ti peaks on the XRD patterns was most likely due to the penetration of the X-rays beyond the TiO2 coating. The TiO2 coating prepared in 10 g/L Na2SiO3 solution included only anatase TiO2 before and after heat treatment. The crystalline phase of the pure TiO2 film is anatase, because it is very difficult for the metastable anatase phase to transform to thermodynamically stable rutile phase due to less heat produced by mild spark discharge on the surface of anode for a low conductivity of the solution. Xiang et al. [10] also reported that TiO2 film was mainly composed of anatase generated during MAO process. Furthermore, it was found that the crystalline phase of the pure TiO2 film after heat treatment was still composed of anatase phase. When the concentration of Na2SiO3 was increased to 20 g/L, the TiO2 coatings included anatase TiO2 and rutile TiO2, and the proportion of anatase phase and rutile phase was 10.28, which was due to a violent discharge on anodic surface and resulted in the transformation of crystalline phase from metastable anatase phase to thermodynamically stable rutile phase. The proportion of anatase phase and rutile phase of this TiO2 coating after heat treatment decreased to 8.45, implying that the metastable anatase phase furhter transformed into thermodynamically stable rutile phase.With the addition of NaVO3 into 20 g/L Na2SiO3 solution, the TiO2 coatings also included anatase TiO2 and rutile TiO2 before and after heat treatment, and the proportion of anatase phase and rutile phase was 1.81 and 0.85, respectively. Compared with the TiO2 coatings without V doping, the proportion of anatase and rutile phase of the TiO2 coatings with V doping significantly decreased due to the change of micro arc discharge characteristics and the function of V element in the coatings [20]. It was also believed that anatase phase of the TiO2 coatings prepared in 20 g/L Na2SiO3 electrolytes with or without NaVO3 addition tended to be transformed to rutile phase after heat treatment. As a result, the TiO2 coatings with different proportion of anatase and rutile phase could present different properties, especially the photocatalytic performance.
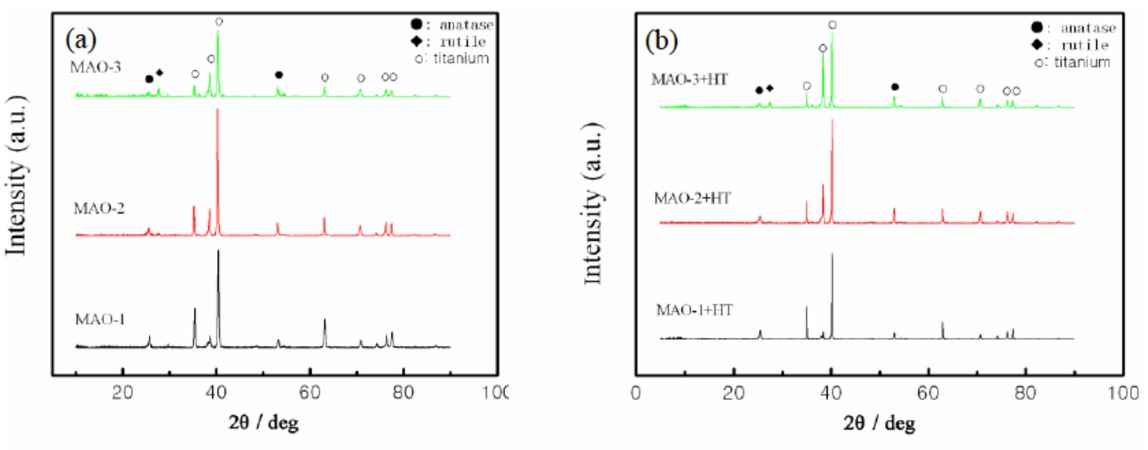
XRD patterns for three MAO coatings formed onto Ti substrates, (a) before heat treatment, (b) after heat treatment
Ratio of anatase and rutile phases of MAO coated Ti substrates before and after heat treatment
| Coatings | Ratio of anatase and rutile |
|---|---|
| MAO-1 | Only anatase |
| MAO-2 | Only anatase |
| MAO-3 | 10.28 |
| MAO-1+HT | 8.45 |
| MAO-2+HT | 1.81 |
| MAO-3+HT | 0.85 |
3.2 Corrosion resistance
In order to study the corrosion resistance of MAO treated Ti substrate when it serves as photocatalytic material, an in corrosion test is employed and the potentiodynamic porlarization curves are used to evaluate the corrosion resistance [21, 22]. The polarization curves of the coated pure Ti substrates in the 3.5 wt.% NaCl solution are shown in Figure 4. Corrosion potential and corrosion current density obtained from Figure 5 by Tafel analysis are shown in Table 3. Based on these electrochemical parameters, it was known that the corrosion current density and corrosion potential of the MAO coated samples after heat treatment all slightly decreased compared with the MAO coated samples before heat treatment, which indicated that the corrosion resistance of the MAO coated samples after heat treatment got better and the corrosion tendency had been intensified [23]. It was known that the metastable anatase phase could be transformed into thermodynamically stable rutile phase of the MAO coating on Ti substrate after heat treatment, which resulted in the enhanced