Abstract
C7H9I5N4O2, monoclinic, P21/c (no. 14), a = 9.46793(10) Å, b = 11.58276(12) Å, c = 16.41497(18) Å, β = 100.844(1)°, Z = 4, V = 1768.00(3) Å3, Rgt(F) = 0.0306, wRref(F2) = 0.0728, T = 130(2) K.
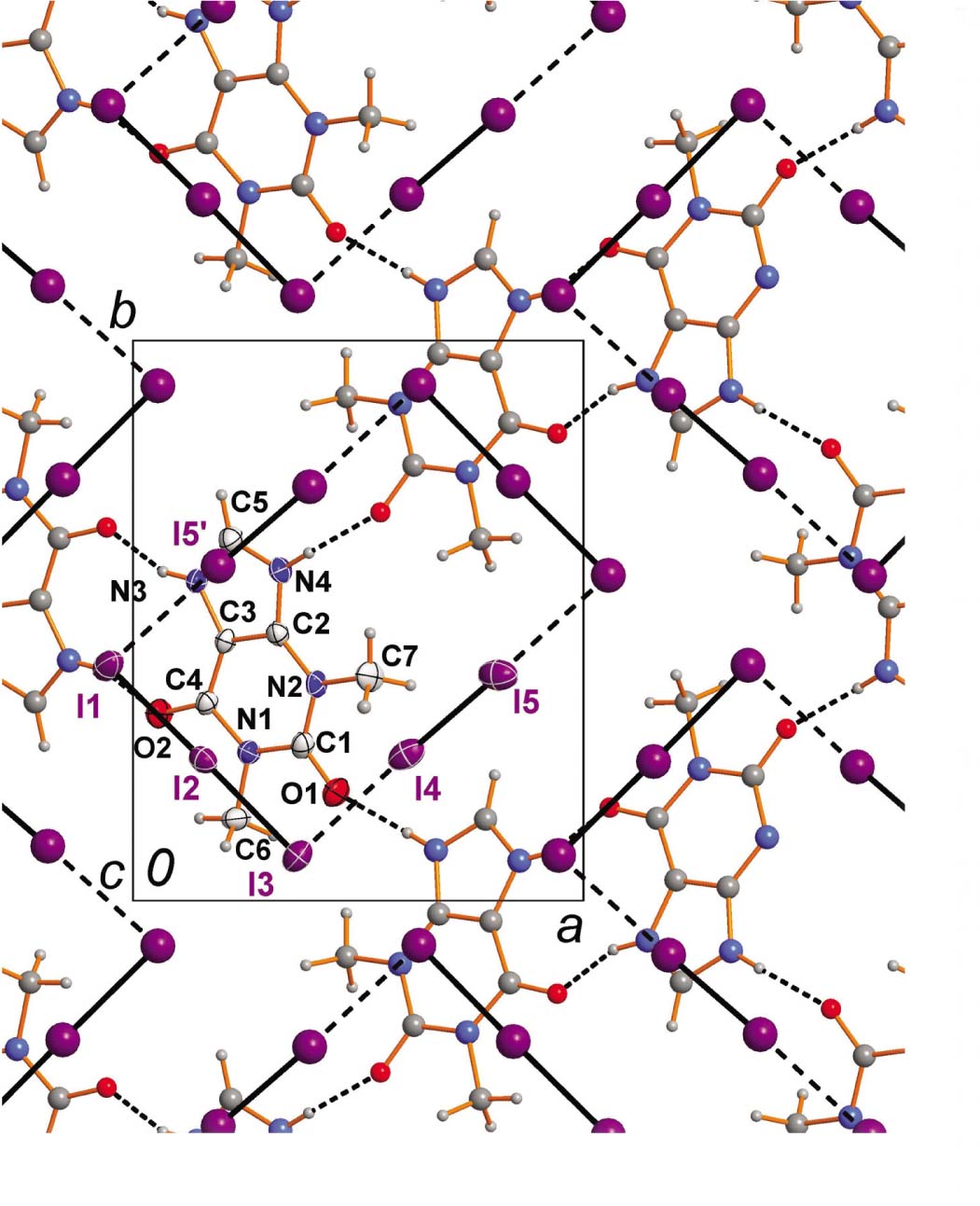
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Black shiny block |
| Size: | 0.34 × 0.27 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 8.80 mm−1 |
| Diffractometer, scan mode: | Xcalibur- EOS, ω |
| θmax, completeness: | 28.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 52671, 4465, 0.030 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4173 |
| N(param)refined: | 174 |
| Programs: | Diamond [1], CrysAlisPRO [2], SHELX [3], [4], [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| I1 | −0.05603(4) | 0.41770(3) | 0.25049(2) | 0.03655(10) |
| I2 | 0.15381(3) | 0.25151(2) | 0.32685(2) | 0.02540(8) |
| I3 | 0.36362(3) | 0.07971(3) | 0.41151(2) | 0.03031(9) |
| I4 | 0.60290(4) | 0.26148(3) | 0.51239(2) | 0.03868(10) |
| I5 | 0.80523(5) | 0.40502(3) | 0.60464(3) | 0.04772(11) |
| O1 | 0.4504(3) | 0.1977(3) | 0.20058(18) | 0.0233(6) |
| O2 | 0.0587(3) | 0.3354(3) | 0.02188(18) | 0.0211(6) |
| N1 | 0.2572(3) | 0.2679(3) | 0.1107(2) | 0.0178(6) |
| N2 | 0.4066(3) | 0.3900(3) | 0.2066(2) | 0.0176(6) |
| N3 | 0.1428(4) | 0.5726(3) | 0.0963(2) | 0.0183(6) |
| H3 | 0.072(7) | 0.592(5) | 0.066(4) | 0.037(16)* |
| N4 | 0.3278(4) | 0.5924(3) | 0.1963(2) | 0.0191(7) |
| H4 | 0.393(7) | 0.624(6) | 0.226(4) | 0.045(18)* |
| C1 | 0.3766(4) | 0.2808(4) | 0.1746(2) | 0.0179(7) |
| C2 | 0.3208(4) | 0.4786(3) | 0.1740(2) | 0.0158(7) |
| C3 | 0.2051(4) | 0.4651(3) | 0.1108(2) | 0.0151(7) |
| C4 | 0.1638(4) | 0.3550(3) | 0.0757(2) | 0.0165(7) |
| C5 | 0.2179(4) | 0.6469(4) | 0.1477(3) | 0.0203(8) |
| H5 | 0.197936 | 0.725295 | 0.149980 | 0.024* |
| C6 | 0.2273(5) | 0.1494(4) | 0.0797(3) | 0.0272(9) |
| H6A | 0.149744 | 0.150503 | 0.032647 | 0.041* |
| H6B | 0.200953 | 0.102392 | 0.122624 | 0.041* |
