Abstract
C27H32O3S, triclinic, P1̄, a = 12.2441(2) Å, b = 14.9350(2) Å, c = 15.2081(3) Å, α = 73.6710(16)°, β = 70.1267(17)°, γ = 70.7828(15)°, V = 2424.41(8) Å3, Z = 4, Rgt(F) = 0.0442, wRref(F2) = 0.1319, T = 297.1(2) K.
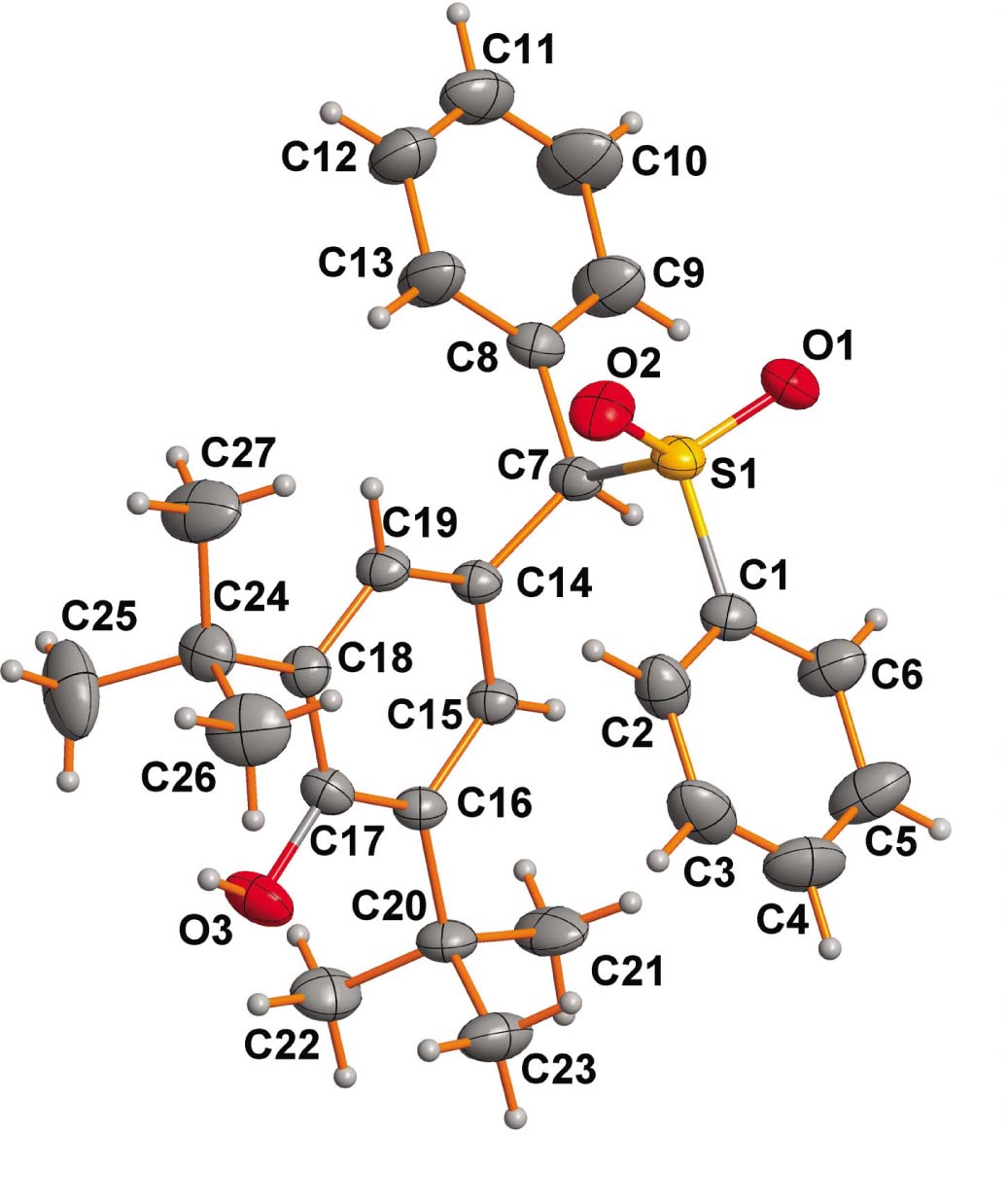
One of the two crystallopraphically molecules foring the asymmetric unit is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.35 × 0.3 × 0.28 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.37 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω-scans |
| θmax, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 42113, 8633, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7324 |
| N(param)refined: | 573 |
| Programs: | CrysAlisPRO [1], SHELX [2], OLEX2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.44215(4) | 0.98322(3) | 0.19627(3) | 0.04365(13) |
| S2 | 0.38627(3) | 0.32632(3) | 0.01628(3) | 0.03848(12) |
| O1 | 0.42680(13) | 1.03546(10) | 0.10413(10) | 0.0606(4) |
| O2 | 0.39315(13) | 1.03550(10) | 0.27304(11) | 0.0608(4) |
| O3 | 0.59990(15) | 0.63910(11) | 0.51725(11) | 0.0656(4) |
| H3 | 0.574275 | 0.662224 | 0.565578 | 0.098* |
| O4 | 0.39557(12) | 0.42414(9) | −0.01926(9) | 0.0513(3) |
| O5 | 0.44495(12) | 0.25817(10) | −0.04659(9) | 0.0512(3) |
| O6 | 0.08672(13) | 0.46209(11) | 0.41683(10) | 0.0624(4) |
| H6A | 0.096791 | 0.514086 | 0.415679 | 0.075* |
| C1 | 0.59806(16) | 0.93277(13) | 0.18464(13) | 0.0435(4) |
| C2 | 0.6428(2) | 0.92150(17) | 0.25992(15) | 0.0594(5) |
| H2 | 0.592218 | 0.941231 | 0.316229 | 0.071* |
| C3 | 0.7645(2) | 0.8802(2) | 0.25027(19) | 0.0750(7) |
| H3A | 0.796068 | 0.871613 | 0.300623 | 0.090* |
| C4 | 0.8382(2) | 0.8522(2) | 0.1672(2) | 0.0803(8) |
| H4 | 0.919832 | 0.824349 | 0.161195 | 0.096* |
| C5 | 0.7925(2) | 0.8649(2) | 0.0927(2) | 0.0832(8) |
| H5 | 0.843526 | 0.846151 | 0.036082 | 0.100* |
| C6 | 0.67104(18) | 0.90549(17) | 0.10060(16) | 0.0621(6) |
| H6 | 0.639844 | 0.913970 | 0.050090 | 0.074* |
| C7 | 0.38258(15) | 0.87788(12) | 0.22931(12) | 0.0404(4) |
| H7 | 0.421374 | 0.843552 | 0.175880 | 0.049* |