corrosion resistance. However, adding 5 g/L NaVO3 into 20 g/L solution, the obtained TiO2 coating showed the worst corrosion resistance due to the increasing micro “defects” and the potential difference between Ti and V elements, which might result in the galvanic corrosion. Besides, the MAO coating doped with V element resulted in the decreased content of O and Ti elements in the coating, which was not also helpful for the improvement of its corrosion resistance. In general, the weakened corrosion resistance of the MAO coating doped with V was due to surface morphologies with some “defects” (pore size), galvanic corrosion between Ti and V elements, and change of Ti and O (Composition of titanium oxide) element content.
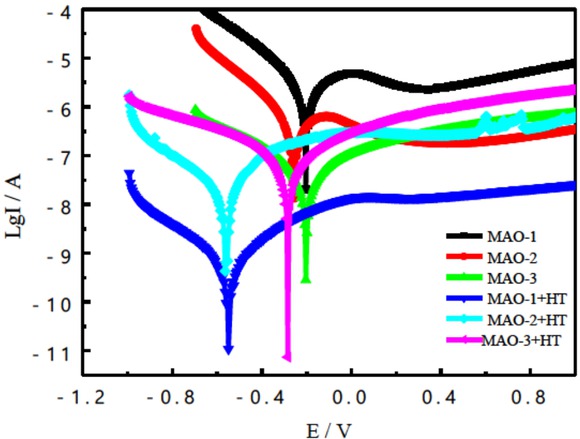
Polarization curves of three MAO coatings before and after heat treatment

Photocatalytic activity of three MAO coatings (a) before heat treament, (b) after heat treatment, (c) catalytic effect of MAO coatings for 5 h
Electrochemical parameters obtained from the polarization curves of Figure 4
| Coatings | Corrosion current density (A/cm2) | Corrosion potential (V) |
|---|---|---|
| MAO-1 | 6.037×10−8 | −0.205 |
| MAO-2 | 8.998×10−8 | −0.2593 |
| MAO-3 | 9.31×10−8 | −0.2061 |
| MAO-1+HT | 1.492×10−9 | −0.5506 |
| MAO-2+HT | 5.922×10−9 | −0.5577 |
| MAO-3+HT | 4.894×10−8 | −0.284 |
3.3 Photocatalytic activity
The photocatalytic activity of the TiO2 coatings on pure Ti before and after heat treatment versus illumination time is shown in Figure 5 (a) and (b). It was found that the photodegradation rate of methylene blue gradually increased along with the illumination time for all the TiO2 coatings before and after heat treatment, and the photodegradation rate increased fast first and then the increasing speed tended to be slow. The photodegradation rate of methylene blue for the TiO2 coatings prepared in 20 g/L Na2SiO3 solution with or without V doping was higher than the TiO2 coating prepared in 10 g/L Na2SiO3 solution before heat treatment, which could be mainly attributed to the mixed phaseand the transfer of photo-generated electron from rutile to anatase. In addition, metal oxides with more structure defects on surface are able to substantially ionosorb oxygen to improve the photocatalytic activity [24]. However, the photocatalytic activity of the TiO2 coating doped with V was lower than the TiO2 coating prepared in 20 g/L Na2SiO3 solution, which might attribute to the proportion of anatase phase and rutile phase. The TiO2 coating doped with V presented the highest photodegradation rate among the three TiO2 coatings after heat treatment due to comprehensive function of the proportion of the mixed phase and the formation of structural defects. The photodegradation efficiencies of the TiO2 coatings before and after heat treatment were shown in Figure 5 (c). Compared with the TiO2 coating doped with V, the photodegradation efficiency of this TiO2 coating after heat treatment had been enhanced. It was believed that the “defects” microstructure, doping V into the coating and the decreasing proportion of anatase and rutile phase resulted in the increasing adsorption of organic matter on the coating surface and enhancing the photocatalytic activity. However, the photodegradation efficiency of the TiO2 coating prepared in 20 g/L Na2SiO3 solution had been deteriorated after heat treatment. In short, the TiO2 coating prepared in 20 g/L Na2SiO3 solution before heat treatment had the best photodegradation efficiency among the all TiO2 coatings, which was determined by a appropriate proportion of anatase and rutile phase to accelerate photo-generated electron transfering from rutile to anatase.