| H6C | 0.311695 | 0.117929 | 0.063472 | 0.041* |
| C7 | 0.5251(5) | 0.4044(4) | 0.2774(3) | 0.0304(10) |
| H7A | 0.527870 | 0.483025 | 0.296131 | 0.046* |
| H7B | 0.614326 | 0.385626 | 0.260806 | 0.046* |
| H7C | 0.510762 | 0.354048 | 0.321557 | 0.046* |
Source of material
All chemicals were obtained from commercial sources and used as purchased. The Raman spectra were measured using a Bruker MULTIRAM spectrometer (Nd: YAG-laser at 1064 nm; InGaAs detector) with an apodized resolution of 8 cm−1 in the region of 4000−70 cm−1. The title compound was synthesized by dissolving 0.18 g (1 mmol) theophylline and 0.25 g (1 mmol) diiodine in 1 mL of 57% aqueous hydroiodic acid. Heating to ∼350 K yielded a dark colored solution, which produced black, block crystals upon slow cooling to room temperature.
Experimental details
A single crystal of the title compound was directly selected from the mother liquor and rapidly transferred into the cold gas-stream (T = 146 K) of the Xcalibur four-circle diffractometer equipped with an EOS detector [2]. An absorption correction (Gaussian method) was applied [2]. The structure solution and the refinement were successfully carried out using the SHELX program system [3], [4], [5]. Hydrogen atoms which are involved in hydrogen bonds were refined freely. All other hydrogen bonds were added using a riding model with fixed Uiso parameters. The maximum residual peak of 3.63 eÅ−3 and the deepest hole of −2.15 eÅ−3 are found 0.79 and 0.68 Å, respectively, from atom I5.
Comment
Nowadays polyiodides (in the 19th century periodides [6]) are defined as the anionic parts of salt structures that fulfil the general formula
From the reaction of theophylline (systematic name: 1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione) with hydroiodic acid in the presence of excess iodine, black, shiny, block crystals of the title compound were obtained. The crystalline material loses its excess of iodine slowly at ambient conditions.
