Abstract
C26H22N4, monoclinic, P21/c (no. 14), a = 11.472(2) Å, b = 6.0805(12) Å, c = 29.787(6) Å, β = 92.448(3)°, V = 2075.9(7) Å3, Z = 4, Rgt(F) = 0.0427, wRref(F2) = 0.0882, T = 296(2) K.
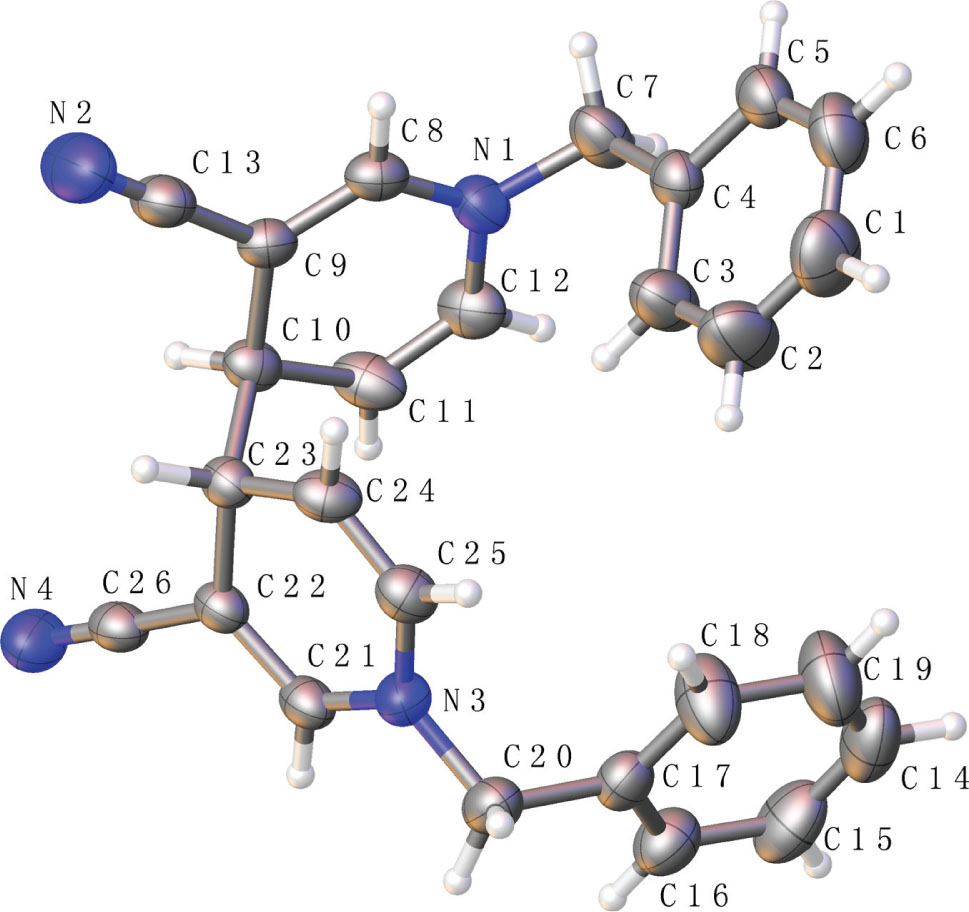
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.25 × 0.22 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 25°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11350, 3661, 0.031 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2871 |
| N(param)refined: | 271 |
| Programs: | Bruker programs [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| N1 | 0.60256(12) | 0.4124(2) | 0.21357(4) | 0.0420(4) |
| N2 | 0.25003(16) | 0.1055(3) | 0.15290(6) | 0.0724(6) |
| N3 | 0.67733(11) | 0.5167(2) | 0.03877(5) | 0.0420(4) |
| N4 | 0.33300(14) | 0.9482(3) | 0.04197(5) | 0.0530(4) |
| C1 | 0.92574(19) | −0.1558(4) | 0.20064(8) | 0.0736(7) |
| H1 | 0.9784 | −0.2599 | 0.1909 | 0.088* |
| C2 | 0.8717(2) | −0.0152(4) | 0.17052(7) | 0.0735(7) |
| H2 | 0.8875 | −0.0251 | 0.1402 | 0.088* |
| C3 | 0.79432(17) | 0.1409(4) | 0.18461(6) | 0.0571(5) |
| H3 | 0.7585 | 0.2359 | 0.1638 | 0.069* |
| C4 | 0.76975(14) | 0.1568(3) | 0.22939(5) | 0.0408(4) |
| C5 | 0.82433(15) | 0.0121(3) | 0.25937(6) | 0.0490(5) |
| H5 | 0.8082 | 0.0197 | 0.2897 | 0.059* |
| C6 | 0.90193(17) | −0.1426(4) | 0.24514(8) | 0.0638(6) |
| H6 | 0.9382 | −0.2381 | 0.2658 | 0.077* |
| C7 | 0.68857(16) | 0.3291(3) | 0.24684(6) | 0.0494(5) |
| H7A | 0.6477 | 0.2671 | 0.2717 | 0.059* |
| H7B | 0.7349 | 0.4516 | 0.2584 | 0.059* |
| C8 | 0.50667(14) | 0.2919(3) | 0.20167(5) | 0.0387(4) |
| H8 | 0.4929 | 0.1635 | 0.2176 | 0.046* |
| C9 | 0.43056(14) | 0.3485(3) | 0.16808(5) | 0.0367(4) |
| C10 | 0.44945(14) | 0.5428(3) | 0.13734(5) | 0.0367(4) |
| H10 | 0.3805 | 0.6377 | 0.1379 | 0.044* |
| C11 | 0.55217(16) | 0.6703(3) | 0.15584(6) | 0.0472(5) |
| H11 | 0.5682 | 0.8048 | 0.1426 | 0.057* |
| C12 | 0.62146(16) | 0.6048(3) | 0.18969(6) | 0.0448(4) |
| H12 | 0.6858 | 0.6910 | 0.1980 | 0.054* |
| C13 | 0.33075(16) | 0.2120(3) | 0.15976(6) | 0.0467(5) |
| C14 | 1.08994(18) | 0.6917(4) | 0.09676(8) | 0.0694(6) |
| H14 | 1.1564 | 0.7246 | 0.1144 | 0.083* |
| C15 | 1.04144(18) | 0.8463(4) | 0.06894(8) | 0.0683(6) |
| H15 | 1.0751 | 0.9851 | 0.0674 | 0.082* |
| C16 | 0.94174(16) | 0.7984(3) | 0.04270(7) | 0.0553(5) |