4 Conclution
The TiO2 coatings prepared in the base electrolyte were smooth and compact, and their surface morphologies changed little after heat treatment. Adding NaVO3 into the base electrolyte, some “cellular” bulges were found on the coating and heat treatment resulted in an increase of micro “defects”and a poor corrosion resistance for this coating.
The coating prepared in 10g/L Na2SiO3 electrolyte was only composed of anatase phase, which was not changed before and after heat treatment. The anatase phase and rutile phase were all found in the two coatings prepared in the 20g/L Na2SiO3 electrolyte or 20g/L Na2SiO3 + 5g/L NaVO3 electrolytes. The ratios of anatase phase and rutile phasewere all decreased after heat treatment.
The TiO2 coating prepared in 20 g/L Na2SiO3 solution showed the best photodegradation efficiency before heat treatment for a appropriate proportion of anatase and rutile phase, but it could be deteriorated after heat treatment. The TiO2 coating doped with V presented the best photodegradation efficiency among the three coatings after heat treatment.
Acknowledgement
The authors gratefully acknowledge the financial support of the Key research and development plan of shaanxi province- Industrial project (Grant No. 2018GY-127).
References
[1] Y.L. Song, P.H. Shao, J.Y. Tian, W.X. Shi, S.S. Gao, J.Y. Qi, X.J. Yan, F.Y. Cui, One-step hydrothermal synthesis of ZnO hollow nanospheres uniformly grown on graphene for enhanced photocatalytic performance, Ceram. Int. 42 (2016) 2074-2078.10.1016/j.ceramint.2015.09.082Suche in Google Scholar
[2] X.W. Lu, X.Z. Li, J.C. Qian, N.M. Miao, C. Yao, Z.G. Chen, Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance, J. Alloys Compd. 661 (2016) 363-371.10.1016/j.jallcom.2015.11.148Suche in Google Scholar
[3] Q.Z. Wang, D.H. Jiao, Y. Bai, J.B. Zhong, L.C. Zhao, X. Yong, J.H. Tong, J.Z. Li, Immobilized Heteropolyacids with zeolite (MCM-41) to enhance photocatalytic performance of BiOBr, Mater. Lett. 161 (2015) 267-270.10.1016/j.matlet.2015.08.111Suche in Google Scholar
[4] J.L. Jia, D. Li, J.F. Wan, X.J. Yu, Characterization and mechanism analysis of graphite/C-doped TiO2 composite for enhanced photocatalytic performance, J. Ind. And Eng. Chem. 33 (2016) 162-169.10.1016/j.jiec.2015.09.030Suche in Google Scholar
[5] Z.M. Wu, X. Tong, P.T. Sheng, W.L. Li, X.H. Yin, J.M. Zou, Q.Y. Cai, Fabrication of high-performance CuInSe2 nanocrystals-modified TiO2 NTs for photocatalytic degradation applications, Appl. Surf. Sci. 351 (2015) 309-315.10.1016/j.apsusc.2015.05.147Suche in Google Scholar
[6] Q. Luo, Q.Z. Cai, X.W. Li, Z.H. Pan, Y.J. LI, X.D. Chen, Q.S. Yan, Preparation and characterization of ZrO2/TiO2 composite photocatalytic film by micro-arc oxidation, T. Nonferr. Metal. Soc. 23 (2013) 2945-2950.10.1016/S1003-6326(13)62818-6Suche in Google Scholar
[7] K.R. Wu, C.H. Hung, C.W. Yeh, J.K. Wu, Microporous TiO2-WO3/TiO2 films with visible-light photocatalytic activity synthesized by micro arc oxidation and DC magnetron sputtering, Appl. Surf. Sci. 263 (2012) 688-695.10.1016/j.apsusc.2012.09.142Suche in Google Scholar
[8] S.C. Di, Y.P. Guo, H.W. Lv, J. Yu, Z.W. Li, Microstructure and properties of rare earth CeO2-doped TiO2 nanostructured composite coatings through micro-arc oxidation, Ceram. Int. 41 ( 2015) 6178-6186.10.1016/j.ceramint.2014.12.134Suche in Google Scholar
[9] W.P. Li, M.Q. Tang, L.Q. Zhu, H.C. Liu, Formation of microarc oxidation coatings on magnesium alloy with photocatalytic performance, Appl. Surf. Sci. 258 (2012) 10017-10021.10.1016/j.apsusc.2012.06.066Suche in Google Scholar