The asymmetric unit of the title compound consists of one N-protonated theophyllinium cation, one triiodide anion and one iodine molecule residing on general positions in the space group P21/c. The geometric parameters of the title cation are very similar to those known from the literature (Tab. 2) [29].
In the title structure each theophyllinium cation donates and accepts two hydrogen bonds to three (crystallographically dependent) adjacent cations via its N—H and C=O functional groups, respectively (see the figure). These hydrogen bonds lead to wavy layers. Six symmetry-related cations form annulated ring motifs (see the figure). The NH⋯O hydrogen-bond parameters are (N⋯O distances of 2.671(4) Å and 2.725(4) Å) in the expected ranges [27].
Between adjacent cationic, hydrogen-bonded theophyllinium layers the polyiodide anions are intercalated (see the figure). Each polyiodide I102− moiety consists of two pentaiodide fragments, which show I–I distances which are in the typical range of single bonds to strong secondary interactions (I1–I2: 2.8787(4) Å; I2–I3: 2.9661(4) Å; I4–I5: 2.7636(6) Å; I3⋯I4: 3.3009(5) Å) [9]. The I5⋯I1′ [′ = 1 − x, 1 − y, 1 – z] distance of 3.6582(6) Å is in the typical range of weaker I⋯I interactions, but significantly shorter than any van der Waals distances in the various scales [30]. In the structure of the known cyclic I102−, the four secondary I⋯I distances are crystallographically constrained and are at 3.441(1) Å [28] fairly accurate within the arithmetic mean of the distances derived for the title structure. Further significant I⋯I interactions can be ruled out as the corresponding iodine to iodine distances (>3.9 Å) are very close the sum of the van der Waals radii. The angles within the I102− dianion are in accord with the expectations (I2–I1–I5′: 77.41(1)°; I2–I3–I4: 98.22(1)°; I4–I3–I1–I5′ [′ = 1 – x, 1 – y, 1 – z]). Finally, it is worth mentioning that the hydrogen-bonded layers show a wavy shape fitting excellently with the needs of the neighboring polyiodide anions.
Within the Raman spectrum of the title compound the lines which are characteristic for the triiodide anion [31] are found at 108(vs) and 145(vs) cm−1. For the solid phase of elemental iodine a I–I valence vibration is generally observed at 180 cm−1 [32]. The Raman spectrum of the title compound shows a very strong line at 172 cm−1. As the iodine molecule is weakly connected to two neighboring halogen bond donors, a small shift of this line to lower wave numbers compared to elemental iodine is expected.
Funding source: Ministry of Innovation, Science and Research of North-Rhine Westphalia and the German Research Foundation (DFG) for financial support is gratefully acknowledged (Xcalibur diffractometer; INST 208/533−1
Award Identifier / Grant number: 162659349
Funding statement: The authors thanks E. Hammes and M. Wyshusek for technical support. Support by the Ministry of Innovation, Science and Research of North-Rhine Westphalia and the German Research Foundation (DFG) for financial support is gratefully acknowledged (Xcalibur diffractometer; INST 208/533−1, project No. 162659349). Funding by the open access fund of the Heinrich-Heine-Universität Düsseldorf is also gratefully acknowledged.