| H16 | 0.9089 | 0.9061 | 0.0239 | 0.066* |
| C17 | 0.89113(14) | 0.5944(3) | 0.04407(6) | 0.0431(4) |
| C18 | 0.94252(19) | 0.4402(4) | 0.07228(8) | 0.0733(7) |
| H18 | 0.9103 | 0.3001 | 0.0736 | 0.088* |
| C19 | 1.0413(2) | 0.4895(5) | 0.09870(9) | 0.0838(8) |
| H19 | 1.0742 | 0.3834 | 0.1178 | 0.101* |
| C20 | 0.78539(14) | 0.5382(3) | 0.01437(6) | 0.0478(5) |
| H20A | 0.7746 | 0.6518 | −0.0083 | 0.057* |
| H20B | 0.7999 | 0.4009 | −0.0010 | 0.057* |
| C21 | 0.59279(14) | 0.6714(3) | 0.03596(5) | 0.0387(4) |
| H21 | 0.6053 | 0.7964 | 0.0188 | 0.046* |
| C22 | 0.49097(14) | 0.6533(3) | 0.05681(5) | 0.0346(4) |
| C23 | 0.46370(14) | 0.4641(3) | 0.08782(5) | 0.0344(4) |
| H23 | 0.3896 | 0.3986 | 0.0771 | 0.041* |
| C24 | 0.55712(15) | 0.2944(3) | 0.08389(5) | 0.0409(4) |
| H24 | 0.5466 | 0.1593 | 0.0978 | 0.049* |
| C25 | 0.65373(15) | 0.3241(3) | 0.06200(5) | 0.0424(4) |
| H25 | 0.7084 | 0.2112 | 0.0621 | 0.051* |
| C26 | 0.40473(15) | 0.8184(3) | 0.04851(5) | 0.0392(4) |
Source of material
Add 1-benzyl-3-cyanopyridinium(0.9 mmol) to 200 mL of water, and then add Na2CO3 (47.2 mmol) and Na2S2O4 (48.8 mmol), and slowly separate the bright yellow solid. Filter and wash with excess water. The solid was recrystallized from approximately equal volumes of ethanol and water to give a solid. Yield: 66% [4]. Some 1-benzyl-3-cyanopyridine-1,4-dihydropyridine (0.2 mmol) was dissolved in 50 mL of 0.1 M NH3−0.1 M NH4Cl aqueous solution. This solution was electrolyzed at −1.20 V using a round mercury pool (area 20 cm2) as a working electrode with a saturated calomel electrode as reference, and a platinum gauze cylinder as a counter electrode. The solution was magnetically stirred and blanketed by a continuous nitrogen flux over its surface. Usually the electrolysis took about 3 h to get completion. After electrolysis, the mercury pool was covered by a yellow precipitate. The whole content of the cell was repeatedly extracted with CH2Cl2. The gummy residue (0.5 g) was recovered by evaporating the dried organic layer was washed with cold water until solidification and the solid (0.4 g) was extracted three times with 10 mL of 95% ethanol at room temperature. Finally, the solution was evaporated to dryness in vacuo to give the title compound as colorless crystals [5].