[10] N. Xiang, R.G. Song, B. Xiang, H. Li, Z.X. Wang, C. Wang, A study on photocatalytic activity of micro-arc oxidation TiO2 films and Ag+/MAO-TiO2 composite films, Appl. Surf. Sci. 347 (2015) 454-460.10.1016/j.apsusc.2015.04.136Suche in Google Scholar
[11] M.R. Bayati, A.Z. Moshfegh, F. Golestani-Fard, On the photocatalytic activity of the sulfur doped titania nano-porous films derived via micro-arc oxidation, Appl. Catal. A- Gen. 389 (2010) 60-67.10.1016/j.apcata.2010.09.003Suche in Google Scholar
[12] J.H. Lee, J.I. Youn, Y.J. Kim, I.K. Kim, K.W. Jang, H.J. Oh, Photocatalytic characteristics of boron and nitrogen doped titania film synthesized by micro-arc oxidation, Ceram. Int. 41 (2015) 11899-11907.10.1016/j.ceramint.2015.05.157Suche in Google Scholar
[13] M.R. Bayati, A.Z. Moshfegh, F. Golestani-Fard, In situ growth of vanadia–titania nano/micro-porous layers with enhanced photocatalytic performance by micro-arc oxidation, Electrochim. Acta 55 (2010) 3093-3102.10.1016/j.electacta.2010.01.045Suche in Google Scholar
[14] Y. Lu, K. Matsuzaka, L. Hao, Y. Hirakawa, H. Yoshida, F.S. Pan, Photocatalytic activity of TiO2/Ti composite coatings fabricated by mechanical coating technique and subsequent heat oxidation, Mater. Sci. Semicond. Process. 16 (2013) 1949-1956.10.1016/j.mssp.2013.07.024Suche in Google Scholar
[15] B. Rahmati, E. Zalnezhad, Ahmed A.D. Sarhan, Z. Kamiab , B. Nasiri Tabrizi, W.A.B.W. Abas, Enhancing the adhesion strength of tantalum oxide ceramic thin film coating on biomedical Ti– 6Al–4V alloy by thermal surface treatment, Ceram. Int. 41 (2015) 13055–13063.10.1016/j.ceramint.2015.07.090Suche in Google Scholar
[16] S. Sado, T. Ueda, K. Ueda, T. Narushima, Formation of TiO2 layers on commercially pure Ti and Ti–Mo and Ti–Nb alloys by two-step thermal oxidation and their photocatalytic activity, Appl. Surf. Sci. 357 (2015) 2198–2205.10.1016/j.apsusc.2015.09.211Suche in Google Scholar
[17] W.Yang, J.L. Wang, D.P. Xu, J.H. Li, T. Chen, Characterization and formation mechanism of grey micro-arc oxidation coatings on magnesium alloy, Surf. Coat. Technol. 283 (2015) 281-285.10.1016/j.surfcoat.2015.10.065Suche in Google Scholar
[18] X. Ma, S.J. Zhu, L.G. Wang, C.X. Ji, C.X. Ren, S.K. Guan, Synthesis and properties of a bio-composite coating formed on magnesium alloy by one-step method of micro-arc oxidation, J. Alloys Compd. 590 (2014) 247-253.10.1016/j.jallcom.2013.12.145Suche in Google Scholar
[19] M.R. Bayatia, A.Z. Moshfegh, F. Golestani-Fard, Synthesis of narrow band gap (V2O5)x–(TiO2)1-x nano-structured layers via micro arc oxidation, Appl. Surf. Sci. 256 (2010) 2903-2909.10.1016/j.apsusc.2009.11.048Suche in Google Scholar
[20] F.Z. Ren, H.Y. Li, Y.X. Wang, J.J. Yang, Enhanced photocatalytic oxidation of propylene over V-doped TiO2 photocatalyst: Reaction mechanism between V5+ and single-electron-trapped oxygen vacancy, Appl. Catal., B 176–177 ( 2015) 160-172.10.1016/j.apcatb.2015.03.050Suche in Google Scholar
[21] S. Durdu, A. Aytaç, M. Usta, Characterization and corrosion behavior of ceramic coating on magnesium by micro-arc oxidation, J. Alloys Compd. 509 (2011) 8601-8606.10.1016/j.jallcom.2011.06.059Suche in Google Scholar
[22] X.Y. Lu, X.G. Feng, Y. Zuo, C.B. Zheng, S. Lu, L. Xu, Evaluation of the micro-arc oxidation treatment effect on the protective performance of a Mg-rich epoxy coating on AZ91D magnesium alloy, Surf. Coat. Technol. 270 (2015) 227-235.10.1016/j.surfcoat.2015.02.052Suche in Google Scholar
[23] C.J. Wang, B.L. Jiang, M. Liu, Y.F. Ge, Corrosion characterization of micro-arc oxidization composite electrophoretic coating on AZ31B magnesium alloy, J. Alloys Compd. 621 (2015) 53-61.10.1016/j.jallcom.2014.09.168Suche in Google Scholar