References
1. Brandenburg, K.: DIAMOND. Visual Crystal Structure Information System. Ver. 4.5.2, Crystal Impact, Bonn, Germany (2018).Search in Google Scholar
2. Oxford Diffraction: CrysAlisPRO, (version 1.171.33.42), Oxford Diffraction Ltd., Oxford, UK (2009).Search in Google Scholar
3. Sheldrick, G. M.: A short history of SHELX. Acta Crystallogr. A64 (2008) 112–122.10.1107/S0108767307043930Search in Google Scholar
4. Sheldrick, G. M.: Crystal structure refinement with SHELXL. Acta Crystallogr. C71 (2015) 3–8.10.1107/S2053229614024218Search in Google Scholar
5. Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B.: ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 44 (2011) 1281–1284.10.1107/S0021889811043202Search in Google Scholar
6. Tilden, W. A.: On the periodides of some of the organic bases. J. Chem. Soc. 18 (1865) 99–105.10.1039/JS8651800099Search in Google Scholar
7. Tebbe, K.-F.: Polyhalogen cations and polyhalide anions, in Homoatomic rings, chains and macromolecules of main-group elements. (Eds.: H. Wood, F. Belger), Elsevier, Amsterdam (1977) 551–606.Search in Google Scholar
8. Blake, A. J.; Devillanova, F. A.; Gould, R. O.; Li, W. S.; Lippolis, V.; Parsons, S.; Radek, C.; Schröder, M.: Template self-assembly of polyiodide networks. Chem. Soc. Rev. 27 (1998) 195–205.10.1039/a827195zSearch in Google Scholar
9. Svensson, P. H.; Kloo, L.: Synthesis, structure, and bonding in polyiodide and metal iodide-iodine systems. Chem. Rev. 103 (2003) 1649–1684.10.1021/cr0204101Search in Google Scholar
10. Kloo, L.; Rosdahl, J.; Svensson, P. H.: On the intra- and intermolecular bonding in polyiodides. Eur. J. Inorg. Chem. 2002 (2002) 1203–1209.10.1002/1099-0682(200205)2002:5<1203::AID-EJIC1203>3.0.CO;2-OSearch in Google Scholar
11. Politzer, P.; Murray, J. S.: Halogen bonding: an interim discussion. ChemPhysChem 14 (2013) 278–294.10.1002/cphc.201200799Search in Google Scholar
12. Politzer, P.; Murray, J. S.; Clark, T.: σ-Hole bonding: a physical interpretation, in halogen bonding I. Vol. 358 (Eds.: P. Metrangolo, G. Resnati), Springer International Publishing, (2015) 19–42.10.1007/128_2014_568Search in Google Scholar
13. Thirman, J.; Engelage, E.; Huber, S. M.; Head-Gordon, M.: Characterizing the interplay of Pauli repulsion, electrostatics, dispersion and charge transfer in halogen bonding with energy decomposition analysis. Phys. Chem. Chem. Phys. 20 (2018) 905–915.10.1039/C7CP06959FSearch in Google Scholar
14. O’Regan, B.; Grätzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353 (1991) 737–739.10.1038/353737a0Search in Google Scholar
15. Correa-Baena, J.-P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T. J.; Grätzel, M.; Hagfeldt, A.: The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 10 (2017) 710–727.10.1039/C6EE03397KSearch in Google Scholar
16. Li, B.; Nie, Z.; Vijayakumar, M.; Li, G.; Liu, J.; Sprenkle, V.; Wang, W.: Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat. Commun. 6 (2015) 6303.10.1038/ncomms7303Search in Google Scholar PubMed PubMed Central
17. Zhao, Y.; Hong, M.; Bonnet Mercier, N.; Yu, G.; Choi, H. C.; Byon, H. R.: A 3.5 V lithium-iodine hybrid redox battery with vertically aligned carbon nanotube current collector. Nano Lett. 14 (2014) 1085–1092.10.1021/nl404784dSearch in Google Scholar PubMed
