Abstract
C31H27F6O6P, triclinic, P1̄ (no. 2), a = 8.0017(2) Å, b = 11.1702(3) Å, c = 17.8353(4) Å, α = 104.9000(10)°, β = 96.5360(10)°, γ = 97.7380(10)°, V = 1508.05(7) Å3, Z = 2, Rgt(F) = 0.0354, wRref(F2) = 0.0896, T = 150(2) K.
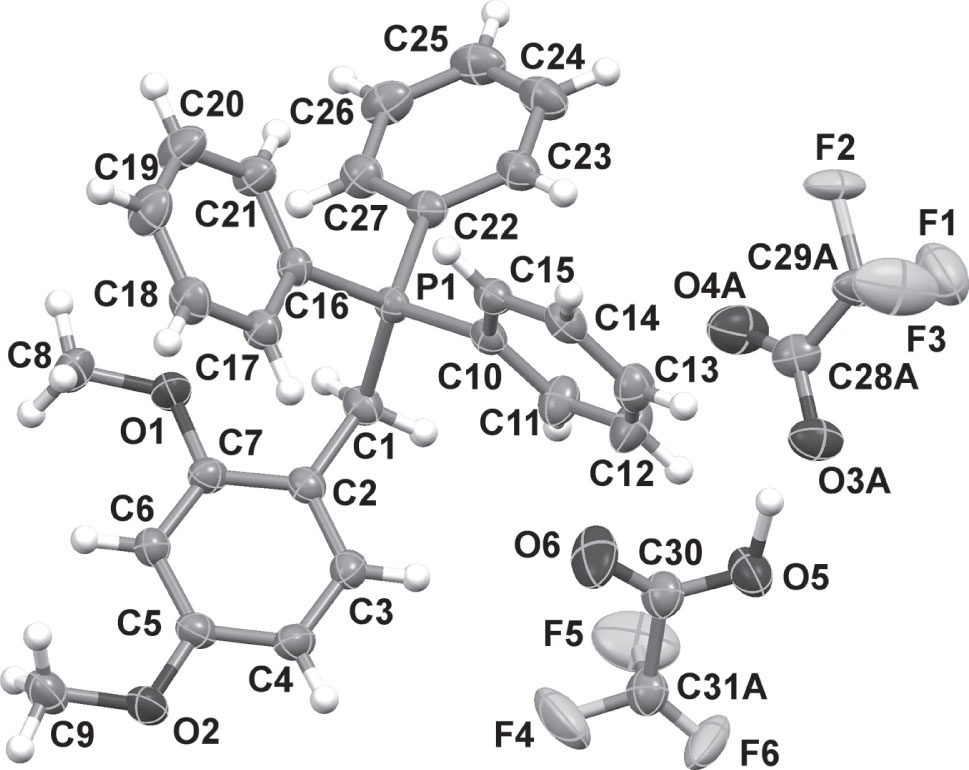
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.40 × 0.23 × 0.17 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 1.53 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 72.37°, 94% |
| N(hkl)measured, N(hkl)unique, Rint: | 49829, 5133, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4610 |
| N(param)refined: | 468 |
| Programs: | Bruker [1], SHELX [2], [3], Mercury [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| P1 | 0.06949(5) | 0.78918(3) | 0.21772(2) | 0.02632(11) |
| O1 | 0.03707(15) | 1.06763(10) | 0.34593(6) | 0.0400(3) |
| O2 | 0.37751(16) | 1.08918(11) | 0.59293(6) | 0.0429(3) |
| O5 | 0.75905(17) | 0.33638(11) | 0.31999(8) | 0.0502(3) |
| H5 | 0.814(3) | 0.320(2) | 0.2597(15) | 0.075* |
| O6 | 0.8437(2) | 0.54398(13) | 0.35006(10) | 0.0679(4) |
| C1 | −0.03351(19) | 0.81364(15) | 0.30501(9) | 0.0316(3) |
| H1A | −0.1293 | 0.8590 | 0.2972 | 0.038* |
| H1B | −0.0828 | 0.7304 | 0.3100 | 0.038* |
| C2 | 0.07919(19) | 0.88550(14) | 0.38097(9) | 0.0297(3) |
| C3 | 0.1485(2) | 0.82724(15) | 0.43441(9) | 0.0335(3) |
| H3 | 0.1267 | 0.7381 | 0.4225 | 0.040* |
| C4 | 0.2484(2) | 0.89640(15) | 0.50453(9) | 0.0366(4) |
| H4 | 0.2950 | 0.8552 | 0.5404 | 0.044* |
| C5 | 0.2799(2) | 1.02649(15) | 0.52208(9) | 0.0333(3) |
| C6 | 0.2123(2) | 1.08769(14) | 0.47020(9) | 0.0321(3) |
| H6 | 0.2344 | 1.1768 | 0.4824 | 0.039* |
| C7 | 0.11191(19) | 1.01646(14) | 0.40035(9) | 0.0309(3) |
| C8 | 0.0908(3) | 1.19879(17) | 0.35556(12) | 0.0556(5) |
| H8A | 0.2158 | 1.2178 | 0.3635 | 0.083* |
| H8B | 0.0488 | 1.2478 | 0.4013 | 0.083* |
| H8C | 0.0447 | 1.2206 | 0.3085 | 0.083* |
| C9 | 0.4233(2) | 1.22261(16) | 0.60905(10) | 0.0449(4) |
| H9A | 0.4845 | 1.2424 | 0.5680 | 0.067* |
| H9B | 0.4969 | 1.2563 | 0.6601 | 0.067* |
| H9C | 0.3197 | 1.2606 | 0.6101 | 0.067* |
| C10 | 0.19682(18) | 0.66755(13) | 0.21458(8) | 0.0277(3) |
| C11 | 0.1486(2) | 0.56850(15) | 0.24577(11) | 0.0408(4) |
| H11 | 0.0461 | 0.5631 | 0.2678 | 0.049* |
| C12 | 0.2509(2) | 0.47789(17) | 0.24452(12) | 0.0494(5) |
| H12 | 0.2185 | 0.4104 | 0.2660 | 0.059* |