[24] A. Sclafani, J.M. Herrmann, Comparison of the Photoelectronic and Photocataly tic Activities of Various Anatase and Rutile Forms of Titania in Pure Liquid Organic Phases and in Aqueous So lutions, J. Phys. Chem. 100 (1996) 13655-13661.10.1021/jp9533584Suche in Google Scholar
© 2020 W. Yang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Microstructure and compressive behavior of lamellar Al2O3p/Al composite prepared by freeze-drying and mechanical-pressure infiltration method
- Al3Ti/ADC12 Composite Synthesized by Ultrasonic Chemistry in Situ Reaction
- Microstructure and photocatalytic performance of micro arc oxidation coatings after heat treatment
- The effect of carbon nanotubes on the mechanical and damping properties of macro-defect-free cements
- Toughening Mechanism of the Bone — Enlightenment from the Microstructure of Goat Tibia
- Characterization of PVC/MWCNTs Nanocomposite: Solvent Blend
- Study on Macroscopic and Mesoscopic Mechanical Behavior of CSG based on Inversion of Mesoscopic Material Parameters
- Bearing properties and influence laws of concrete-filled steel tubular arches for underground mining roadway support
- Comparing Test Methods for the Intra-ply Shear Properties of Uncured Prepreg Tapes
- Investigation of Microstructural, Mechanical and Corrosion Properties of AA7010-TiB2 in-situ Metal Matrix Composite
- A Comparative Study of Structural Changes in Conventional and Unconventional Machining and Mechanical Properties Evaluation of Polypropylene Based Self Reinforced Composites
- Research on Influence mechanism of composite interlaminar shear strength under normal stress
- Mechanical properties of geopolymer foam at high temperature
- Synthesis and mechanical properties of nano-Sb2O3/BPS-PP composites
- Multiscale acoustic emission of C/SiC mini-composites and damage identification using pattern recognition
- Modifying mechanical properties of Shanghai clayey soil with construction waste and pulverized lime
- Relationship between Al2O3 Content and Wear Behavior of Al+2% Graphite Matrix Composites
- Static mechanical properties and mechanism of C200 ultra-high performance concrete (UHPC) containing coarse aggregates
- A Parametric Study on the Elliptical hole Effects of Laminate Composite Plates under Thermal Buckling Load
- Morphology and crystallization kinetics of Rubber-modified Nylon 6 Prepared by Anionic In-situ Polymerization
- Effects of Elliptical Hole on the Correlation of Natural Frequency with Buckling Load of Basalt Laminates Composite Plates
- Effect of interphase parameters on elastic modulus prediction for cellulose nanocrystal fiber reinforced polymer composite
- Mixed Matrix Membranes prepared from polysulfone and Linde Type A zeolite
- Fabrication and low-velocity impact response of pyramidal lattice stitched foam sandwich composites
- Design and static testing of wing structure of a composite four-seater electric aircraft
- CSG Elastic Modulus Model Prediction Considering Meso-components and its Effect
- Optimization of spinning parameters of 20/316L bimetal composite tube based on orthogonal test
- Chloride-induced corrosion behavior of reinforced cement mortar with MWCNTs
- Statistical Law and Predictive Analysis of Compressive Strength of Cemented Sand and Gravel
- Young’s modulus and Poisson’s ratio of the deformable cement adhesives
- Reverse localization on composite laminates using attenuated strain wave
- Impact of reinforcement on shrinkage in the concrete floors of a residential building