18. Walbaum, C.; Pantenburg, I.; Meyer, G.: Penta-, Hepta- und Oktaiodid-Anionen in Salzen mit Erdalkalimetall-Kronenether-Kationen. Z. Naturforsch. B65 (2010) 1077–1083.10.1515/znb-2010-0904Search in Google Scholar
19. Walbaum, C.; Pantenburg, I.; Junk, P.; Deacon, G. B.; Meyer, G.: Bulky cations and four different polyiodide anions in [Lu(Db18c6)(H2O)3(thf)6]4(I3)2(I5)6(I8)(I12). Z. Anorg. Allg. Chem. 636 (2010) 1444–1446.10.1002/zaac.201000112Search in Google Scholar
20. Peuronen, A.; Rinta, H.; Lahtinen, M.: N⋯I halogen bonding supported stabilization of a discrete pseudo-linear [I12]2− polyiodide. CrystEngComm 17 (2015) 1736–1740 and references cited therein.10.1039/C4CE01866DSearch in Google Scholar
21. van Megen, M.; Reiss, G. J.: I62− Anion composed of two asymmetric triiodide moieties: a competition between halogen and hydrogen bond. Inorganics 1 (2013) 3–13.10.3390/inorganics1010003Search in Google Scholar
22. van Megen, M.; Jablonka, A.; Reiss, G. J.: Synthesis, structure and thermal decomposition of a new iodine inclusion compound in the 2,2-dimethylpropane-1,3-diamine/HI/I2 system. Z. Naturforsch. B69 (2014) 753–760.10.5560/znb.2014-4088Search in Google Scholar
23. Reiss, G. J.: Two iodine-rich (dimethylphosphoryl)methanaminium iodides. Z. Kristallogr. CM 232 (2017) 789–795.10.1515/zkri-2017-2071Search in Google Scholar
24. Reiss, G. J.; Leske, P. B.: The twinned crystal structure of bis(4-aminopyridin-1-ium) iodide triiodide, C20H28I8N8. Z. Kristallogr. NCS 229 (2014) 452–454 and references cited.10.1515/ncrs-2014-0193Search in Google Scholar
25. Reiss, G. J.; van Megen, M.: Two new polyiodides in the 4,4′-bipyridinium diiodide/iodine system. Z. Naturforsch. B67 (2012) 5–10.10.5560/ZNB.2012.67b0005Search in Google Scholar
26. Reiss, G. J.: I5− polymers with a layered arrangement: synthesis, spectroscopy, and structure of a new polyiodide salt in the nicotine/HI/I2 system. Z. Naturforsch. B70 (2015) 735–740.10.1515/znb-2015-0092Search in Google Scholar
27. Merkelbach, J.; Majewski, M. A.; Reiss, G. J.: Crystal structure of caffeinium triiodide – caffeine (1/1), C16H21I3N8O4. Z. Kristallogr. NCS 233 (2018) 941–944.10.1515/ncrs-2018-0125Search in Google Scholar
28. Wieczorrek, C.: Isolierte I102−-Ringe, ein neues Strukturelement in der Polyiodid-Chemie. Acta Crystallogr. C56 (2000) 1082–1084.10.1107/S0108270100008817Search in Google Scholar
29. Buist, A. R.; Kennedy, A. R.; Manzie, C.: Four salt phases of theophylline. Acta Crystallogr. C70 (2014) 220–224.10.1107/S2053229614000825Search in Google Scholar
30. Hu, S.-Z.; Zhou, Z.-H.; Xie, Z.-X.; Robertson, B. E.: A comparative study of crystallographic van der Waals radii. Z. Kristallogr. CM 229 (2014) 517–523.10.1515/zkri-2014-1726Search in Google Scholar
31. Deplano, P.; Ferraro, J. R.; Mercuri, M. L.; Trogu, E. F.: Structural and Raman spectroscopic studies as complementary tools in elucidating the nature of the bonding in polyiodides and in donor I2 adduct. Coord. Chem. Rev. 188 (1999) 71–95.10.1016/S0010-8545(98)00238-0Search in Google Scholar
32. Congeduti, A.; Nardone, M.; Postorino, P.: Polarized Raman spectra of a single crystal of iodine. Chem. Phys. 256 (2000) 117–123.10.1016/S0301-0104(00)00085-9Search in Google Scholar
© 2019 Guido J. Reiss, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 Public License.
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3