Experimental details
All hydrogen atoms were placed in the calculated positions and all the non-hydrogen atoms were refined anisotropically.
Discussion
Reactions that couple two aromatic rings to make biaryls are among the most widely used processes in the pharmaceutical industry [6], [7]. Coupling of pyridines results in heterobiaryls, a privileged pharmacophore found in commercial drugs as well as numerous therapeutic candidates [8], [9], [10]. The title molecule is depicted in the figure. When the 3-position is a strong electron-withdrawing group such as a cyano group, the reaction is relatively easy to occur, and the methylene group in the para position can be activated by an electrochemical method to form a title compound. However, due to the steric hindrance effect, the two benzyl arrangements are not completely identical. In the crystal structure, several important bond angle data are derived as follows C8—N1—C12 = 117.59(14)°, C9—C10—C11 = 108.18(13)°, C22—C23—C24 = 107.97(13)°, C21—N3—C25 = 117.5(14)°. The bond lengths and angles are in the expected ranges.
Acknowledgements
We gratefully acknowledge support by the Science Research Foundation of North China University of Science and Technology. Startup Foundation for Docotors of North China University of Science and Technology.
References
1. BRUKER. SAINT. Version 8.23B., Bruker AXS Inc., Madison, WI, USA (2013).Search in Google Scholar
2. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
4. Brown, A.; Fisher, H. F.: A comparison of the glutamate dehydrogenase catalyzed oxidation of NADPH by trinitrobenzenesulfonate with the uncatalyzed reaction. J. Am. Chem. Soc. 98 (1976) 5682–5688.10.1021/ja00434a046Search in Google Scholar PubMed
5. Carelli, I.; Cardinali, M. E.; Casini, A.: Electrochemical reduction of 3-cyano-1-methylpyridinium iodide, a nicotinamide adenine dinucleotide model compound. J. Org. Chem. 41 (1976) 3967–3969.10.1021/jo00887a007Search in Google Scholar PubMed
6. Brown, D. G.; Bostrom, J.: Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59 (2015) 4443–4458.10.1021/acs.jmedchem.5b01409Search in Google Scholar PubMed
7. Roughley, S. D.; Jordan, A. M.: The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54 (2011) 3451–3479.10.1021/jm200187ySearch in Google Scholar PubMed
8. Capdeville, R.; Buchdunger, E.; Zimmermann, J.: Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 1 (2002) 493.10.1038/nrd839Search in Google Scholar PubMed
9. Roecker, A. J.; Mercer, S. P.; Schreier, J. D.: Discovery of 5′′-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3′′-terpyridine-3′-carboxamide (MK-1064): a selective orexin 2 receptor antagonist (2-SORA) for the treatment of insomnia. ChemMedChem 9 (2014) 311–322.10.1002/cmdc.201300447Search in Google Scholar PubMed
10. Martina, S. D.: Etoricoxib: A highly selective COX-2 inhibitor. Ann. Pharmacother. 39 (2005) 854–862.10.1345/aph.1E543Search in Google Scholar PubMed
© 2019 Hao Zhu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3