| C8 | 0.24962(16) | 0.91356(13) | 0.23391(13) | 0.0445(4) |
| C9 | 0.2162(2) | 0.9211(2) | 0.15421(18) | 0.0750(7) |
| H9 | 0.274338 | 0.901516 | 0.100299 | 0.090* |
| C10 | 0.0965(3) | 0.9576(3) | 0.1531(2) | 0.0970(10) |
| H10 | 0.075705 | 0.963206 | 0.098009 | 0.116* |
| C11 | 0.0094(2) | 0.98515(19) | 0.2313(2) | 0.0741(7) |
| H11 | −0.070470 | 1.009889 | 0.230059 | 0.089* |
| C12 | 0.0411(2) | 0.9760(2) | 0.3109(2) | 0.0820(8) |
| H12 | −0.017871 | 0.993658 | 0.365205 | 0.098* |
| C13 | 0.1603(2) | 0.9406(2) | 0.31261(17) | 0.0757(7) |
| H13 | 0.180137 | 0.935045 | 0.368114 | 0.091* |
| C14 | 0.42527(14) | 0.81073(12) | 0.31344(12) | 0.0366(3) |
| C15 | 0.51366(14) | 0.72653(12) | 0.29622(12) | 0.0373(4) |
| H15 | 0.534256 | 0.708979 | 0.237497 | 0.045* |
| C16 | 0.57255(14) | 0.66747(12) | 0.36373(12) | 0.0387(4) |
| C17 | 0.53812(16) | 0.69617(13) | 0.45185(12) | 0.0422(4) |
| C18 | 0.44690(15) | 0.77936(13) | 0.47342(12) | 0.0412(4) |
| C19 | 0.39255(15) | 0.83563(13) | 0.40164(12) | 0.0413(4) |
| H19 | 0.332374 | 0.891689 | 0.413507 | 0.050* |
| C20 | 0.67560(16) | 0.57738(13) | 0.33906(14) | 0.0465(4) |
| C21 | 0.6930(2) | 0.56318(18) | 0.23892(17) | 0.0732(7) |
| H21A | 0.619538 | 0.556883 | 0.234599 | 0.110* |
| H21B | 0.755886 | 0.505805 | 0.226319 | 0.110* |
| H21C | 0.714498 | 0.617954 | 0.193001 | 0.110* |
| C22 | 0.6491(2) | 0.48547(14) | 0.40810(18) | 0.0635(6) |
| H22A | 0.640025 | 0.490752 | 0.471815 | 0.095* |
| H22B | 0.714415 | 0.430763 | 0.391173 | 0.095* |
| H22C | 0.576243 | 0.477263 | 0.404598 | 0.095* |
| C23 | 0.79472(18) | 0.58929(17) | 0.3414(2) | 0.0703(6) |
| H23A | 0.810676 | 0.647136 | 0.298168 | 0.106* |
| H23B | 0.858966 | 0.534594 | 0.322870 | 0.106* |
| H23C | 0.788803 | 0.593575 | 0.404796 | 0.106* |
| C24 | 0.41070(19) | 0.81336(15) | 0.56872(13) | 0.0539(5) |
| C25 | 0.3702(4) | 0.7370(2) | 0.65414(17) | 0.1053(11) |
| H25A | 0.305630 | 0.719574 | 0.646479 | 0.158* |
| H25B | 0.343231 | 0.762502 | 0.711412 | 0.158* |
| H25C | 0.436444 | 0.680798 | 0.658175 | 0.158* |
| C26 | 0.5155(3) | 0.8403(2) | 0.5792(2) | 0.0852(8) |