Articles in the same Issue
- Frontmatter
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-5-fluorophenol, C14H13FN2O
- Crystal structure of (E)-2-(1-((2-aminophenyl)imino)ethyl)-4,6-dichlorophenol, C14H12Cl2N2O
- The crystal structure of (E)-1-(4-methoxyphenyl)-3-(2-nitrophenyl)triaz-1-ene C8H8N2O4
- Crystal structure of (E)-2-(((6-bromopyridin-2-yl)methylene)amino)-3′,6′-bis(ethylamino)-2′,7′-dimethylspiro[isoindoline-1,9′-xanthen]-3-one—methanol (1:1), C32H30N5O2Br ⋅ CH4O
- Crystal structure of 2,4-pentanedione bis(2,4-dinitrophenylhydrazone), C17H16N8O8
- Crystal structure of sodium morpholine-4-carbodithioate, (C5H12NNaO3S2)
- Crystal structure of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluorido phosphate), C16H28F12N4P2
- Crystal structure of 5-(4-chlorophenyl)-3-(4-fluorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazole, C21H16ClFN2
- Crystal structure of catena-poly[diaqua-bis(3-carboxy-5-methoxybenzoato-κO)-(1,2-bis(imidazol-1-yl)ethane-κ2N:N′)cobalt(II)], C26H28CoN4O12, [Co(C9H6O5)2(H2O)2(C8H10N4)]
- The crystal structure of 3-cyclohexyl-1,5-dioxaspiro[5.5]undecane-2,4-dione, C15H22O4
- Crystal structure of (2,4-dimethoxybenzyl)triphenylphosphonium trifluoroacetate — trifluoroacetic acid (1/1), C31H27F6O6P
- Crystal structure of 4-tert-butyl-1-(2,6-dimethylphenyl)-1H-1,2,3-triazole, C14H19N3
- Crystal structure of 1,1′-methylenebis(4-tert-butylpyridinium) tetrachloridocobaltate(II) – dichloromethane (1:1), C20H30Cl6CoN2
- Crystal structure of (4,4′-(ethane-1,2-diylbis((nitrilo)(2-furylmethylylidene)))bis(3-methyl-1-phenyl-1H-pyrazol-5-olato-κ4N,N′,O,O′))-nickel(II)), C32H26N6NiO4
- Synthesis and crystal structure of bis{((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ2N,O}copper(II), C44H38CuN4O4
- Crystal structure of catena-poly[diaqua-bis(3,5-dichloropyridine-4-carboxylato-κ1O)-bis(μ2-4,4′-bipyridine-κ2N:N′)cobalt(II)], C22H16Cl4CoN4O6
- The crystal structure of 2-(4-chloro-2,6-dinitrophenyl)-1-(4-chloro-3,5-dinitrophenyl)diazene 1-oxide, C12H4Cl2N6O9
- The crystal structure of 3-(1H-benzo[d]imidazol-2-yl)-7-chloro-1-cyclopropyl-6-fluoro-1,4-dihydroquinolin — dimethylsulfoxide (1/1), C21H19ClFN3O2S
- The crystal structure of dichlorido-bis(1-butyl-1H-imidazole-κN)zinc(II), C14H24Cl2ZnN4
- (Z)-N-tert-butyl-1-(2-(3,5-dichlorobenzamido)phenyl) methanimine oxide, C18H18Cl2N2O2
- Crystal structure of diaqua-bis(3-carboxy-5-bromoisophthalato-κO)-bis(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-benzo[d]imidazol-3-ium-κN)nickel(II) bis(3-carboxy-5-bromoisophthalate), C66H54Br4N8NiO18
- Crystal structure of poly[aqua(μ2-5-methoxyisophthalato-κ2O,O′:O′′)-(1,2-bis(imidazol-1′-yl)ethane-κ2N:N′)cobalt(II), C34H36Co2N8O12
- Crystal structure of poly[diaqua-bis(μ2-1-(4-(1H-imidazol-1-yl)benzyl)-1H-1,2,4-triazole-κ2N:N′)manganese(II)] terephthalate tetrahydrate, MnC32H38N10O10
- Crystal structure of the fluorescent fipronil derivative 5,5′-(methylenebis(azanediyl))bis(1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carbonitrile), C25H6N8O2Cl4F12S2
- Crystal structure of the phosphorescent complex diethyldithiophosphonato-κ2S,S′-bis(2-phenylpyridinato-κ2C,N)iridium(III), C26H26N2O2PS2Ir
- The crystal structure of 4,10-diethoxy-6H,12H-6,12-epoxydibenzo[b,f][1,5]dioxocine, C18H18O5
- Crystal structure of dichlorido-bis(N-benzyl-2-(quinolin-8-yloxy)acetamide-κ2N,O)copper(II) — ethyl acetate (1/1), C38H36N4O6Cl2Cu