| C13 | 0.3997(2) | 0.48451(16) | 0.21233(10) | 0.0410(4) |
| H13 | 0.4701 | 0.4225 | 0.2126 | 0.049* |
| C14 | 0.4459(2) | 0.58100(15) | 0.17987(9) | 0.0352(4) |
| H14 | 0.5474 | 0.5848 | 0.1570 | 0.042* |
| C15 | 0.34510(19) | 0.67248(14) | 0.18048(9) | 0.0310(3) |
| H15 | 0.3768 | 0.7386 | 0.1577 | 0.037* |
| C16 | 0.20992(18) | 0.92615(13) | 0.21348(9) | 0.0286(3) |
| C17 | 0.3580(2) | 0.97019(14) | 0.26932(9) | 0.0331(3) |
| H17 | 0.3800 | 0.9302 | 0.3095 | 0.040* |
| C18 | 0.4720(2) | 1.07237(15) | 0.26554(11) | 0.0404(4) |
| H18 | 0.5725 | 1.1029 | 0.3034 | 0.048* |
| C19 | 0.4399(2) | 1.13031(17) | 0.20663(12) | 0.0469(4) |
| H19 | 0.5187 | 1.2004 | 0.2043 | 0.056* |
| C20 | 0.2944(2) | 1.08674(17) | 0.15146(12) | 0.0472(4) |
| H20 | 0.2734 | 1.1270 | 0.1113 | 0.057* |
| C21 | 0.1787(2) | 0.98458(15) | 0.15435(10) | 0.0366(4) |
| H21 | 0.0786 | 0.9546 | 0.1162 | 0.044* |
| C22 | −0.10081(18) | 0.74348(14) | 0.13589(9) | 0.0302(3) |
| C23 | −0.1226(2) | 0.62720(15) | 0.08078(9) | 0.0361(4) |
| H23 | −0.0456 | 0.5708 | 0.0853 | 0.043* |
| C24 | −0.2574(2) | 0.59387(18) | 0.01919(10) | 0.0456(4) |
| H24 | −0.2733 | 0.5140 | −0.0183 | 0.055* |
| C25 | −0.3689(2) | 0.67595(19) | 0.01180(11) | 0.0471(4) |
| H25 | −0.4599 | 0.6529 | −0.0311 | 0.056* |
| C26 | −0.3480(2) | 0.79176(18) | 0.06683(11) | 0.0437(4) |
| H26 | −0.4248 | 0.8480 | 0.0617 | 0.052* |
| C27 | −0.2155(2) | 0.82591(16) | 0.12934(10) | 0.0373(4) |
| H27 | −0.2024 | 0.9048 | 0.1676 | 0.045* |
| C30 | 0.7771(2) | 0.44883(16) | 0.36147(11) | 0.0400(3) |
| F1a | 0.7135(8) | 0.1103(5) | 0.0367(3) | 0.0834(16) |
| F2a | 0.7951(8) | 0.2615(7) | −0.0037(5) | 0.0502(13) |
| F3a | 0.9784(9) | 0.1835(9) | 0.0567(5) | 0.093(3) |
| F4b | 0.7305(10) | 0.5663(5) | 0.4853(4) | 0.096(2) |
| F5b | 0.5160(4) | 0.4666(6) | 0.40791(18) | 0.0952(19) |
| F6b | 0.6833(11) | 0.3743(3) | 0.4649(3) | 0.098(2) |
| C29Aa | 0.8671(14) | 0.2222(12) | 0.0676(10) | 0.0472(14) |
| C31Aa | 0.7242(8) | 0.4582(4) | 0.4419(3) | 0.0400(3) |
| F1Ac | 0.8189(12) | 0.1002(4) | 0.0515(3) | 0.109(2) |
| F2Ac | 0.8006(11) | 0.2598(9) | 0.0086(6) | 0.105(3) |
| F3Ac | 1.0280(8) | 0.2465(9) | 0.0677(5) | 0.114(3) |
| F4Ad | 0.6921(12) | 0.5674(5) | 0.4755(4) | 0.098(3) |
| F5Ad | 0.5769(10) | 0.3735(6) | 0.4324(4) | 0.129(5) |
| F6Ad | 0.8293(12) | 0.4279(8) | 0.4904(3) | 0.135(3) |