| H26A | 0.583908 | 0.785273 | 0.576811 | 0.128* |
| H26B | 0.492440 | 0.860367 | 0.639058 | 0.128* |
| H26C | 0.535814 | 0.892225 | 0.528194 | 0.128* |
| C27 | 0.3066(3) | 0.9051(2) | 0.57383(19) | 0.0898(9) |
| H27A | 0.330508 | 0.956483 | 0.523762 | 0.135* |
| H27B | 0.286128 | 0.923224 | 0.634360 | 0.135* |
| H27C | 0.238105 | 0.893104 | 0.566580 | 0.135* |
| C28 | 0.23134(15) | 0.32853(14) | 0.06189(12) | 0.0426(4) |
| C29 | 0.19861(18) | 0.24253(16) | 0.09473(15) | 0.0535(5) |
| H29 | 0.256180 | 0.184187 | 0.087568 | 0.064* |
| C30 | 0.0787(2) | 0.2444(2) | 0.13855(17) | 0.0671(6) |
| H30 | 0.055094 | 0.187017 | 0.161623 | 0.080* |
| C31 | −0.00560(19) | 0.3317(2) | 0.14778(18) | 0.0754(7) |
| H31 | −0.086094 | 0.332757 | 0.176980 | 0.090* |
| C32 | 0.0275(2) | 0.4165(2) | 0.1147(2) | 0.0781(7) |
| H32 | −0.030488 | 0.474841 | 0.120771 | 0.094* |
| C33 | 0.14709(19) | 0.41577(17) | 0.07210(16) | 0.0627(6) |
| H33 | 0.170420 | 0.473225 | 0.050652 | 0.075* |
| C34 | 0.43714(14) | 0.27618(12) | 0.12415(11) | 0.0345(3) |
| H34 | 0.428427 | 0.209951 | 0.144327 | 0.041* |
| C35 | 0.57092(14) | 0.26685(12) | 0.09930(11) | 0.0371(4) |
| C36 | 0.64811(17) | 0.17622(16) | 0.09203(17) | 0.0604(6) |
| H36 | 0.616727 | 0.124492 | 0.100805 | 0.072* |
| C37 | 0.7710(2) | 0.16083(19) | 0.0720(2) | 0.0765(7) |
| H37 | 0.821466 | 0.099134 | 0.068118 | 0.092* |
| C38 | 0.81849(18) | 0.23681(19) | 0.05778(17) | 0.0677(6) |
| H38 | 0.901143 | 0.227219 | 0.043610 | 0.081* |
| C39 | 0.74286(19) | 0.32671(18) | 0.06469(17) | 0.0620(5) |
| H39 | 0.774672 | 0.378211 | 0.055869 | 0.074* |
| C40 | 0.61994(17) | 0.34248(15) | 0.08457(14) | 0.0512(5) |
| H40 | 0.570076 | 0.404415 | 0.088030 | 0.061* |
| C41 | 0.35119(13) | 0.32898(12) | 0.20331(11) | 0.0351(3) |
| C42 | 0.33631(14) | 0.42514(12) | 0.20223(11) | 0.0384(4) |
| H42 | 0.385528 | 0.459089 | 0.152745 | 0.046* |
| C43 | 0.24985(14) | 0.47287(12) | 0.27299(11) | 0.0382(4) |
| C44 | 0.17607(14) | 0.41899(13) | 0.34580(11) | 0.0400(4) |
| C45 | 0.18751(14) | 0.32121(12) | 0.34910(11) | 0.0377(4) |