- Synthesis and crystal structure of bis{4-methyl-2-((E)-((4-((E)-1-(benzyloxyimino)ethyl)phenyl)imino)methyl)phenolato-κ3N,O,O}copper(II), C92H84Cu2N8O8
- The crystal structure of 1,3,5-trinitro-4,6-diazidobenzene, C6HN9O6
- Crystal structure 1-cinnamyl-2-((Z)-styryl)-1H-benzo[d]imidazole — methanol (1/1), C24H20N2 ⋅ CH4O
- The crystal structure of poly[m2-aqua-tetraaqua-bis(m9-4-formylbenzene-1,3-disulfonato)tetrasodium(I) hydrate, C14H18O19S4Na4
- Crystal structure of 2-((2,8-bis(trifluoromethyl)quinolin-4-yl)(hydroxy)methyl)piperidin-1-ium trifluoroacetate, [C17H17F6N2O][C2F3O2]
- The crystal structure of bis(ferrocenecarboxylato-κ2O,O′)bis[4-(dimethylamino)pyridine-κN]copper(II) — acetonitrile(1/2), C40H44CuO4Fe2N6
- Crystal structure of poly[di-μ2-aqua)-diaqua-bis(μ6-4,4′,4′′-(benzene-1,3,5-triyltris(oxy))tribenzoato-κ6O1:O2:O3:O3:O5:O6)tricadmium(II)] dihydrate, C54H42Cd3O24
- The crystal structure of dichlorido(1,3-bis(2,6-diisopropyl-phenyl)-1H-3λ4-imidazol-2-yl)(3-phenyl-pyridine-κN)palladium(IV), C38H45N3Cl2Pd
- The crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-phenyl-1,3,5-triazine, C12H8ClN3O
- The crystal structure of 2,6-di-tert-butyl-4-(phenyl(phenylsulfonyl)methyl)phenol, C27H32O3S
- Crystal structure of bis{μ2-bis{(((((1-methoxyimino)ethyl)phenyl)imino)methyl)-2-phenolato-κ3N,O:O}copper(II)}, C68H68Cu2N8O8
- Crystal structure of catena-poly[tetraaqua-bis(μ2-2-(4-carboxylatophenoxy)benzoato-κ2O:O′)-pentakis(pyridine-κ1N)dinickel(II)], C53H47N5Ni2O13
- Synthesis and crystal structure of 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-5-(3-methoxy-benzylamino)-4-trifluoromethanesulfinyl-1H-pyrazole-3-carbonitrile, C20H12N4Cl2F6O2S
- Redetermination of the crystal structure of bis(μ2-di-ethyldithiocarbamato-κ3S,S′:S;κ3S:S: S′)-hexacarbonyl-di-rhenium(I), C16H20N2O6Re2S4
- The crystal structure of (E)-N′-((2-hydroxynaphthalen-1-yl)methylene)-2-phenylacetohydrazide, C19H16O2N2
- Crystal structure of 6-hydroxy-4,8,11b-trimethyltetradecayhdro-8,11-epoxy-6a,9-methanocyclohepta[a]naphthalene-4-carboxylic acid – methanol (1/1), C20H30O4
- The crystal structure of aqua-bis(3-acetyl-2-oxo-2H-chromen-4-olato-κ2O,O′)zinc(II) monohydrate, C22H18O10Zn
- Crystal structure of poly[bis(μ2-4-bromoisophthalate-κ2O:O′)-tris(μ2-1-(3-((1H-1,2,4-triazol-1-yl)methyl)benzyl)-1H-1,2,4-triazole-κ2N:N′)dicobalt(II)] monohydrate, C26H23CoN9O5Br
- A cyclic I102− anion in the layered crystal structure of theophyllinium pentaiodide, C7H9I5N4O2
- Crystal structure of catena-poly[diaqua-bis(μ2-4-((4-(pyridin-2-ylmethoxy)phenyl)diazenyl)benzoato-κ3O,O′:N)cadmium(III)], Cd(C19H14O3N3)2(H2O)
- Crystal structure of catena-poly[(μ2-4,4′-bipyridyl-κN,N′)-bis(O,O′-dimethyldithiophosphato-κS)-zinc(II)], {C14H20N2O4P2S4Zn}n
- Crystal structure of 3-amino-2-hydroxy-6-methoxybenzamide hydrate, C16H22N4O7
- Crystal structure of hemikis(cyclohexane-1,4-diammonium) (pyridine-2-carboxylate), [C6H16N2]0.5[C6H4NO2]
- Crystal structure of 2-chloro-4-(prop-2-yn-1-yloxy)-6-(thiophen-2-yl)-1,3,5-triazine, C10H6ClN3OS
- The crystal structure of 3-butyl-1-methyl-1H-imidazol-3-ium catena-poly[tris(μ2-bromido-κ2Br:Br)lead(II)], C8H15Br3N2Pb
- Crystal structure of 3-(5-amino-1H-1,2,4-triazol-3-yl)-1-(piperidin-1-yl)propan-1-one, C10H17N5O