| C29Bc | 0.8284(12) | 0.2159(10) | 0.0567(8) | 0.0472(14) |
| C31Bc | 0.6683(7) | 0.4599(4) | 0.4292(3) | 0.0400(3) |
| O3Ae | 0.87085(17) | 0.26823(14) | 0.19818(9) | 0.0476(4) |
| O4Ae | 0.7084(2) | 0.37043(16) | 0.13586(9) | 0.0637(5) |
| C28Ae | 0.8026(2) | 0.2969(2) | 0.13907(12) | 0.0382(4) |
| O4Bf | 0.787(4) | 0.344(3) | 0.178(2) | 0.0637(5) |
| O3Bf | 0.866(3) | 0.152(3) | 0.1747(14) | 0.0476(4) |
| C28Bf | 0.830(5) | 0.236(4) | 0.148(3) | 0.0382(4) |
aOccupancy: 0.465(8), bOccupancy: 0.530(8), cOccupancy: 0.544(8), dOccupancy: 0.470(8), eOccupancy: 0.950(2), fOccupancy: 0.050(2).
Source of material
All reagents were obtained from Sigma-Aldrich and used without further purification. 2,4-Dimethoxybenzyl alcohol (0.42 g, 2.5 mmol, 1 eq.) was added to a solution of triphenylphosphine (1.31 g, 5 mmol, 2 eq.) in dry toluene (5 mL) at 0 °C under an argon atmosphere. Trifluoroacetic acetic anhydride (0.35 mL, 2.5 mmol, 1 eq.) in dry toluene (5 mL) was subsequently added drop-wise to the reaction mixture. The reaction mixture was allowed to reach room temperature, after which it was heated to and kept at 60 °C for 16 hours. Precipitation with the addition of Et2O (100 mL), followed by recrystallization from DCM, gave the title compound (85%, 1.10 g), as light yellow block-like crystals: 1H-NMR (600 MHz, CDCl3) δH [ppm] 7.78 (3H, br t, J = 7.5 Hz, H-4′), 7.63 (6H, ddd, J = 7.7, 7.5 Hz, JPH = 3.6 Hz, H-3′ and H-5′), 7.48 (6H, br dd, JPH = 12.3 Hz, J = 7.7 Hz, H-2′ and H-6′), 6.91 (1H, dd, J = 8.4 Hz, JPH = 2.8 Hz, H-6), 6.33 (1H, dd, J = 8.4, 2.1 Hz, H-5), 6.19 (1H, d, J = 2.1 Hz, H-3), 4.45 (2H, d, JPH = 13.0 Hz, -CH2-), 3.37 (3H, s, -OMe), 3.19 (3H, s, -OMe); 13C-NMR (151 MHz, CDCl3) δC [ppm] 162.9 (d, JPC = 3.3 Hz, C-4), 160.9 (q, JFC = 36.7 Hz, -C=O), 158.8 (d, JPC = 4.8 Hz, C-2), 135.7 (d, JPC = 2.5 Hz, C-4′), 134.4 (d, JPC = 9.6 Hz, C-2′ and C-6′), 132.8 (d, JPC = 5.4 Hz, C-6), 130.7 (d, JPC = 12.4 Hz, C-3′ and C-5′), 118.7 (d, JPC = 85.2 Hz, C-1′), 116.7 (q, JFC = 290.8 Hz, -CF3), 107.1 (d, JPC = 8.9 Hz, C-1), 105.7 (s, C-5), 99.0 (s, C-3), 56.0 (4-OMe), 55.3 (2-OMe), 25.6 (d, JPC = 49.5 Hz, -CH2-); 31P-NMR (151 MHz, CDCl3) δP [ppm] 19.7 (s, -PPh3); 19F-NMR (151 MHz, CDCl3) δF [ppm] −81.90 (s, -CF3); HRMS (ES): m/z [M]+ required for [C9H11O2PPh3]+: 413.1670, found 413.1663.