| C46 | 0.27686(14) | 0.27869(12) | 0.27659(11) | 0.0354(3) |
| H46 | 0.287213 | 0.213982 | 0.277237 | 0.042* |
| C47 | 0.23521(16) | 0.58057(13) | 0.26831(12) | 0.0434(4) |
| C48 | 0.3296(2) | 0.62068(15) | 0.18443(16) | 0.0610(5) |
| H48A | 0.408602 | 0.583763 | 0.189644 | 0.092* |
| H48B | 0.319410 | 0.687000 | 0.185313 | 0.092* |
| H48C | 0.320098 | 0.616414 | 0.125758 | 0.092* |
| C49 | 0.2521(2) | 0.59557(17) | 0.35844(15) | 0.0650(6) |
| H49A | 0.191002 | 0.575951 | 0.413094 | 0.097* |
| H49B | 0.246084 | 0.662593 | 0.352738 | 0.097* |
| H49C | 0.329901 | 0.557535 | 0.365707 | 0.097* |
| C50 | 0.1115(2) | 0.64063(16) | 0.25369(18) | 0.0676(6) |
| H50A | 0.106197 | 0.634488 | 0.194216 | 0.101* |
| H50B | 0.101924 | 0.707259 | 0.252925 | 0.101* |
| H50C | 0.049397 | 0.617587 | 0.304805 | 0.101* |
| C51 | 0.10364(16) | 0.26347(14) | 0.42672(12) | 0.0450(4) |
| C52 | 0.1136(2) | 0.25884(18) | 0.52594(13) | 0.0650(6) |
| H52A | 0.195319 | 0.229128 | 0.528273 | 0.097* |
| H52B | 0.062213 | 0.221457 | 0.573089 | 0.097* |
| H52C | 0.089611 | 0.323047 | 0.538499 | 0.097* |
| C53 | −0.02700(18) | 0.30923(18) | 0.42156(16) | 0.0622(6) |
| H53A | −0.051803 | 0.374499 | 0.431456 | 0.093* |
| H53B | −0.078437 | 0.272679 | 0.469951 | 0.093* |
| H53C | −0.032263 | 0.309069 | 0.360050 | 0.093* |
| C54 | 0.1359(2) | 0.15955(16) | 0.41246(16) | 0.0685(7) |
| H54A | 0.129750 | 0.159960 | 0.351087 | 0.103* |
| H54B | 0.081588 | 0.125779 | 0.461171 | 0.103* |
| H54C | 0.216744 | 0.127670 | 0.416184 | 0.103* |
Source of material
To a stirring solution of 4-benzylidene-2,6-di-tert-butylcyclohexa-2,5-dienol (177 mg, 0.6 mmol) in anhydrous toluene (2 mL) were added sodium benzenesulfinate (82 mg, 0.5 mmol) and acetic acid (30 mg, 0.5 mmol). The mixture was stirred at 65 °C. After the completion of reaction, monitored by TLC, the mixture was washed by water, dried over by anhydrous magnesium sulfate and concentrated in vacuo. The residue was then purified by flash chromatography (petroleum ether/ethyl acetate 10:1) on silica gel to afford the title compound as white solid in yield 75%.