- Novel multi-zone self-heated composites tool for out-of-autoclave aerospace components manufacturing
- Effect of notch on static and fatigue properties of T800 fabric reinforced composites
- Electrochemical Discharge Grinding of Metal Matrix Composites Using Shaped Abrasive Tools Formed by Sintered Bronze/diamond
- Fabrication and performance of PNN-PZT piezoelectric ceramics obtained by low-temperature sintering
- The extension of thixotropy of cement paste under vibration: a shear-vibration equivalent theory
- Conventional and unconventional materials used in the production of brake pads – review
- Inverse Analysis of Concrete Meso-constitutive Model Parameters Considering Aggregate Size Effect
- Finite element model of laminate construction element with multi-phase microstructure
- Effect of Cooling Rate and Austenite Deformation on Hardness and Microstructure of 960MPa High Strength Steel
- Study on microcrystalline cellulose/chitosan blend foam gel material
- Investigating the influence of multi-walled carbon nanotubes on the mechanical and damping properties of ultra-high performance concrete
- Preparation and properties of metal textured polypropylene composites with low odor and low VOC
- Calculation Model for the Mixing Amount of Internal Curing Materials in High-strength Concrete based on Modified MULTIMOORA
- Electric degradation in PZT piezoelectric ceramics under a DC bias
- Cushioning energy absorption of regular polygonal paper corrugation tubes under axial drop impact
- Erratum
- Study on Macroscopic and Mesoscopic Mechanical Behavior of CSG based on Inversion of Mesoscopic Material Parameters
- Effect of interphase parameters on elastic modulus prediction for cellulose nanocrystal fiber reinforced polymer composite
- Statistical Law and Predictive Analysis of Compressive Strength of Cemented Sand and Gravel
- Retraction
- Assessment of nano-TiO2 and class F fly ash effects on flexural fracture and microstructure of binary blended concrete
Artikel in diesem Heft
- Regular Articles
- Microstructure and compressive behavior of lamellar Al2O3p/Al composite prepared by freeze-drying and mechanical-pressure infiltration method
- Al3Ti/ADC12 Composite Synthesized by Ultrasonic Chemistry in Situ Reaction
- Microstructure and photocatalytic performance of micro arc oxidation coatings after heat treatment
- The effect of carbon nanotubes on the mechanical and damping properties of macro-defect-free cements
- Toughening Mechanism of the Bone — Enlightenment from the Microstructure of Goat Tibia
- Characterization of PVC/MWCNTs Nanocomposite: Solvent Blend
- Study on Macroscopic and Mesoscopic Mechanical Behavior of CSG based on Inversion of Mesoscopic Material Parameters
- Bearing properties and influence laws of concrete-filled steel tubular arches for underground mining roadway support
- Comparing Test Methods for the Intra-ply Shear Properties of Uncured Prepreg Tapes
- Investigation of Microstructural, Mechanical and Corrosion Properties of AA7010-TiB2 in-situ Metal Matrix Composite
- A Comparative Study of Structural Changes in Conventional and Unconventional Machining and Mechanical Properties Evaluation of Polypropylene Based Self Reinforced Composites
- Research on Influence mechanism of composite interlaminar shear strength under normal stress
- Mechanical properties of geopolymer foam at high temperature
- Synthesis and mechanical properties of nano-Sb2O3/BPS-PP composites
- Multiscale acoustic emission of C/SiC mini-composites and damage identification using pattern recognition