- Crystal structure of aqua-2,2′,2′′-(((nitrilo-κN-tris(ethane-2,1-diyl))tris(azanylylidene-κ3N′,N′′,N′′′))tris(methanylylidene))tris(4-chlorophenolato-κ3O,O′,O′′)neodymium(III), C27H26Cl3N4NdO4
- Crystal structure of dichlorido-(μ2-2,2′-(diazene-1,2-diyl)bis(benzen-1-ido)-κ2C:C′)dimercury(II), C12H8Cl2Hg2N2
- Crystal structure of (3E,5E)-3,5-bis(4-cyanobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C27H18FN3O3S
- Crystal structure of dichlorido(pyridine-κN)(2,4,6-tri-2-pyridyl-1,3,5-triazine-κ3N2,N1,N6)nickel(II), C23H17Cl2N7Ni
- Redetermination of the crystal structure of tetrakis(4-chlorobenzyl)tin(IV), C28H24Cl4Sn
- The crystal structure of 2,6-bis(pyridin-1-ium-3-ylmethyl)hexahydro-4,8-ethenopyrrolo-[3,4-f] isoindole-1,3,5,7-tetrone tetrachloridocuprate(II) monohydrate, C24H24Cl4CuN4O5
- Crystal structure of cyclo-[octaaqua-tetrakis(μ2-5,5′-(1H-imidazole-4,5-diyl)bis(tetrazol-2-ido)-κ4N,N′,N′′,N′′′)tetramagnesium(II)], C20H24N40O8Mg4
- The crystal structure of a matrine derivative, 13-(4-Cl-pyrrole)-matrine, C18H26ClN4O
- Crystal structure of (dibenzyl sulphoxide-κO)bis(2-chlorobenzyl-κC1)dichloridotin(IV), C28H26Cl4OSSn
- Crystal structure of catena-poly[(μ2-azido-κ2N:N)(μ2-4-cyanobenzoato-κ2O:O′)-(μ2-methanol-κ2O:O)copper(II)], C9H8CuN4O3
- Crystal structure of 1,1′-dibenzyl-3,3′-dicyano-1,1′,4,4′-tetrahydro-4,4′-bipyridine, C26H22N4
- Crystal structure of (2-bromobenzyl)((1-bromonaphthalen-2-yl)methyl)sulfane, C18H14Br2S
- Crystal structure of 2-(4-ammoniocyclohexyl)-3-(pyridin-2-yl)imidazo[1,5-a]pyridin-2-ium 2-[(2-carboxylatophenyl)disulfanyl]benzoate dihydrate, [C18H22N4][C14H8O4S2] ⋅ 2H2O
- Crystal structure of (E)-N-((3R,5S,10S, 13S,14S,17S)-17-((S)-1-(dimethylamino)ethyl)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl)-2-methylbut-2-enamide – water – methanol (1/1/1), C29H54N2O3
- Crystal structure of methyl 2-(4-(3-(2,4-difluorophenyl)pyrazolo[1,5-a]pyrimidin-6-yl)phenyl)acetate, C21H15F2N3O2
- Crystal structure of poly[triaqua-(μ4-benzene-1,3,5-tricarboxylato-κ5O1,O2:O3:O4:O5)-(μ2-5-(3-pyridyl)tetrazolato-κ2N1:N3)dizinc(II)], C15H13N5O9Zn2
- Crystal structure of N-(3-methylphenyl)(propan-2-yloxy)carbothioamide, C11H15NOS
- Crystal structure of poly[(μ2-1,3-bis(imidazol-1-ylmethyl)benzene-κ2N:N′)(nitrato-κ1O)cadmium(II)] — water (2/1), C28H32CdN10O7
- Crystal structure of 4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione, C8H7N3S
- Crystal structure of benzyltrichloridobis(1H-pyrazole-κ2N)tin(IV), C13H15Cl3N4Sn
- Crystal structure of chlorido-4-fluorobenzyl-bis(2-methylquinolin-8-olato-κ2N,O)tin(IV), C27H22ClFN2O2Sn
- Crystal structure of tetrakis(O,O′-diisopropyldithiophosphato-κ2S,S′)-(μ2-1,2-bis(4-pyridylmethylene)hydrazine-κ2N:N′)zinc(II), C36H66N4O8P4S8Zn2
- Crystal structure of tetrabutylammonium 4,4-oxydibenzoate – boric acid – water (1/2/6) C46H98B2N2O17
- Redetermination of the crystal structure of catena-poly[[tribenzyltin(IV)]-(μ2-pyridine-4-carboxylato-κ2N:O)], C27H25NO2Sn
- The synthysis and crystal structure of cyclohexyl 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-((trifluoromethyl)sulfinyl)-1H-pyrazole-3-carboxylate, C18H15N3Cl2F6O3S
- The crystal structure of 5,7-bis(2-hydroxyethoxy)-2-phenyl-4H-chromen-4-one, C19H18O6
- Synthesis and crystal structure of (±)-Ethyl 5′-(difluoromethyl)-2-oxo-4′,5′-dihydrospiro[indoline-3,3′-pyrazole]-4′-carboxylate, C14H13F2N3O3