Experimental details
All hydrogen atoms were placed geometrically on calculated positions using a riding model with the help of the SHELX program [2].
Comment
The diarylmethyl sulfones as one of the most important sulfur-containing compounds are widely present in many biologically active natural products and pharmaceuticals [4], [5], [6], [7]. For example, 2-(1-((4-chlorophenyl)sulfonyl)cyclohexyl)-1,4-difluorobenzene as a γ-secretase inhibitor show high potential in the treatment of Alzheimer’s disease [4]. 4,4′,4′′-((Phenylsulfonyl)methanetriyl)tris(fluorobenzene) is useful as a potassium channel modulator [5]. 8-((Di(naphthalen-2-yl)methyl)sulfonyl)-4-methyl-1-(naphthalen-2-yl)-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepine as an antidepressant drug has a wider therapeutic spectrum and can exert sufficient therapeutic effects in a short period [6]. 9-((4-Methoxyphenyl)sulfonyl)-9H-fluorene is a very selective and effective antimalarial agent, which shows pronounced activity against human skin cancer cells [7]. In addition, diarylmethylamines are also widely used as important synthetic intermediates in medicinal chemistry and materials science [8], [9], [10]. Our group has maintained a keen interest in the synthesis of the sulfones [11], [12] and diarymethyl derivatives [13]. We first synthesized the 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol and obtained the crystal of it. Then we submitted the data to CCDC on 28th September, 2017. One month later, Zhu’s group deposited another monoclinic crystal structure of the same compound with P21/c space group with the Cambridge Crystallographic Data Center (CCDC no.: 1583037) [14]. There is only one molecule in the asymmetric unit of this monoclinic structure. Herein, we reported the crystal structure of the title compound. The geometry and labeling for the crystal structure of it is depicted in the figure. The title structure crystallizes in triclinic space group P1̄. Thus, we obtained another modification of the title compound.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21402159
Funding source: Central Universities
Award Identifier / Grant number: XDJK2014C037
Award Identifier / Grant number: XDJK2015C021
Funding source: Chongqing Postdoctoral Science Foundation
Award Identifier / Grant number: Xm2016110
Funding source: Doctoral Foundation of Southwest Univ.
Award Identifier / Grant number: SWU113083
Award Identifier / Grant number: SWU113060
Funding source: ChongQing Industry Polytechnic College
Award Identifier / Grant number: GZY201719-YA
Award Identifier / Grant number: GZY2018-GGRC-56
Funding statement: We are grateful to the National Natural Science Foundation of China (Grant No. 21402159), the Fundamental Research Funds for the Central Universities (XDJK2014C037; XDJK2015C021), the Chongqing Postdoctoral Science Foundation (Xm2016110), the Doctoral Foundation of Southwest Univ. (SWU113083; SWU113060) and the School-level scientific research projects and PhD Key Talents Project Foundation of ChongQing Industry Polytechnic College (GZY201719-YA; GZY2018-GGRC-56).