- Modifying mechanical properties of Shanghai clayey soil with construction waste and pulverized lime
- Relationship between Al2O3 Content and Wear Behavior of Al+2% Graphite Matrix Composites
- Static mechanical properties and mechanism of C200 ultra-high performance concrete (UHPC) containing coarse aggregates
- A Parametric Study on the Elliptical hole Effects of Laminate Composite Plates under Thermal Buckling Load
- Morphology and crystallization kinetics of Rubber-modified Nylon 6 Prepared by Anionic In-situ Polymerization
- Effects of Elliptical Hole on the Correlation of Natural Frequency with Buckling Load of Basalt Laminates Composite Plates
- Effect of interphase parameters on elastic modulus prediction for cellulose nanocrystal fiber reinforced polymer composite
- Mixed Matrix Membranes prepared from polysulfone and Linde Type A zeolite
- Fabrication and low-velocity impact response of pyramidal lattice stitched foam sandwich composites
- Design and static testing of wing structure of a composite four-seater electric aircraft
- CSG Elastic Modulus Model Prediction Considering Meso-components and its Effect
- Optimization of spinning parameters of 20/316L bimetal composite tube based on orthogonal test
- Chloride-induced corrosion behavior of reinforced cement mortar with MWCNTs
- Statistical Law and Predictive Analysis of Compressive Strength of Cemented Sand and Gravel
- Young’s modulus and Poisson’s ratio of the deformable cement adhesives
- Reverse localization on composite laminates using attenuated strain wave
- Impact of reinforcement on shrinkage in the concrete floors of a residential building
- Novel multi-zone self-heated composites tool for out-of-autoclave aerospace components manufacturing
- Effect of notch on static and fatigue properties of T800 fabric reinforced composites
- Electrochemical Discharge Grinding of Metal Matrix Composites Using Shaped Abrasive Tools Formed by Sintered Bronze/diamond
- Fabrication and performance of PNN-PZT piezoelectric ceramics obtained by low-temperature sintering
- The extension of thixotropy of cement paste under vibration: a shear-vibration equivalent theory
- Conventional and unconventional materials used in the production of brake pads – review
- Inverse Analysis of Concrete Meso-constitutive Model Parameters Considering Aggregate Size Effect
- Finite element model of laminate construction element with multi-phase microstructure
- Effect of Cooling Rate and Austenite Deformation on Hardness and Microstructure of 960MPa High Strength Steel
- Study on microcrystalline cellulose/chitosan blend foam gel material
- Investigating the influence of multi-walled carbon nanotubes on the mechanical and damping properties of ultra-high performance concrete
- Preparation and properties of metal textured polypropylene composites with low odor and low VOC
- Calculation Model for the Mixing Amount of Internal Curing Materials in High-strength Concrete based on Modified MULTIMOORA
- Electric degradation in PZT piezoelectric ceramics under a DC bias
- Cushioning energy absorption of regular polygonal paper corrugation tubes under axial drop impact
- Erratum
- Study on Macroscopic and Mesoscopic Mechanical Behavior of CSG based on Inversion of Mesoscopic Material Parameters
- Effect of interphase parameters on elastic modulus prediction for cellulose nanocrystal fiber reinforced polymer composite
- Statistical Law and Predictive Analysis of Compressive Strength of Cemented Sand and Gravel
- Retraction
- Assessment of nano-TiO2 and class F fly ash effects on flexural fracture and microstructure of binary blended concrete