Experimental details
The structure was solved by direct methods with the SHELXS-97 program [2], using the ShelXle [3] interface. Molecular graphics were done using ORTEP-3 [4]. All hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms. The C—Haromatic and C—Hmethyl bond distances were restrained to 0.95 Å and 0.95 Å with Uiso(Haromatic) = 1.2Ueq and Uiso(Hmethyl) = 1.5Ueq of the parent atom, respectively. The CF3 groups appear disordered (Table 2).
Comment
Since their discovery, organic phosphonium cationic compounds have found widespread use in synthesis as phase transfer catalysts [5] and as reagents for Wittig [6] and Appel [7] reactions. In terms of biological applications, a range of phosphonium cations have also been found active as anti-bacterial agents against several different types of microorganisms. [8] The multi-functional use of these quaternary phosphorous compounds therefore continues to attract interest into their syntheses and applications.
The title complex crystallized in the triclinic space group P1̄ and Z = 2. A single cation-anion pair, along with a hydrogen-bonded solvent molecule is contained within the asymmetric unit. The phosphonium cation exhibits a slightly distorted tetrahedral geometry with typical C—P—C bond angles of 105.69(7)° – 113.65(7)°, where both the largest and smallest angle is enclosed between the alkyl and a phenyl group. A slightly longer bond of P1—C1 = 1.8198(16) Å is observed, which corresponds to the bound alkyl group, whereas slightly shorter bonds were observed for the bound phenyl groups (1.7956(16) Å – 1.7983(15) Å). The least-squares planes defined by the carbon atoms of the phenyl moieties intersect at angles of 63.53(17)°, 63.89(16)°, and 84.16(17)°. Interestingly, face-to-face π–π interactions between the 2,4-dimethoxyarene ring systems is observed with a centroid-to-centroid distance of 3.631 Å. In addition, alternating edge-to-edge π–π interactions are observed between the same ring systems at a distance of 5.089 Å, at an angle of ca. 46.9°. The disordered moiety of the trifluoroacetato anion forms a strong O5—H5⋯O3 hydrogen bond (H5—O3 = 2.429 Å), at an angle of ca. 155°. The fluorine atoms of the CF3-group of both the acetate and acetic acid molecules are disordered over two unique positions which have been modelled with occupation factors of 0.456 and 0.530, respectively. All other geometric parameters are within the expected ranges.
Acknowledgements
Financial assistance from the South African National Research Foundation (SA NRF) and The University of the Free State (UFS) is gratefully acknowledged.
References
1. Bruker. APEX3, SAINT and SADABS. Bruker AXS Inc., Madison, WI (2012).Search in Google Scholar
2. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
3. Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44 (2011) 1281–1284.10.1107/S0021889811043202Search in Google Scholar PubMed PubMed Central
4. Macrae, C. F.; Bruno, I. J.; Chisholm, J. A.; Edgington, P. R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P. A.: Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 41 (2008) 466–470.10.1107/S0021889807067908Search in Google Scholar
5. Liu, S.; Kumatabara, Y.; Shirakawa, S.: Chiral quaternary phosphonium salts as phase-transfer catalysts for environmentally friendly benign asymmetric transformations. Green Chem. 18 (2016) 331–341.10.1039/C5GC02692JSearch in Google Scholar
6. Ding, W.; Hu, J.; Jin, H.; Xiaochun, Y.; Wang, S.: One-pot synthesis of α,β-unsaturated esters, ketones, and nitriles from alcohols and phosphonium salts. Synthesis 50 (2018) 107–118.10.1055/s-0036-1590904Search in Google Scholar
7. Byrne, P. A.; Rajendran, K. V.; Muldoon, J.; Gilheany, D. G.: A convenient and mild chromatography-free method for the purification of the products of Wittig and Appel reactions. Org. Biomol. Chem. 10 (2012) 3531–3537.10.1039/c2ob07074jSearch in Google Scholar PubMed
8. Li, L.; Zhou, H.; Gai, F.; Chi, X.; Zhao, Y.; Zhang, F.; Zhao, Z.: Synthesis of quaternary phosphonium N-chloramine biocides for antimicrobial applications. RSC Adv. 7 (2017) 13244–13249.10.1039/C6RA24954JSearch in Google Scholar
© 2019 Jireh B.M. Smit et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3