References
1. Agilent Technologies: CrysAlisPRO Software system, version 1.171.39.29c. Agilent Technologies UK Ltd, Oxford, UK (2017).Suche in Google Scholar
2. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Suche in Google Scholar PubMed
3. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H.: OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42 (2009) 339–341.10.1107/S0021889808042726Suche in Google Scholar
4. Churcher, I.; Beher, D.; Best, J. D.; Castro, J. L.; Clarke, E. E.; Gentry, A.; Harrison, T.; Hitzel, L.; Kay, E.; Kerrad, S.; Lewis, H. D.; Morentin-Gutierrez, P.; Mortishire-Smith, R.; Oakley, P. J.; Reilly, M.; Shaw, D. E.; Shearman, M. S.; Teall, M. R.; Williams, S.; Wrigley, J. D. J.: 4-Substituted cyclohexyl sulfones as potent, orally active γ-secretase inhibitors. Bioorg. Med. Chem. Lett. 16 (2006) 280–284.10.1016/j.bmcl.2005.10.009Suche in Google Scholar PubMed
5. Gouliaev, A. H.; Sloek, F. A.; Teuber, L.; Demnitz, J.: Preparation of benzene derivatives as potassium channel inhibitors. Patent WO2003059873A1, (2003).Suche in Google Scholar
6. Ito, N.; Kurimura, M.; Yamauchi, T.; Segawa, C.; Sasaki, H.; Tai, K.; Arai, K.; Shinohara, T.: Substituted benzo[1,4]diazepine derivatives as antidepressants and their preparation. Patent WO2009145357A1, (2009).Suche in Google Scholar
7. Langler, R. F.; Paddock, R. L.; Thompson, D. B.; Crandall, I.; Ciach, M.; Kain, K. C.: Selected sulfonyl compounds as anticancer/antimalarial agents. Aust. J. Chem. 56 (2003) 1127–1133.10.1071/CH03073Suche in Google Scholar
8. Duxbury, D. F.: The photochemistry and photophysics of triphenylmethane dyes in solid and liquid media. Chem. Rev. 93 (1993) 381–433.10.1021/cr00017a018Suche in Google Scholar
9. Shchepinov, M. S.; Korshun, V. A. Recent applications of bifunctional trityl groups. Chem. Soc. Rev. 32 (2003) 170–180.10.1039/b008900lSuche in Google Scholar PubMed
10. Nair, V.; Thomas, S.; Mathew, S. C.; Abhilash, K. G.: Recent advances in the chemistry of triaryl- and triheteroarylmethanes. Tetrahedron 62 (2006) 6731–6747.10.1016/j.tet.2006.04.081Suche in Google Scholar
11. Guan, X. Y.; Liu, S. S.; Zhang, L. D.; Guo, X.; Ma, X.; Liu, Z. Q.: The crystal structure of N-((3,5-di-tert-butyl-4-hydroxyphenyl) (phenyl)methyl)-4-methylbenzenesulfonamide, C28H35N3S. Z. Kristallogr. NCS 233 (2018) 259–261.10.1515/ncrs-2017-0257Suche in Google Scholar
12. Guan, X. Y.; Zhang, L. D.; Liu, Z. Q.; Guo, X.; Ma, X.: The crystal structure of (E)-N-benzyl-N′-benzylidene-4-methylbenzenesul fonohydrazide, C21H20N2O2S. Z. Kristallogr. NCS 233 (2018) 153–154.10.1515/ncrs-2017-0256Suche in Google Scholar
13. Guan, X. Y.; Zhang, L. D.; You, P. S.; Liu, S. S.; Liu, Z. Q.: 1,6-Conjugate sulfonylation of para-quinone methides: an expedient approach to unsymmetrical gem-diarylmethyl sulfones. Tetrahedron Lett. 60 (2019) 244–247.10.1016/j.tetlet.2018.12.023Suche in Google Scholar
14. Liu, T.; Liu, J.; Xia, S.; Meng, J.; Shen, X.; Zhu, X.; Chen, W.; Sun, C.; Cheng, F.: Catalyst-free 1,6-conjugate addition/aromatization/sulfonylation of para-quinone methides: facile access to diarylmethyl sulfones. ACS Omega 3 (2018) 1409–1415.10.1021/acsomega.7b01745Suche in Google Scholar PubMed PubMed Central
© 2019 Zhang-Qin Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Artikel in diesem Heft
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3
Artikel in diesem Heft
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3


